Abstract
The ovarian follicle that contains one single oocyte is the fundamental functional tissue unit of mammalian ovary. Therefore, isolation and in vitro culture of ovarian follicles to obtain fertilizable oocytes are regarded as a promising strategy for women to combat infertility. In this communication, we performed a brief survey of studies on microfluidic encapsulation of ovarian follicles in core–shell hydrogel microcapsules for biomimetic 3D culture. These studies highlighted that recapitulation of the mechanical heterogeneity of the extracellular matrix in ovary is crucial for in vitro culture to develop early pre-antral follicles to the antral stage, and for the release of cumulus–oocyte complex (COC) from antral follicles in vitro. The hydrogel encapsulation-based biomimetic culture system and the microfluidic technology may be invaluable to facilitate follicle culture as a viable option for restoring women’s fertility in the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the national survey of family growth conducted by the Centers for Disease Control and Prevention (CDC), impaired reproduction or fertility due to compromised ovarian function affects ~11% (or ~6.7 million) of reproductive-age women in USA.8 The ovary is a critical organ of the female reproductive system and the ovarian follicle is the fundamental functional tissue unit of mammalian ovaries. For humans, it has been long recognized that females are born with a maximum number (~106) of follicles that not only are non-renewable but also degenerate over time4,7,27,40,62: Less than ~30% of the follicles can survive to puberty, and it continues to decline during adulthood (particularly after ~35 years old) to the point of extinction at ~50 years old. Therefore, infertility is a headache for professional women who want to delay childbearing. Moreover, many women are infertile either because of genetic ovarian disorders, or due to exposure to environmental/occupational hazards and/or aggressive medical treatments (e.g., radiation and chemotherapy) that compromise the ovarian function.3,14,18,25,35,37,47,57
Banking ovarian tissue containing healthy follicles by cryopreservation for future orthotopic transplantation has been investigated to preserve the fertility of women. This approach, however, entails the risk of re-introducing malignant cells (e.g., circulating tumor cells in the blood of ovary or primary ovarian cancer cells) back into patients.19,26,42,50 Another option is to treat the ovary first with exogenous follicle stimulating hormone (FSH) to over-stimulate the follicle growth, and then with exogenous luteinizing hormone (LH) to induce superovulation of the follicles and release oocytes. The oocytes are often further in vitro fertilized (IVF) to obtain embryos for cryopreservation to preserve fertility, because oocytes are more difficult to cryopreserve than embryos.21,44,45,65 However, embryo cryopreservation may not be suitable for women without partners or younger women who survive cancer therapies, and can be associated with significant legal, moral, ethical, and religious issues.15,20,41,56,58,59,72 Moreover, the multiple hormonal treatments can both delay the treatment of malignant diseases and create a risk for some patients with hormone-responsive diseases.60
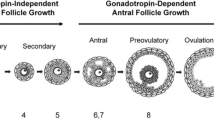
To resolve the aforementioned challenges, isolation of ovarian follicles for in vitro culture has been regarded as a promising strategy to restore female fertility. Pre-antral follicles have been plated in a small drop of medium immersed in biocompatible oil on the 2D flat bottom surface of Petri dish for culture.16,38,54 As recently demonstrated in mice,67 pre-antral follicles have also been cultured in homogeneous 3D hydrogel of alginate. This technique has now been applied to many species including nonhuman primates70,71 and women.66,68 In mice, an interpenetrating network of alginate and fibrin has been shown to be a better system than that of alginate alone because the degradation of fibrin over time allows the expansion of the growing pre-antral follicles during culture with reduced resistance,36,51,52,69 which mimics the soft medullary region in the ovary.63,64 Inspired by studies on culturing embryonic stem cells to form embryoid body, a recent study reported in vitro culture of murine pre-antral follicles in hanging drops and found the hanging-drop method is better than the conventional 2D and even the homogeneous 3D alginate hydrogel culture systems, in terms of preventing premature oocyte release and follicle degeneration.61 This could be attributed partially to the nearly zero resistance to the growing follicles in the fluidic hanging drops of culture medium. However, none of these culture systems recapitulate the native milieu in which the growing follicles span both the harder cortex and softer medulla (i.e., the mechanical heterogeneity) during their development in the ovary in vivo.6,10,28,46,64 As schematically illustrated in Fig. 1, the ovary consists of two distinct layers of tissues10,28: the outer cortex and the inner medulla and the former is much stiffer than the latter. Therefore, efforts have been made recently to produce biomimetic ovarian microtissue that recapitulates the mechanical heterogeneity for biomimetic 3D culture of rodent ovarian follicles, as detailed below.
A schematic illustration of the mammalian ovary showing the development of follicles at various stages and the difference in mechanical properties between the ovarian cortex and medulla, together with the biomimetic ovarian microtissue that recapitulates the mechanical heterogeneity experienced by follicles in the ovary. 1, primordial follicles; 2, primary follicles; 3–4, pre-antral follicles; 5–6, antral follicles; 7, cumulus–oocyte complex (COC); and 8–10, corpus luteum. The figure is reprinted and redrawn from Ref. 10 with permission from Elsevier.
In this work, we performed a brief survey of studies conducted in our laboratory1,10 on microfluidic encapsulation of ovarian follicles in biomimetic ovarian microtissues that recapitulate the mechanical heterogeneity (note: not the exact composition) in the ovarian cortex versus medulla for biomimetic 3D culture of rodent ovarian follicles.
Microfluidic Fabrication of Biomimetic Ovarian Microtissue
A non-planar poly(dimethylsiloxane) (PDMS) microfluidic device was developed for encapsulating early pre-antral follicle in core–shell microcapsule to generate the biomimetic ovarian microtissue.10 A schematic top view of the microchannel system is given in Fig. 2a. Figure 2b shows a zoom-in view of the non-planar design of the flow-focusing junction where W1 × H1 = 200 × 200 μm, W2 × H2 = 80 × 300 μm, and W3 × H3 = 200 × 400, and the corresponding real image (top view) of the flow-focusing junction. The non-planar microchannel was fabricated using a multilayer (3-step UV exposure) SU-8 fabrication technique.2
Microfluidic generation of biomimetic ovarian microtissue and on-chip extraction of the microtissue from oil emulsion into isotonic aqueous solution: (a), a schematic overview of the microchannel system; (b), a 3D zoom-in view and real image of the dispatching channel and flow-focusing junction (FFJ) showing the non-planar design of the FFJ; (c), an image of the extraction channel at its entrance; and (d), an image of the extraction channel at its end. The symbol q represents the flow rate of the aqueous core flow of sodium alginate; I-1, I-2, I-3, I-4, and I-5 represent the five inlets; O-1 and O-2 represent the two outlets; and λ ≤ 1. The figure is reprinted and redrawn from Ref. 10 with permission from Elsevier.
To generate the ovarian microtissue, collagen (and/or alginate) core solution with individually isolated follicles from deer mice (Peromyscus), sodium alginate shell solution, and the emulsion of mineral oil and aqueous calcium chloride solution were injected into the device from the I-1, I-2, and I-3 inlets, respectively. At the flow-focusing junction, the aqueous core and shell solutions were pinched into droplets by the oil emulsion flow due to the Rayleigh–Plateau instability.23,32,49 Sodium alginate solution in the shell was crosslinked (i.e., hardened) to form calcium alginate hydrogel by the aqueous calcium chloride solution in the oil emulsion once they are in contact, but mainly during traveling in the downstream serpentine channel.2,29 The aqueous core flow is arranged in the center of alginate shell flow both horizontally and vertically (Figs. 2a and 2b). Furthermore, a dispatching channel shortly at the upstream of the flow-focusing junction was designed to increase the distance between neighboring follicles by injecting the same solution as the core fluid via the I-4 inlet (Figs. 2a and 2b). With a dispatching flow rate of being 60% (λ) of the core flow rate, it is possible to encapsulate one follicle in each microcapsule or microtissue with high efficiency (>97%).10
Because it is time consuming, ineffective, and cell damaging to extract the ovarian microtissue dispersed in the oil emulsion off chip by multiple centrifugation (in oil emulsion) and washing (in culture medium),31,34 an extraction channel at the downstream of the serpentine channel (Fig. 2a) was further designed to achieve efficient on-chip extraction of the microtissue from the oil emulsion into an isotonic aqueous solution. This is done by introducing an aqueous flow of the isotonic extraction solution through inlet I-5 into the extraction channel so that the channel is partially (half–half) occupied by the oil emulsion and aqueous extraction solution with a stable interface between them.31,34 As the Reynolds number for the microfluidic flow is much less than one (~0.1), the movement of microparticles is dictated by viscous force and the microtissue would travel along with the oil emulsion if undisturbed.5,13,17 To extract the microtissue from oil into the aqueous extraction solution, an expansion design was used for the extraction channel that is narrow (400 μm) at the beginning part (Fig. 2c) and wide (800 μm) at the end (Fig. 2d). Because the diameter of the microtissue is ~350 μm that is only slightly less than the width (400 μm) of the extraction channel at its entrance, the gelled/hardened microtissue is forced to penetrate through the oil–water interface and partially immersed in the aqueous extraction solution once it enters the extraction channel (Fig. 2c). Since the interfacial tension between the microtissue (hydrophilic) and oil (hydrophobic) is high while it is nearly zero between the microtissue and aqueous extraction solution (hydrophilic), the microtissue should experience an unbalanced force due to the difference in interfacial tension on its surface.31,34 Consequently, the microtissue can be pushed away from the oil emulsion into aqueous extraction solution further downstream in the extraction channel as the channel width increases (Figs. 2c and 2d). The expansion design at the exit also makes it more convenient to collect the microtissue by allowing them going straight into a 500 μm of aqueous channel to exit from O-1, while the oil flow turns into a side channel to exit from O-2 (Fig. 2d).
Development of Early Pre-antral Follicles Under 3D Culture in Biomimetic Ovarian Microtissue
The biomimetic ovarian microtissues were cultured in ovarian stromal cells-conditioned medium9 in 96-well plate (one in each well) for observing their development. The conditioned medium was made by incubating ovarian stromal cells isolated from one Peromyscus ovary in a 60 mm culture dish with 5 mL of α-MEM–glutamax medium supplemented with 10% (v/v) heat-inactivated FBS and 1% (v/v) penicillin–streptomycin solution, at 37 °C in 5% CO2 air for 2 days. The procedure was repeated once to obtain a total of 10 mL of the medium, which was further supplemented with 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, and 100 mIU/mL recombinant human follicle stimulating hormone (FSH) to produce the ovarian stromal cell-conditioned medium (CM) for further use. The ovarian stromal cells were isolated by dissociating the cells from the Peromyscus ovary using trypsin and type I collagenase at 37 °C, filtering through a 40 μm filter, centrifuging at 390 g, and culturing in a 60 mm dish in 5 mL of DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 5% CO2 air for 20 h.9
Typical images showing an early secondary pre-antral follicle in the biomimetic ovarian microtissue with a 0.5% collagen (Col) core and 2% alginate (Alg) shell on day 0 and its development to the antral stage are given in Fig. 3a. The fibrous collagen core enclosed in the alginate shell is evident, which is further confirmed by scanning electron microscopy (Fig. 3b).1,10 The defining feature of an antral follicle is that it contains a cumulus–oocyte complex (COC) inside a fluid-filled antral cavity (day 9, Fig. 3a). The mechanical properties (elastic modulus) of the various core and shell materials in the various ovarian microtissues generated for culturing the early pre-antral follicles are shown in Fig. 3c. The modulus of regular alginate as the shell material was constantly more than 10 times higher than that of all core materials. The modulus of oxidized alginate (O-Alg) decreases with culture time and after ~7 days, it is similar to the highest modulus of the core materials.
Qualitative and quantitative data showing the crucial role of mechanical heterogeneity in regulating the follicle development: (a), typical images showing the development of an early preantral follicle under biomimetic 3D culture in ovarian microtissue with a 2% alginate shell and 0.5% collagen core; (b), the collagen fiber in the 0.5% collagen core viewed by scanning electron microscope (SEM); (c), elastic modulus of various shell and core materials used for fabricating the ovarian microtissue; (d), quantitative data of the percentage of development to the antral stage of early preantral follicles cultured under various conditions; and (e), production of estradiol by the growing follicles under the two best culture conditions. *p < 0.05. Alg: alginate; O-Alg: oxidized alginate; and Col: collagen. The figure is reprinted and redrawn from Refs. 28 and 10 with permission from Elsevier (for panels a and d) and from Ref. 1 with permission from Wiley (for panels b, c, and e).
Quantitative data on the development of the early pre-antral follicles to the antral stage, both under miniaturized 3D culture in the various ovarian microtissue made of the different core (gray) and shell (blue or red) materials and under 2D culture, are shown in Fig. 3d. No antral follicles developed under 2D culture. For 3D culture, the core/shell materials significantly affect the development. A 0.5% collagen (with no alginate) core together with a 2% alginate shell results in the highest development. Moreover, the culture condition with 0.5% collagen (0% alginate) as the core together with a 2% oxidized alginate shell gives 0% development. These data indicates the crucial role of mechanical heterogeneity in regulating the follicle growth, as the only difference between the regular and oxidized alginate is that the modulus of the latter decreases to that similar to the core materials in ~7 days (Fig. 3c). In addition, the development in the microtissue (2% alginate shell) with less collagen (0.1%) is not as good as that in the microtissue with more collagen (0.5%), suggesting that sufficient cell adhesion in the extracellular matrix is also important for follicle development.
Production of estradiol is an important molecular event accompanying follicle development to the antral stage. As shown in Fig. 3e, culturing early secondary pre-antral follicles in the optimal microtissue with a 0.5% collagen core and 2% alginate shell produces significantly more estradiol than the second best (according to the percentage of follicle development to the antral stage, Fig. 3d) culture condition with a 0.5% collagen + 0.5% alginate core and 2% alginate shell. These data further demonstrate the crucial role of mechanical heterogeneity in regulating the follicle function and growth at the molecular level.
Release of COCs from Antral Follicles Developed from Early Pre-antral Follicles Via Breaking the Cortex for Obtaining Fertilizable Oocytes
As illustrated in Fig. 1a, following development to the antral stage in vivo is the event of ovulation, a delicate physicochemical process that results in the release of a COC from each mature follicle. Although the exact mechanisms that regulate the ovulation process are still not fully understood, it is well accepted in contemporary literature that ovulation is triggered by the surge of luteinizing hormone (LH) from the pituitary gland. This LH surge activates a cascade of epidermal growth factor (EGF) mediated signaling pathways to induce luteinization of granulosa cells and expansion of cumulus cells, which is necessary for the release of the COC out of the follicle and ovary.22,24,43,48,53,73 With this in mind, 6 out of the 17 antral follicles obtained by culturing early secondary pre-antral follicle in the microtissue with a 0.5% collagen core and 2% alginate shell (Fig. 3d) were treated with LH and EGF (LH+EGF+), leaving the remaining 11 antral follicles without LH or EGF treatment (LH−EGF−). Fewer antral follicles were used for the LH+EGF+ group because according to the conventional theory, the probability of ovulation from this group should be much higher than that for the LH−EGF− group.
A typical image showing the release of a COC from the biomimetic ovarian microtissue via breaking apart the cortex (i.e., the alginate hydrogel shell) and leave behind presumably a corpus luteum-like structure is given in Fig. 4a. It is worth noting that this is the first study that achieved the release of COC in vitro in a biomimetic fashion via breaking apart the cortex-like hydrogel shell. Unexpectedly, only 1 out of the 6 antral follicles treated with LH and EGF released COC while the release of a COC was observed for all the 11 antral follicles without LH or EGF treatment (Fig. 4b). Of note, all the antral follicles were highly viable,1,10 and the antral follicles were treated for the release of a COC with the well-established dose of LH and EGF.22,24,43,48,53,73 Interestingly, only 1 out of 3 antral follicles obtained from culturing early pre-antral follicles in the sub-optimal microtissue with a 0.5% alginate core and 2% alginate shell released COC under the LH−EGF− culture. These data suggest the crucial role of mechanical heterogeneity in the ovary in regulating the release of COC and possibly ovulation although further characterization studies such as those reported in the literature55 are need to confirm the latter. These results support the hypothesis that mechanical properties of the extracellular matrix, i.e., rigidity, regulate follicle selection and/or health, and are consistent with the literature suggesting that disruption of the normal physical microenvironment in the ovary may cause ovarian disorders such as premature ovarian failure (POF) and polycystic ovary syndrome (PCOS).39,64
Release of cumulus–oocyte complex in vitro via breaking apart the cortex (i.e., the alginate hydrogel shell) and in vitro maturation and activation of oocytes: (a), a typical image showing the release of cumulus–oocyte complex (COC) from the biomimetic ovarian microtissue leaving behind a corpus luteum-like structure; (b), the effect of luteinizing hormone (LH) and epidermal growth factor (EGF) on the release of COCs; (c) typical image of an MII oocyte obtained by in vitro culture of early pre-antral follicles in the biomimetic ovarian microtissue showing the characteristic 1st polar body and mitotic spindle; and (d) image of a two-cell embryo developed from the MII oocyte after parthenogenetic activation. The figure is reprinted and redrawn from Ref. 10 with permission from Elsevier (for panels a and b) and from Ref. 1 with permission from Wiley (for panels c and d).
In vitro maturation (IVM) of the COCs was further performed using an optimized protocol that we developed by adding both leukemia inhibition factor (LIF) and epidermal growth factor (EGF) into the conventional medium for the IVM of the COCs.11,12 Although none of the oocytes in the COCs from the sub-optimal culture conditions developed to the metaphase II (MII) stage, 5 MII oocytes out of 11 COCs (45.5%) were obtained by culturing early pre-antral follicles in the optimal biomimetic ovarian microtissue with a 0.5% collagen core and 2% alginate hydrogel shell. A typical image of the MII oocytes is given in Fig. 4c, showing its characteristic meiotic spindles in the cytoplasm and first polar body. Moreover, the MII oocytes obtained by the biomimetic culture of early pre-antral follicles and IVM of the COCs could be further activated to obtain 2-cell stage embryo (Fig. 4d).1 In other words, fertilizable MII oocytes can be obtained by culturing early pre-antral and possibly primary follicles in the biomimetic ovarian microtissue.
Outlook of Future Directions
Future research on this topic may be in the following three directions: First, the microfluidic device shown in Fig. 2 can only be used to extract all microcapsules (including empty microcapsules) from oil emulsion into aqueous extraction solution on chip with no selectivity for the biomimetic ovarian microtissue. As a result, the few (often <~100) biomimetic ovarian microtissues are buried in thousands of empty microcapsules and it is difficult to find the microtissues for retrieval manually off chip. Consequently, the retrieval efficiency is often less than ~50% even after hours of search. This low efficiency and tediousness may not be acceptable in the clinic and it is desired to improve the retrieval efficiency to more than 90% without tedium. Therefore, it is important to further develop the microfluidic device with the capability of selectively extracting the biomimetic ovarian microtissues on chip without any labeling, to achieve high retrieval efficiency of the microtissues and eliminate the need of manual search. For example, this could be done by integrating an optical or electrical sensor in the microfluidic device to detect the follicle-laden microcapsules for extraction, based on the possible difference in optical and electrical properties between ovarian follicles and the encapsulating hydrogel. It is worth noting that the microfluidic device should be cost effective without the need of expensive/specialized instrument for its wide application.
Second, the aforementioned pioneering studies reveal the crucial role of mechanical heterogeneity in regulating the development of ovarian follicles.1,10 However, the percentage of development to the antral stage is still not high, indicating the design of the biomimetic ovarian microtissue needs further development. For example, the materials for making the core and shell of the microtissue could be further optimized in terms of their compositions and physicochemical properties in mimicking the ovarian medulla and cortex. Furthermore, the biomimetic ovarian microtissues should be tested using follicles of primates and humans to ascertain their superiority to the conventional 2D and 3D culture approaches for facilitating follicle development and ovulation.
Lastly, our recent studies reveal that alginate hydrogel microencapsulation is exceptional in not only inhibiting ice formation during cooling, but also suppressing devitrification (i.e., forming ice in vitrified solutions) and the growth of ice crystals during warming.30,33,74 Therefore, biomimetic ovarian microtissues with an alginate hydrogel shell could improve the outcome of follicle cryopreservation that is important for the long-term preservation of women’s fertility, which is worthy of further investigation. This is because most follicles from (at least) young cancer survivors will need to be extracted from cryopreserved ovarian tissue, or from isolated follicles that are then cryopreserved should this prove to be a more successful option.
Conclusions
In summary, modern nonplanar microfluidic technology was successfully utilized to produce biomimetic ovarian microtissue that could recapitulate the mechanical heterogeneity in the extracellular matrix of ovarian tissue. This mechanical heterogeneity was found to play a crucial role in regulating follicle growth. With further development, the nonplanar microfluidic technology and biomimetic ovarian microtissue system could be valuable for facilitating follicle culture and preservation as a feasible option of assisted reproduction to restore women’s fertility in the clinic.
References
Agarwal, P., J. K. Choi, H. Huang, S. Zhao, J. Dumbleton, J. Li, and X. He. A biomimetic core-shell platform for miniaturized 3d cell and tissue engineering. Part. Part. Syst. Charact. 32:809–816, 2015.
Agarwal, P., S. Zhao, P. Bielecki, W. Rao, J. K. Choi, Y. Zhao, J. Yu, W. Zhang, and X. He. One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3d culture of pluripotent stem cells. Lab Chip 13:4525–4533, 2013.
Andersen, C. Y., S. G. Kristensen, T. Greve, and K. T. Schmidt. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 8:595–608, 2012.
Barnett, K. R., C. Schilling, C. R. Greenfeld, D. Tomic, and J. A. Flaws. Ovarian follicle development and transgenic mouse models. Hum. Reprod. Update 12:537–555, 2006.
Baroud, C. N., F. Gallaire, and R. Dangla. Dynamics of microfluidic droplets. Lab Chip 10:2032–2045, 2010.
Berkholtz, C. B., L. D. Shea, and T. K. Woodruff. Extracellular matrix functions in follicle maturation. Semin. Reprod. Med. 24:262–269, 2006.
Broekmans, F. J., M. R. Soules, and B. C. Fauser. Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. 30:465–493, 2009.
CDC. National Survey of Family Growth. http://www.cdc.gov/nchs/nsfg.htm. Accessed 2016.
Choi, J. K., P. Agarwal, and X. He. In vitro culture of early secondary preantral follicles in hanging drop of ovarian cell-conditioned medium to obtain MII oocytes from outbred deer mice. Tissue Eng. Part A 19:2626–2637, 2013.
Choi, J. K., P. Agarwal, H. Huang, S. Zhao, and X. He. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 35:5122–5128, 2014.
Choi, J. K., and X. He. In vitro maturation of cumulus–oocyte complexes for efficient isolation of oocytes from outbred deer mice. PLoS ONE 8:e56158, 2013.
Choi, J. K., and X. He. Improved oocyte isolation and embryonic development of outbred deer mice. Sci. Rep. 5:12232, 2015.
Cubaud, T., and T. G. Mason. Capillary threads and viscous droplets in square microchannels. Phys. Fluids 20:053302, 2008.
De Vos, M., J. Smitz, and T. K. Woodruff. Fertility preservation in women with cancer. Lancet 384:1302–1310, 2014.
Deepinder, F., and A. Agarwal. Technical and ethical challenges of fertility preservation in young cancer patients. Reprod. Biomed. Online 16:784–791, 2008.
Desai, N., A. Alex, F. AbdelHafez, A. Calabro, J. Goldfarb, A. Fleischman, and T. Falcone. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 8:119, 2010.
Di Carlo, D., J. F. Edd, K. J. Humphry, H. A. Stone, and M. Toner. Particle segregation and dynamics in confined flows. Phys. Rev. Lett. 102(9):094503, 2009.
Donnez, J., and M. M. Dolmans. Cryopreservation and transplantation of ovarian tissue. Clin. Obstet. Gynecol. 53:787–796, 2010.
Donnez, J., P. Jadoul, J. Squifflet, A. Van Langendonckt, O. Donnez, A. S. Van Eyck, C. Marinescu, and M. M. Dolmans. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract. Res. Clin. Obstet. Gynaecol. 24:87–100, 2010.
Eroglu, A., M. Toner, and T. L. Toth. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil. Steril. 77:152–158, 2002.
Fabbri, R. Cryopreservation of human oocytes and ovarian tissue. Cell Tissue Bank 7:113–122, 2006.
Fan, H. Y., Z. L. Liu, M. Shimada, E. Sterneck, P. F. Johnson, S. M. Hedrick, and J. S. Richards. Mapk3/1 (Erk1/2) in ovarian granulosa cells are essential for female fertility. Science 324:938–941, 2009.
Garstecki, P., M. J. Fuerstman, H. A. Stone, and G. M. Whitesides. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip 6:437–446, 2006.
Gilchrist, R. B., D. G. Mottershead, and J. G. Thompson. Oocyte maturation and ovulation: an orchestral symphony of signaling. Aust. Biochem. 42(1):8–11, 2011.
Goswami, D., and G. S. Conway. Premature ovarian failure. Hum. Reprod. Update 11:391–410, 2005.
Grynberg, M., M. Poulain, S. Sebag-Peyrelevade, S. le Parco, R. Fanchin, and N. Frydman. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil. Steril. 97:1260–1268, 2012.
Hassold, T., and P. Hunt. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2:280–291, 2001.
He, X., and T. L. Toth. In vitro culture of ovarian follicles from peromyscus. Semin. Cell Dev. Biol. 61:140–149, 2017.
Hoffman, A. S. Hydrogels for biomedical applications. Ann. N. Y. Acad. Sci. 944:62–73, 2001.
Huang, H., J. K. Choi, W. Rao, S. Zhao, P. Agarwal, G. Zhao, and X. He. Alginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-CPA cell vitrification. Adv. Funct. Mater. 25:6839–6850, 2015.
Huang, H., and X. He. Interfacial tension based on-chip extraction of microparticles confined in microfluidic stokes flows. Appl. Phys. Lett. 105:143704, 2014.
Huang, H., and X. He. Fluid displacement during droplet formation at microfluidic flow-focusing junction. Lab Chip 15:4197–4205, 2015.
Huang, H., and X. He. Microscale materials and devices for cell cryopreservation by vitrification. In: Multiscale Technologies for Cryomedicine-Implementation from Nano to Macroscale, edited by X. He, and J. C. Bischof. Singapore: World Scientific, 2016, pp. 101–124.
Huang, H., M. Sun, T. Heisler-Taylor, A. Kiourti, J. Volakis, G. Lafyatis, and X. He. Stiffness-independent highly efficient on-chip extraction of cell-laden hydrogel microcapsules from oil emulsion into aqueous solution by dielectrophoresis. Small 11:5369–5374, 2015.
Jeruss, J. S., and T. K. Woodruff. Preservation of fertility in patients with cancer. N. Engl. J. Med. 360:902–911, 2009.
Jin, S. Y., L. Lei, A. Shikanov, L. D. Shea, and T. K. Woodruff. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil. Steril. 93:2633–2639, 2010.
Jin, M., Y. Yu, and H. Huang. An update on primary ovarian insufficiency. Sci. China Life Sci. 55:677–686, 2012.
Kim, I. W., S. P. Gong, C. R. Yoo, J. H. Choi, D. Y. Kim, and J. M. Lim. Derivation of developmentally competent oocytes by the culture of preantral follicles retrieved from adult ovaries: maturation, blastocyst formation, and embryonic stem cell transformation. Fertil. Steril. 92:1716–1724, 2009.
Ma, X., L. Fan, Y. Meng, Z. Hou, Y. D. L. Mao, W. Wang, W. Ding, and J. Y. Liu. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol. Hum. Reprod. 13:527–535, 2007.
Malizia, B. A., M. R. Hacker, and A. S. Penzias. Cumulative live-birth rates after in vitro fertilization. N. Engl. J. Med. 360:236–243, 2009.
Mandelbaum, J., J. Belaisch-Allart, A. M. Junca, J. M. Antoine, M. Plachot, S. Alvarez, M. O. Alnot, and J. Salat-Baroux. Cryopreservation in human assisted reproduction is now routine for embryos but remains a research procedure for oocytes. Hum. Reprod. 13(Suppl 3):161–174, 1998; discussion 175–177.
Meirow, D., I. Hardan, J. Dor, E. Fridman, S. Elizur, H. Ra’anani, E. Slyusarevsky, N. Amariglio, E. Schiff, G. Rechavi, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum. Reprod. 23:1007–1013, 2008.
Park, J. Y., Y. Q. Su, M. Ariga, E. Law, S. L. Jin, and M. Conti. Egf-like growth factors as mediators of Lh action in the ovulatory follicle. Science 303:682–684, 2004.
Porcu, E., R. Fabbri, G. Damiano, R. Fratto, S. Giunchi, and S. Venturoli. Oocyte cryopreservation in oncological patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 113(Suppl 1):S14–S16, 2004.
Porcu, E., and S. Venturoli. Progress with oocyte cryopreservation. Curr. Opin. Obstet. Gynecol. 18:273–279, 2006.
Rodgers, R. J., I. L. van Wezel, H. F. Irving-Rodgers, T. C. Lavranos, C. M. Irvine, and M. Krupa. Roles of extracellular matrix in follicular development. J. Reprod. Fertil. Suppl. 54:343–352, 1999.
Santos, R. R., C. Amorim, S. Cecconi, M. Fassbender, M. Imhof, J. Lornage, M. Paris, V. Schoenfeldt, and B. Martinez-Madrid. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim. Reprod. Sci. 122:151–163, 2010.
Scaramuzzi, R. J., D. T. Baird, B. K. Campbell, M. A. Driancourt, J. Dupont, J. E. Fortune, R. B. Gilchrist, G. B. Martin, K. P. McNatty, A. S. McNeilly, et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 23:444–467, 2011.
Seemann, R., M. Brinkmann, T. Pfohl, and S. Herminghaus. Droplet based microfluidics. Rep. Prog. Phys. 75(1):01660, 2012.
Shaw, J. M., J. Bowles, P. Koopman, E. C. Wood, and A. O. Trounson. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum. Reprod. 11:1668–1673, 1996.
Shikanov, A., M. Xu, T. K. Woodruff, and L. D. Shea. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 30:5476–5485, 2009.
Shikanov, A., M. Xu, T. Woodruff, and L. Shea. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J. Vis. Exp. 49:e2695, 2011.
Shimada, M., I. Hernandez-Gonzalez, I. Gonzalez-Robayna, and J. A. S. Richards. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol. Endocrinol. 20:1352–1365, 2006.
Silva, J. R., R. van den Hurk, and J. R. Figueiredo. Ovarian follicle development in vitro and oocyte competence: advances and challenges for farm animals. Domest. Anim. Endocrinol. 55:123–135, 2016.
Skory, R. M., Y. Xu, L. D. Shea, and T. K. Woodruff. Engineering the ovarian cycle using in vitro follicle culture. Hum. Reprod. 30:1386–1395, 2015.
Steptoe, P. C., and R. G. Edwards. Birth after the reimplantation of a human embryo. Lancet 2:366, 1978.
Telfer, E. E., and M. B. Zelinski. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil. Steril. 99:1523–1533, 2013.
Trounson, A., and L. Mohr. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 305:707–709, 1983.
Tucker, M., P. Morton, and J. Liebermann. Human oocyte cryopreservation: a valid alternative to embryo cryopreservation? Eur. J. Obstet. Gynecol. Reprod. Biol. 113(Suppl 1):S24–S27, 2004.
Wallace, W. H., R. A. Anderson, and D. S. Irvine. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 6:209–218, 2005.
Wang, W., Y. Tang, L. Ni, T. Jongwutiwes, H.-C. Liu, and Z. Rosenwaks. A modified protocol for in vitro maturation of mouse oocytes from secondary preantral follicles. Adv. Biosci. Biotechnol. 3:57–74, 2012.
Woodruff, T. K. Making eggs: is it now or later? Nat. Med. 14:1190–1191, 2008.
Woodruff, T. K., and L. D. Shea. The role of the extracellular matrix in ovarian follicle development. Reprod. Sci. 14:6–10, 2007.
Woodruff, T. K., and L. D. Shea. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J. Assist. Reprod. Gen. 28:3–6, 2011.
Wright, D. L., A. Eroglu, M. Toner, and T. L. Toth. Use of sugars in cryopreserving human oocytes. Reprod. Biomed. Online 9:179–186, 2004.
Xiao, S., J. Zhang, M. M. Romero, K. N. Smith, L. D. Shea, and T. K. Woodruff. In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 5:17323, 2015.
Xu, M., A. Banc, T. K. Woodruff, and L. D. Shea. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 103:378–386, 2009.
Xu, M., S. L. Barrett, E. West-Farrell, L. A. Kondapalli, S. E. Kiesewetter, L. D. Shea, and T. K. Woodruff. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum. Reprod. 24:2531–2540, 2009.
Xu, M., A. T. Fazleabas, A. Shikanov, E. Jackson, S. L. Barrett, J. Hirshfeld-Cytron, S. E. Kiesewetter, L. D. Shea, and T. K. Woodruff. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol. Reprod. 84:689–697, 2011.
Xu, M., E. R. West-Farrell, R. L. Stouffer, L. D. Shea, T. K. Woodruff, and M. B. Zelinski. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol. Reprod. 81:587–594, 2009.
Xu, J., M. Xu, M. P. Bernuci, T. E. Fisher, L. D. Shea, T. K. Woodruff, M. B. Zelinski, and R. L. Stouffer. Primate follicular development and oocyte maturation in vitro. Adv. Exp. Med. Biol. 761:43–67, 2013.
Zeilmaker, G. H., A. T. Alberda, I. van Gent, C. M. Rijkmans, and A. C. Drogendijk. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil. Steril. 42:293–296, 1984.
Zhang, M., Y. Q. Su, K. Sugiura, G. Xia, and J. J. Eppig. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–369, 2010.
Zhang, W., G. Yang, A. Zhang, L. X. Xu, and X. He. Preferential vitrification of water in small alginate microcapsules significantly augments cell cryopreservation by vitrification. Biomed. Microdevices 12:89–96, 2010.
Acknowledgments
This work was partially supported by grants from NIH (R01EB012108 and R01EB023632).
Conflict of Interest
The author declares no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this review article. No animal studies were carried out by the authors for this review article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
Rights and permissions
About this article
Cite this article
He, X. Microfluidic Encapsulation of Ovarian Follicles for 3D Culture. Ann Biomed Eng 45, 1676–1684 (2017). https://doi.org/10.1007/s10439-017-1823-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1823-7