Abstract
Retinal Vein Occlusion (RVO) is a blinding disease caused by one or more occluded retinal veins. Current treatment methods only focus on symptom mitigation rather than targeting a solution for the root cause of the disorder. Retinal vein cannulation is an experimental eye surgical procedure which could potentially cure RVO. Its goal is to dissolve the occlusion by injecting an anticoagulant directly into the blocked vein. Given the scale and the fragility of retinal veins on one end and surgeons’ limited positioning precision on the other, performing this procedure manually is considered to be too risky. The authors have been developing robotic devices and instruments to assist surgeons in performing this therapy in a safe and successful manner. This work reports on the clinical translation of the technology, resulting in the world-first in-human robot-assisted retinal vein cannulation. Four RVO patients have been treated with the technology in the context of a phase I clinical trial. The results show that it is technically feasible to safely inject an anticoagulant into a \(100\,{\mu} {\rm m}\)-thick retinal vein of an RVO patient for a period of 10 min with the aid of the presented robotic technology and instrumentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background
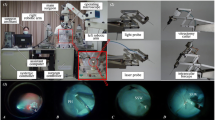
Vitreoretinal surgery is a branch within ophthalmologic microsurgery that encompasses a range of demanding tasks at the posterior pole of the eye. Figure 1 depicts the typical surgical scene during a vitreoretinal intervention. Depending on the surgical procedure, a number of incisions are created in the sclera and equipped with valved trocars through which the required surgical instruments are inserted. Throughout the procedure, the surgeon is able to visualise the surgical workspace by looking through a stereoscopic microscope, located directly above the patient’s eye. Often, an additional wide-angle viewing system is used to enlarge the field of view. The workspace is illuminated by means of a handheld light probe or by self-maintained chandelier light.
The VR operating scene. (a) The surgeon’s posture with respect to the patient and the microscope. (b) Close-up on the surgeon’s hands and on the wide-angle viewing system. (c) Extraocular close-up demonstrating the incision ports and the trocars. (d) Intraocular close-up showing the patient’s retina and a forceps instrument.
Over the past two decades, technological innovation in the field of surgical instrumentation has enabled vitreoretinal surgeons to perform a wide range of novel treatment methods. Despite this progress, the standard of care for a number of highly prevalent retinal diseases, such as Retinal Vein Occlusion (RVO), remains inadequate as the envisioned treatment methods require complex surgical actions exceeding the human skill. RVO is a retinal condition where clots inside retinal veins distort the oxygen delivery throughout the retina (Fig. 2). The disease leads to partial or nearly complete blindness depending on the location of the clot, caused by branch RVO (BRVO) or central RVO (CRVO) respectively. RVO is the second most common retinal vascular disease, affecting an estimated 16.4 million people worldwide.17 Currently, the standard of care is focused on complications induced by the sudden stop in retinal circulation rather than on the occlusion itself, which is the root cause of the disease. Consequently, RVO patients need to be treated on a periodic basis with costly therapies, such as intravitreal injections and laser photocoagulation, in order to offer limited symptom relief.21 A promising potentially curative treatment method is retinal vein cannulation, also known as retinal endovascular surgery (REVS).21 During REVS, the surgeon aims at dissolving the clot by injecting an anticoagulant directly into the occluded vein (Fig. 2).
When using this technique, some surgeons reported good results while others experienced significant complications and could not confirm a gain in visual acuity.3,20 The high complication rate is considered to be strongly correlated with the associated surgical complexity. The targeted veins have a thickness ranging from 30 to 400 μm and are extremely fragile. Two types of unintended surgical motions inhibit the safe insertion of the microneedle into the occluded vein. Firstly, the surgeon’s hand tremor causes the tip of the microneedle to vibrate with a root mean square amplitude in the order of \(180\,{\mu} {\rm m}\).16 Secondly, forces applied on the incision with the surgical instrument, which are tangential to the sclera, cause the eye to rotate (Fig. 3a). The subsequent motion of the targeted vein further impedes the safe insertion of the microneedle. Lastly, after the microneedle is correctly placed, the surgeon must precisely maintain its position throughout the injection phase, which can take up to 10 min.20 These technical difficulties and the associated risk of complications have hindered further clinical research on the potential of REVS as a curative treatment for RVO. This has inspired research institutes around the globe to develop robotic assistance devices that offer features such as precision enhancement, eye stabilisation and hand-free tool immobilisation to enable the safe application of REVS.
State-Of-The-Art
A fair number of robotic prototypes have been developed with the purpose of enabling complex vitreoretinal therapies such as REVS.18 These systems can be subdivided into three categories: hand-held devices, comanipulation systems and telemanipulation systems. Hand-held devices aim at providing precision enhancement by actively compensating hand tremor and by offering limited motion scaling. A leading example is the Micron system developed at Carnegie Mellon University.12 The device consists of a 6-DOF (Degree of Freedom) miniature Stewart platform, positioned between the handle and the surgical tool, and an optical tracking system. While offering adequate precision enhancement, hand-held devices are limited with regard to providing eye stabilisation and hand-free instrument immobilisation, which are desired features in the context of REVS. In the case of comanipulation systems, the surgical instrument is simultaneously held by the surgeon and by a grounded robotic device. The surgeon retains direct control over the instrument motion, while the system provides assistive features such as precision enhancement, eye stabilisation and instrument immobilisation. Precision enhancement typically results from a combination of tremor compensation and the generation of motion-opposing forces that reduce the instrument speed. Eye stabilisation is implemented by limiting the DOFs of the instrument, either mechanically or by means of software, to three rotations about the incision and a translation through the incision (Fig. 3b). This technique significantly reduces the net tangential force imposed on the incision, hereby minimising intra-operative eye rotations. One of the defining systems in this field is the Steady-Hand robotic system developed at Johns Hopkins University.10 A 6-DOF non-backdrivable robotic platform is controlled with the aid of a 6-DOF force sensor, mounted close to the instrument handle, and an admittance controller. In the case of telemanipulation systems, the surgeon is physically decoupled from the surgical instrument. The instrument is fixed to a robotic platform, which is indirectly controlled by the surgeon with a joystick. In the context of vitreoretinal surgery, telemanipulation systems and comanipulation systems can offer the same surgically relevant features. However, telemanipulation systems typically rely on motion scaling to implement the precision enhancement, instead of motion-opposing forces. One of the leading telemanipulation systems has been developed at TU Eindhoven,13 a 7-DOF non-backdrivable robotic platform controlled with a 4-DOF backdrivable joystick. Other examples of vitreoretinal robotic platforms within these three categories have been reported in literature.1,11,14,15,19 Despite the vast amount of research conducted on robot-assisted REVS, none of the developed robotic platforms has ever been used in the context of an in-human REVS study.
Over the years, the authors have been developing state-of-the-art robotic assistance devices and instruments for vitreoretinal surgery at the University of Leuven.5,6,7,8,9,21 Research has been focused on enabling the safe application of REVS. Both a comanipulation system and a telemanipulation system have been constructed (Fig. 4), offering a more than tenfold precision enhancement, eye stabilisation, and hand-free tool immobilisation. The performance and usability of these systems has been thoroughly compared by vitreoretinal surgeons in an ex-vivo experimental campaign on REVS. The study learned that both technologies allow surgeons to perform REVS in a reliable manner.5 However, preference by the users was clearly given to the comanipulation system as it allows the surgeon to retain direct control over the instrument, is more intuitive to use, and leaves a smaller footprint in the operating room (OR). For this reason, research efforts have been further focused on the clinical translation of the comanipulation system. This work reports on the world-first in-human robot-assisted REVS. Firstly, the architecture of the previously reported comanipulation system will be briefly reviewed and the adopted surgical workflow will be detailed. Secondly, this work elaborates on the preclinical porcine REVS studies and on the clinical in-human REVS trial that have been conducted.
Materials and Methods
This section briefly reviews the architecture of the robotic assistance device and details the adopted surgical workflow to explain the clinical use of the system.
Architecture Robotic Assistance Device
Figure 4a depicts the comanipulated robotic assistance device, as previously reported by the authors.9 It consists of two subsystems: the surgical system and the alignment system. The surgical system, to which the surgical instrument is fixed, assists the surgeon in performing the treatment. The device keeps the eye from rotating during the intervention, enhances the surgeon’s precision more than tenfold, and immobilises the instrument upon request. The alignment system is used to pre-operatively position the surgical system with respect to the patient. The key features of the robotic assistance device are implemented as follows.
Eye Stabilisation
The surgical system minimizes intra-operative eye rotations by limiting the number of DOFs of the instrument from six to four (Fig. 3b). These are two rotations \(\phi\) and \(\theta\) about the incision, a rotation \(\psi\) about the instrument axis, and a translation R along the direction of the instrument axis as well as through the incision. These four DOFs are centred at a remote center of motion (RCM), a point distal from the robotic assistance device. The RCM is implemented by means of a novel mechanism, incorporated in the surgical system (Fig. 6a).5 Adopting this particular mechanism ensures the compactness of the tool holder, which is necessary given the confined surgical workspace and to enable easy handling of the instrument. The alignment system is used to pre-operatively align the RCM of the surgical system with the incision. It consists of an active Cartesian positioning stage which is connected to the head support of the operating table (Fig. 6b). The alignment procedure is performed by the surgeon with the aid of a pair of foot pedals, i.e., the alignment pedal and the control pedal, as well as a calibration tool to indicate the location of the RCM, as explained further in this work.
(a) Overview on the kinematics of the alignment system and the surgical system. (b) Schematics depicting the alignment of the RCM with the entry point in the sclera by using the alignment system and the calibration tool. (c) Schematics depicting the use of the surgical system during intraocular surgery.
Precision Enhancement
Comanipulated robotic systems can enhance the surgical precision by rendering motion-opposing forces of which the magnitude increases with the speed of motion. A steady-state instrument speed is reached when the motion-opposing force and the applied force of the surgeon equal in magnitude. A higher ratio between the motion-opposing force level and the speed level reduces the steady-state instrument speed when the applied force by the surgeon, i.e., the effort, is held constant. The speed reduction affects both the involuntary instrument vibrations due to hand tremor forces as well as the voluntary instrument motion, hereby enabling a stable and reliable retinal approach. This strategy is typically implemented with the aid of a non-backdrivable device that is controlled using a handle-mounted force-sensor in an admittance scheme. The force sensor is used to measure the surgeon’s intention and to set the velocity of the device accordingly.4 In contrast, the presented robotic assistance device consists of a backdrivable architecture, hereby omitting the need for a multi-DOF force sensor.9 This enables the surgeon to directly move the system, while motion-opposing forces are being generated as a function of the current instrument velocity.
Instrument Immobilisation
Motion of the robotic assistance device can be enabled and disabled with the aid of the control pedal. While pressing the corresponding button on this foot pedal, the surgeon can freely move the instrument within the available workspace. Once the instrument is correctly positioned, the surgeon releases the button upon which the system will lock the instrument into place with an accuracy of \(10\,{\mu} {\rm m}\) within 30 ms.
Surgical Workflow
A specific surgical workflow is followed when performing REVS with the clinical robotic technology (Fig. 5). The process encompasses the execution of the following four successive steps: set-up procedure, pre-operative alignment, intra-operative usage, and post-operative usage.
Set-Up Procedure
Before the onset of surgery, the robotic system is set up in the OR by two trained staff members. The installation and the operational check-up procedure of the device take approximately 15 min to complete. Next, the patient is brought into the OR and is placed on the surgical table. Subsequently, general anaesthesia is applied, after which the patient and the robotic system are covered with sterile drapes. Lastly, a sterile stainless steel instrument holder is attached to the draped surgical system. After completion of the set-up procedure, the surgeon manually performs a number of required steps, such as placement of the valved trocars and vitrectomy.
Pre-Operative Alignment
After completion of the preparatory steps, the surgeon activates the alignment mode of the robotic assistance device with the aid of the control pedal. This allows the surgeon to align the RCM of the surgical system with the entry point in the sclera to prevent unintended intra-operative eye rotations. The alignment procedure encompasses three steps. Firstly, the pose of the surgical system is set to a predefined kinematic configuration and the tool holder is equipped with a calibration tool. In this setting, the tip of the calibration tool visually indicates the position of the RCM (Fig. 6b). Secondly, the surgeon uses the alignment system to move the surgical system such that the tool tip, and thus the RCM, coincides with the intended entry point in the sclera (Fig. 6c). The motion of the alignment system is controlled with the aid of the alignment pedal and the control pedal. The alignment pedal allows to set the direction of motion, while the speed of motion is regulated with the control pedal. Thirdly, once the RCM coincides with the entry point, the surgeon uses the control pedal to store the pose of the alignment system into the memory of the controller. Once the alignment procedure is completed, the surgeon again uses the alignment system to position the surgical system at a safe distance from the patient’s head. After having the calibration tool replaced with the cannulation instrument, the surgical system is moved back to the stored alignment position.
Intra-Operative Usage
After completion of the pre-operative alignment, the state of the robotic assistance device is switched from alignment mode to surgery mode using the control pedal. At this point, the surgeon can start performing retinal vein cannulation with the aid of the robotic assistance device and the cannulation instrument. The instrument consists of a metallic-coated glass micropipette of which the tip has a length of \(500 \,{\mu} {\rm m}\), an angulation of 30°, and an outer diameter of \(30 \,{\mu} {\rm m}\).21 During insertion into and extraction out from the eye, the needle tip is covered with a retractable outer tube. The coating ensures the visibility of the needle tip. The angulation lowers the angle of attack between the needle tip and the vein, hereby effectively reducing the risk of double puncturing the vessel.9 The surgeon starts by taking hold of the instrument handle and subsequently unlocks the motion of the surgical system with the aid of the control pedal. The instrument is inserted into the eye through the entry port in the sclera (Fig. 6c). With the aid of both the high-precision robotic assistance device and the visual feedback received from the surgical microscope, the needle tip is carefully inserted into the targeted vein. Once correctly positioned, the surgeon again disables the motion of the surgical system to immobilise the instrument and subsequently releases the handle. At this point, the prolonged injection of the anticoagulant into the occluded vein is initiated. Once the injection is completed, the surgeon again unlocks the surgical system and removes the needle out of the eye.
Post-Operative Usage
Once the instrument is removed out of the eye, the alignment mode is reactivated and the surgical system is moved away from the patient’s head. Subsequently, the cannulation instrument is removed from the tool holder. Finally, the surgeon manually performs a series of conventional surgical steps, such as removal of the trocars and suturing of the sclerotomy.
Results
The clinical translation process of the developed technology entails a broad range of milestones: technical validation on both component and system level, system performance characterisation during animal trials, generation of the required documentation, and regulatory and standards compliance. This section first focuses on the system performance based on the results of the concluding ex-vivo and in-vivo porcine trials. Secondly, a brief overview is presented on the results of the first in-human robot-assisted REVS study.
Ex-Vivo Porcine Trial
The robotic assistance device has been extensively tested by performing retinal vein cannulation on enucleated porcine eyes. The experimental campaign was used to investigate the efficacy of the precision enhancement and the hand-free tool immobilisation offered by the system. The eye stabilisation feature was not tested here as the dissected eyes remain stationary on top of a lab table.
Firstly, the precision enhancement was validated by a trained operator performing a total of 80 puncture attempts in 20 enucleated porcine eyes. A puncture attempt was defined as being successful when the operator succeeded in safely inserting the needle into the vessel. This was verified by briefly injecting demineralised water and subsequently inspecting whether the present blood remnants were flushed out of the vessel. In contrast, when a vessel was double punctured, the blood remnants remained stationary, hereby indicating an unsuccessful puncture. At the onset of each puncture attempt, the needle tip was positioned at approximately 25 mm from the targeted vein upon which a time recording was initiated. The recording was stopped once an attempt was deemed to be successful or unsuccessful. Figure 7 summarizes the results of the experimental campaign. Each puncture attempt is depicted as either a green or red coloured box, indicating a successful and unsuccessful puncture respectively. Further, the required time to perform the puncture is reported inside each box. The distribution of the puncture time is illustrated by means of a box plot. A total of 78 successful punctures were made, with a median puncture time of 20.2 s. The two unsuccessful punctures occurred near the start of the experiment. The user indicated that in both cases a misjudgement of the needle tip depth was made due to the limited experience at this point in the experiment. Nonetheless, an overall success rate of 97.5% was obtained with the aid of the precision enhancement that is offered by the robotic system.
Results of an experimental campaign on robot-assisted retinal vein punctures using enucleated porcine eyes. (a) Successful and unsuccessful punctures are indicated with green and red boxes respectively. The time to perform the puncture is indicated inside each box. (b) A box plot indicating the puncture time distribution.
Secondly, the hand-free tool immobilisation was validated by performing five prolonged infusions into \(100\,{\mu} {\rm m}\)-thick retinal veins of five enucleated porcine eyes. Injection periods up to 10 min are targeted during REVS. During the experiment, infusion periods of 15 min were used, hereby including a minimum safety margin of \(50\%\). An infusion was deemed to be successful when the demineralised water was injected into the vessel throughout the full infusion period. This was verified in a similar manner as with the puncture campaign. An overall success rate of \(100\%\) was obtained during the experiment, which illustrates the efficacy of the tool immobilisation feature of the robotic system.
In-Vivo Porcine Trial
Throughout the development of the robotic assistance device and the cannulation instrument, the feasibility of performing safe and successful REVS with the technology has been carefully assessed in the context of an in-vivo porcine study. The study was approved by the Ethical Committee for Animal Research at Medanex Clinic (EC MxCl-2013-017), and all experiments were done in accordance with the ARVO statement on the use of animals in ophthalmic and vision research. A total of 24 retinal vein cannulations have been performed by two expert VR surgeons on 12 domesticated pigs. Initial outcomes of this trial were published by Willekens et al., who reported on the first 18 performed porcine in-vivo retinal vein cannulations.21 The diameter of the targeted veins ranged between 100 and 150 μm. The trials were conducted following the previously described surgical workflow, hereby mimicking in-human robot-assisted REVS as closely as possible. Ocriplasmin, an anti-coagulant developed by Thrombogenics, or saline solution was used as injection agent, and RVO was induced in each porcine eye by creating a laser-induced blood clot. An in-vivo cannulation attempt was considered as being successful when the needle tip was safely inserted into the vein and a stable infusion could be maintained for at least 3 min. Figure 8 shows a successful retinal vein cannulation, illustrated by the blood washout during the injection phase. Overall, 21 cannulation attempts out of 24 were successful, resulting in a 87.5\(\%\) success rate. Only three cannulation attempts were unsuccessful, all of which occurred when the development phase of the technology was still ongoing. In two instances, laser-induced clot creating failed, leading to excessive intraocular bleeding. In one instance, intraocular needle breakage occurred due to induced instrument motion caused by excessive torsion on a stiff infusion line. This was mitigated by significantly reducing the torsional stiffness of the infusion line. The finalized technology was validated during the last six in-vivo cannulation attempts, where a \(100\%\) success rate was achieved. The study shows that REVS can be performed safely and with a high procedural success rate with the aid of the developed technology.
In-Human Clinical Trial
Based on the promising results of the preclinical research, a phase I clinical study (NCT02747030) was approved to investigate the safety and feasibility of performing robot-assisted REVS to treat CRVO. On the 12th of January 2017, the authors succeeded in successfully performing the world’s first in-human robot-assisted REVS at the University Hospital of Leuven.2 At the time of this writing, four CRVO patients have been treated. In all cases, the surgeon was able to inject Ocriplasmin into the targeted retinal vein, having an estimated diameter of 100 to 150 μm. Injection periods up to 10 min were achieved. The study shows that it is possible to perform REVS with the aid of the developed technology in conjunction with a state-of-the-art stereoscopic microscope and proper intraocular lighting. A detailed report on the clinical outcome of the study will be published in the near future.
Discussion
REVS is a challenging VR procedure, which could potentially cure RVO. Its clinical application is hindered because of the scale and fragility of retinal veins on one end and surgeons’ limited positioning precision on the other. The authors have a long track record in the development of robotic assistance devices and instrumentation for VR surgery. One of the targeted applications is REVS, where the technology can offer precision enhancement, eye stabilisation, and hand-free tool immobilisation. Both comanipulation and telemanipulation control techniques have been thoroughly investigated in the context of ex-vivo experimental campaigns. VR surgeons expressed their preference for comanipulation when assessing the performance and usability of the two techniques. Benefits of comanipulation over telemanipulation for VR surgery have also been recognized by other key research groups: simplicity, lower system cost, easier integration into the OR, and preservation of the direct connection between the surgeon and the instrument.10 Two key design considerations distinguish the KU Leuven comanipulation system from other existing VR comanipulation systems. Firstly, a novel 4-DOF RCM mechanism is incorporated into the surgical system to provide intra-operative eye stabilisation. Using this patented technology results in a superior compactness of the tool holder compared to other existing RCM mechanisms. This effectively enhances the ergonomics of handling the instrument. Secondly, the surgical system is fully backdrivable, hereby reducing the overall technical complexity and improving the safety compared to non-backdrivable systems. Further, the authors are the first to develop a VR comanipulation system that is suited for clinical use and to present a dedicated surgical workflow for robot-assisted REVS. The benefit of using the technology for REVS has been demonstrated extensively in the context of preclinical investigations. Success rates as high as 97.5 and \(100\%\) were achieved with the finalised technology during ex-vivo and in-vivo porcine trials respectively. These promising results formed the foundation for the approval of the first in-human study on robot-assisted REVS. At the time of this writing, four CRVO patients have received a robot-assisted REVS treatment with the developed technology. In all four cases, the surgeon was able to safely perform REVS with the aid of the developed technology, making this first in-human study a technical success. Robot-assisted REVS has shown to be a convincing use case to demonstrate the surgical benefits and potential of the robotic comanipulation system. A detailed report on the short-term clinical outcomes of the performed study will be published in the near future. Long-term effects of the application of REVS on the visual acuity of RVO patients will be investigated in a follow-up clinical study. Furthermore, it is believed that the device could be equally used to improve the quality of existing VR therapies or to enable the application of other ultra-precision VR treatments. Future efforts will therefore also be aimed at exploring the potential of the robotic comanipulation system in novel use cases.
References
Das, H., T. I. M. Ohm, C. Boswell, R. O. B. Steele, and G. Rodriguez. Robot-Assisted Microsurgery Development at JPL. In: Information Technologies in Medicine, Vol. II, pp. 85–99, 2001.
Donald, T. Robot assists with injections for RVO. Retina Today, pp. 59–61, 2017.
Feltgen, N., B. Junker, H. Agostini, and L. L. Hansen. Retinal Endovascular Lysis in Ischemic Central Retinal Vein Occlusion. One-Year Results of a Pilot Study. Ophthalmology 114:716–724, 2007.
Fleming, I., M. Balicki, J. Koo, I. Iordachita, B. Mitchell, J. Handa, G. Hager, and R. Taylor. Cooperative robot assistant for retinal microsurgery. In: Medical Image Computing and Computer-Assisted Intervention MICCAI 2008. MICCAI 2008. Lecture Notes in Computer Science, pp. 543–550, 2008.
Gijbels, A., D. Reynaerts, and E. B. Vander Poorten. Design of 4-DOF parallelogram-based RCM mechanisms with a translational DOF implemented distal from the end-effector. In: Advances on Theory and Practice of Robots and Manipulators: Proceedings of Romansy 2014 XX CISM-IFToMM Symposium on Theory and Practice of Robots and Manipulators, pp. 103–111, 2014.
Gijbels, A., E. B. Vander Poorten, B. Gorissen, A. Devreker, P. Stalmans, and D. Reynaerts. Experimental validation of a robotic comanipulation and telemanipulation system for retinal surgery. In: 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, pp. 144–150, 2014.
Gijbels, A., E. B. Vander Poorten, P. Stalmans, and D. Reynaerts. Development and experimental validation of a force sensing needle for robotically assisted retinal vein cannulations. In: 2015 IEEE International Conference on Robotics and Automation (ICRA), pp. 2270–2276, 2015.
Gijbels, A., E. B. Vander Poorten, P. Stalmans, H. Van Brussel, and D. Reynaerts. Design of a teleoperated robotic system for retinal surgery. In: Proceedings of IEEE International Conference on Robotics and Automation, pp. 2357–2363, 2014.
Gijbels, A., K. Willekens, L. Esteveny, P. Stalmans, D. Reynaerts, and E. B. Vander Poorten. Towards a clinically applicable robotic assistance system for retinal vein cannulation. In: 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), pp. 284–291, 2016.
He, X., D. Roppenecker, D. Gierlach, M. Balicki, K. Olds, P. Gehlbach, J. Handa, R. Taylor, and I. Iordachita. Toward clinically applicable steady-hand eye robot for vitreoretinal surgery. In: Proceedings of the ASME 2012 International Mechanical Engineering Congress & Exposition (IMECE2012), 88384, pp. 145–153, 2012.
Kummer, M. P., J. J. Abbott, B. E. Kratochvil, R. Borer, A. Sengul, and B. J. Nelson. OctoMag: an electromagnetic system for 5-DOF wireless micromanipulation. In: 2010 IEEE International Conference on Robotics and Automation, pp. 1610–1616, 2010.
Maclachlan, R. A., B. C. Becker, J. C. Tabar, G. W. Podnar, L. A. Lobes, C. N. Riviere, J. Cuevas Tabares, G. W. Podnar, L. A. Lobes, and C. N. Riviere. Micron: an actively stabilized handheld tool for microsurgery. IEEE Trans. Robot. 28:195–212, 2012.
Meenink, H. C. M., R. Hendrix, P. C. J. N. Rosielle, M. Steinbuch, H. Nijmeijer, and M. De Smet. A master-slave robot for vitreo-retinal eye surgery. In: Proceedings of the euspen International Conference, June, pp. 3–6. 2010.
Meenink, H. C. M., R. Hendrix, P. C. J. N. Rosielle, M. Steinbuch, H. Nijmeijer, and M. De Smet. A master-slave robot for vitreo-retinal eye surgery. In: Proceedings of the euspen International Conference, June, pp. 3–6, 2010.
Noda, Y., Y. Ida, S. Tanaka, T. Toyama, M. F. Roggia, Y. Tamaki, N. Sugita, M. Mitsuishi, and T. Ueta. Impact of Robotic Assistance on Precision of Vitreoretinal Surgical Procedures. PLoS ONE 8:e54116, 2013.
Riviere, C., and P. Jensen. A study of instrument motion in retinal microsurgery. In: Proceedings of the 22nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, vol. 1, pp. 59–60, 2000.
Rogers, S., R. L. Mcintosh, B. Grad, D. Journ, N. Cheung, L. Lim, J. J. Wang, P. Mitchell, J. W. Kowalski, H. Nguyen, and T. Y. Wong. The Prevalence of Retinal Vein Occlusion: Pooled Data from Population Studies from the United States, Europe, Asia, and Australia. Population Studies, vol. 117, pp. 1–14, 2011.
Tsui, I., A. Tsirbas, C. W. Mango, S. D. Schwartz, and J.-P. Hubschman. Robotic surgery in ophthalmology. In: Robot Surgery, edited by S. H. Baik, Chapter 10, Rijeka: InTech2010.
Wei, W., R. Goldman, N. Simaan, H. Fine, and S. Chang. Design and theoretical evaluation of micro-surgical manipulators for orbital manipulation and intraocular dexterity. In: 2007 IEEE International Conference on Robotics and Automation, April, pp. 3389–3395, 2007.
Weiss, J. N., T. E. D. C. S. Group, W. Green, C. Chan, G. Hutchins, J. Terry, A. Vine, M. Samama, B. Allf, E. de Juan, G. Steinkamp, L. Hattenbach, I. Scharrer, and C. Ohrloff. Treatment of central retinal vein occlusion by injection of tissue plasminogen activator into a retinal vein. Am. J. Ophthalmol. 126:142–144, 1998.
Willekens, K., A. Gijbels, L. Schoevaerdts, L. Esteveny, T. Janssens, J. H. M. Feyen, C. Meers, D. Reynaerts, E. B. Vander Poorten, and P. Stalmans. Robot-assisted retinal vein cannulation in an in vivo porcine retinal vein occlusion model. Acta Ophthalmol. 95:270–275, 2017.
Acknowledgments
This research was funded by the University of Leuven, an Innovation Mandate of Flanders Innovation & Entrepreneurship (HBC.5016.0250), an SB Fellowship of the Research Foundation Flanders (1S41517N) and Agentschap voor Innovatie door Wetenschap en Technologie (O&O 145046). Thrombogenics sponsored both the in-vivo preclinical study and the phase I clinical study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Robert Merrifield oversaw the review of this article.
Andy Gijbels and Jonas Smits are designated as co-first authors.
Rights and permissions
About this article
Cite this article
Gijbels, A., Smits, J., Schoevaerdts, L. et al. In-Human Robot-Assisted Retinal Vein Cannulation, A World First. Ann Biomed Eng 46, 1676–1685 (2018). https://doi.org/10.1007/s10439-018-2053-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2053-3