Abstract
New therapies are desperately needed for human central nervous system (CNS) regeneration to circumvent the lack of innate regenerative ability following traumatic injuries. Previously attempted therapies have been stymied by barriers to CNS regeneration largely because of protective mechanisms such as the blood brain barrier, inhibitory molecules, and glial scar formation. The application of electric stimulation (ES) has shown promise for enhancing peripheral nervous system regeneration, but is in its infancy in CNS regeneration. The objective of this study is to better understand how short duration ES can be harnessed to direct adult neural stem progenitor cell (NSPC) neurogenesis, neurite extension, and maturation. Herein, NSPCs were exposed to physiological levels of electrical stimulation of 0.53 or 1.83 V/m (applied power supply setting of 1.2 and 2.5 V) of direct current (DC) for 10 min/days for 2 days with a total differentiation time of 3 days. Culturing conditions consisted of either mitogenic growth factors or the neuronal differentiation factor interferon-γ (IFN-γ). Stimulated NSPCs showed lengths that were over five times longer than unstimulated controls (112.0 ± 88.8 μm at 0.53 V/m vs. 21.3 ± 8.5 μm for 0 V/m with IFN-γ) with the longest neurites reaching up to 600 µm. Additionally, ES resulted in mature neuronal morphologies and signs of differentiation through positive βIII tubulin, neuronal nuclei (NeuN), and better organized filamentous-actin (f-actin) staining with growth cone formation. Additionally, the neurites and soma of stimulated NSPCs showed increases in intracellular Ca2+ during stimulation, signifying the presence of functional neurons capable of electrical conductance and communication with other cells. Our study demonstrates that short stimulation times (10 min/ day) result in significant neurite extension of stem cells in a quick time frame (3 days). This ES modality is potentially advantageous for promoting axon re-growth at an injury site using delivered adult stem cells; however, significant work still remains to understand both the delivery approach of cells as well as ES application in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain and spinal cord injuries affect over 2 million people in the US annually and are generally irreversible.1 Failure to heal central nervous system (CNS) injuries is largely due to intrinsic inhibition of both axonal re-growth and interconnection past the site of injury.6,11,14,48,51 Many approaches are being tested to heal CNS injuries; however, a completely successful methodology generating functional restoration has yet to be discovered. For example, electric fields occur endogenously and have long been used for both healing and pain management in medical practice.50 Additionally, neural stem progenitor cells (NSPCs) are an attractive cell source for tissue regenerative purposes because of their ability to differentiate into the three primary cell types of the CNS: astrocytes, oligodendrocytes and neurons.28,32,47 While recent studies have demonstrated an impact of electrical stimulation on stem cells,33 little is known about the influence of electrical stimulation (ES) on NSPCs.
Endogenous electrical fields in the CNS promote cell migration, cell death, limb growth, and are vital during initial developmental stages.19,31,35,38 Bioelectricity in the brain is due to spatial changes of small ion secretions from individual cells, driving extracellular flow of ions and establish voltage gradients in tissues.35 EFs are also present in the native injury response, where endogenous levels of 42–150 V/m have been recorded.29 Based on the literature, applied EFs can range up to a maximum of 33 V/m to 437 V/m depending on the animal model chosen and specific cell type of interest.3,16,34,36 During development, and to a limited degree in adulthood, stem cells migrate in the brain from their origin to their site of maturation, due in part to bioelectricity.38 Without normal electrical fields in the brain, developmental abnormalities are more likely to form.31,44 Ultimately, the physiological currents that exist in the CNS play a key role in cell function and likely affect stem cell responses.
In addition to bioelectricity, cytokines such as interferon gamma (IFN-γ), epidermal growth factor (EGF), and fibroblast growth factor (FGF) play an important role in innate immunity and in the development, preservation, and regeneration of the brain. Previous studies have revealed that IFN-γ can be used as a differentiation factor for NSPCs and functions to significantly enrich neuronal populations over oligodendrocytes or astrocytes.26–28 EGF and FGF are commonly used to expand NSPCs in number due to their mitogenic capacity.10,24 However, it is difficult to pinpoint an exact range that should be applied to facilitate neuronal maturation and axon extension after CNS trauma.
The combined treatment of NSPCs with ES and known differentiation molecules has not been thoroughly studied. By utilizing both mitogenic and differentiation cues, we aim to determine if DC ES can override and/or provide synergistic responses by directing both neurite extension and the cell fate of NSPCs in culture. Therefore, the primary objective of this study was to determine the ability of DC ES to enhance neurite extension and guidance of NSPCs and their differentiation into neurons with the addition of IFN-γ or in the presence of EGF and FGF.26–28 This regime can potentially be used in the future in combination with other current tissue engineered therapies. Based on previous work with both primary neurons and stem cells, we hypothesized that short DC ES would enhance outgrowth of NSPC neurites, cause alignment, and increase maturation as evidenced by mature axon/growth cone formation and expression of neuronal proteins.
Materials and Methods
Cell Culture and Isolation
NSPCs were isolated from the subventricular zone (SVZ) of 6 wk old adult female Wistar rat (Charles River, Wilmington, MA, USA) forebrains and expanded as neurospheres in growth medium containing neurobasal (NBM; Life Technologies, Grand Island, NY, USA), 100 μg/mL penicillin streptomycin (PS; Life Technologies), 2 mM l-glutamine (l-glut; Life Technologies), B-27 (Life Technologies), 0.02 μg/mL heparin (Sigma-Aldrich, St. Louis, MI, USA), 20 ng/mL of EGF (Sigma-Aldrich) and 20 ng/mL FGF (Peprotech, Rocky Hill, NJ, USA) as described previously.52
NSPC Stimulation Regime
ES chambers consisted of a 63 mm long non-conductive open silicone rectangular box with a 75 × 25 mm glass slide bottom. Platinum electrodes were adhered to the silicone frame and connected to a DC power source (MPJA, West Palm Beach, FL, USA). The platinum electrodes had a width 7.0 ± 1.0 mm and height of 11 ± 0.48 mm with a separation of 54 ± 0.88 mm between each electrode. Glass coverslips in 24-well culture plates were used as controls. Before experimentation, control coverslips and stimulation chambers were coated with 50 µg/mL poly-d-lysine (PDL; BD Biosciences, Bedford, MA, USA) and 5 µg/mL laminin (Life Technologies, Carlsbad, CA). Cells were seeded at 40,000 cells/cm2 in growth medium overnight and cultured in either differentiation or growth medium the following day. Differentiation media contained NBM, 150 ng/mL IFN-γ for neuronal specification (Peprotech), 100 μg/mL PS, 2 mM l-glut and B-27. Stimulation was performed in a copper-lined humidified CO2 incubator (HERAcell 150i; Thermo Scientific, Sunnyvale, CA, USA) at 37 °C and 5% CO2 providing effective shielding from any external interference. After about 10 h in differentiation medium, cells were stimulated in PBS (pH = 7.4) at 0.53 or 1.83 V/m (applied power supply setting of 1.2 and 2.5 V) for 10 min/ days for 2 days (3 days total differentiation), followed by replacement of the proper medium. The EFs we selected for this study was finalized after preliminary cell studies (Suppl. Figs. 1, 2), as well as the fact that an electrochemical reaction occurred above 1.83 V/m. EF strength was calculated by measuring the voltage in the stimulation media and dividing it by the distance between the measuring electrodes. The platinum plates did demonstrate some capacitance response, so the voltage used for calculations was the equilibrium value after 10 min. The measured voltage was used to calculate current (using the resistivity of PBS) as 0.040 mA for the 0.53 V/m and 0.14 mA for 1.83 V/m. Resistivity was calculated by utilizing a VWR Digital Conductivity Meter (VWR, Radnor, PA, USA).
NSPCs Length, Polarity and Differentiation Analysis
Live phase contrast images were obtained 24 h after the final stimulation (at 3 days) to allow quantification of neurite outgrowth and polarity. Images were obtained at random areas throughout the culture area, and approximately 6–10 images were taken per group. All primary neurites of cells in each image taken were included in analysis. Images were analyzed with ImageJ software. The length of each neurite and angle was determined by measuring from the soma to the end of each process, or measured to the termination of a neurite at a neighboring cell. After imaging, cells were fixed with 3.7% paraformaldehyde for 5 min, and then permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) in PBS (pH = 7.4) for 5 min for immunohistochemistry. In PBS, a 10% goat serum (Sigma) block was applied for 1 h at room temperature (RT). Samples were again washed with PBS and primary antibody monoclonal mouse anti-βIII tubulin (1:500; Covance, Princeton, NJ, USA) or mouse anti-nestin (1:10; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) was left on overnight at 4 °C. Cells were washed three times in PBS for 15 min each wash, then the secondary antibody goat anti-mouse IgG Alexa-Fluor 546 (1:400; Life Technologies) was incubated for at least 1 h at RT. Samples were washed for 15 min in PBS three times then blocked again with 10% donkey serum (Sigma) in PBS for 1 h at RT. After rinsing samples with PBS, primary antibody rabbit anti-neuronal nuclei (NeuN; 1:800; Millipore, Billerica, MA, USA) was incubated with anti-βIII tubulin samples and Alexa-Fluor 488 phalloidin (for filamentous-actin (f-actin); 1:200; Life Technologies) was co-stained with anti-nestin samples overnight at 4 °C. After 15 min PBS washes, secondary antibody donkey anti-rabbit IgG Alexa-Fluor 488 (1:400; Life Technologies) was added to anti-NeuN samples for at least 1 h at RT. Following three more 15 min PBS washes, all nuclei were stained with Hoechst 33342 (10 μM; Life Technologies) for 10 min. Coverslips were mounted with prolong gold (Life Technologies) and imaged with an Olympus IX81 florescent microscope (Tokyo, Japan).
Live Time-Lapse Imaging
Additional live brightfield imaging was performed using AxioVision software with an incubated Zeiss microscope (San Diego, CA, USA) held at 37 °C at time points before, during, and after ES on day 2. Cells were seeded and stimulated as described above. On day 2, chambers were placed on the microscope stage and imaged for 30 min with voltage turned on at 10 min and off at 20 min.
NSPC Metabolic Activity Assay
PrestoBlue reagent (Life Technologies) was used to assess cell activity before and after stimulation according to the manufacturer’s instructions. The assay measures the reducing capacity of cells, which is tied to metabolic function. Cells were seeded overnight at 40,000 cells/cm2 at day 0; before the first medium change (day 1) and after the final ES (day 3), PrestoBlue reagent (diluted in the appropriate media) was incubated with the same NSPCs at 37 °C for 45 min. Fluorescence intensity (I) was read (Ex/Em: 555 nm/590 nm) at 45 min for cells and controls using an Infinite M200 spectrophotometer (Tecan, Männedorf, Switzerland). Since PrestoBlue measures the reducing environment of the cell, dithiothreitol (DTT, Chem-Impex International, Wood Dale, IL, USA), at concentrations of 50, 100, and 1000 µM, was used as a positive control to normalize the data. The fold change in activity was calculated as I day0/I day3. After the incubation period, cells were washed with PBS and either stimulated with an EF or fixed for IHC analysis, depending on the time point.
Calcium Tracking Experiments
NSPCs were seeded at 40,000 cells/cm2 in silicone isolators (JTR13R-2.0-Press-To-Seal Silicone Isolator; Grace Bio-Labs, Bend, OR, USA) inside of 60 mm petri dishes between platinum wires spaced 45 mm apart. PDL and laminin were adsorbed to the petri dish prior to cell seeding as described previously. After overnight culture in growth medium cells were incubated in differentiation media containing 150 ng/mL IFN-γ. No ES was performed until calcium fluctuation measurements on day 2.
Prior to analysis on day 2, silicone isolators were removed and Fluo-4, AM (Life Technologies) molecular probe was prepared at 3 mM in dimethyl sulfoxide (DMSO; BDH, West Chester, PA USA) and was mixed with an equal amount of 20 w/v % of Pluronic F-127 (Life Technologies) also dissolved in DMSO. This solution was added to Hank’s balanced salt solution (HBSS; Life Technologies) to ensure a final concentration of 3 μM for Fluo-4, AM and 2 w/v % for Pluronic F-127. NSPCs were incubated with this mixture for 1 h and then washed in HBSS for at least 1 h. During ES and imaging, Leibovitz media (L-15; Life Technologies) replaced HBSS. Calcium changes were observed using a 40× objective on an upright Olympus FV1000 microscope with Olympus Fluoview software that included settings for Fluo-4 acquisition (494 nm excitation, 506 nm emission). The confocal settings were optimized to reduce photobleaching and potential phototoxic effects with the laser power kept consistently at 10% and a 2 microsecond/pixel dwell time. About 10 cells per group were examined and a representative video and images were obtained for each group. Each interval lasted 5 min and 10 s, with initially no stimulation for 10 s, followed by 3 min of stimulation at 0.04 V/m in separate experiments, then 2 min of no stimulation. Additionally, sequential increases from 0.04 to 0.2 to 13.7 V/m (1.1, 2.2, 4.5 V applied) for approximately 1 min each were performed in place of the 3 min stimulation step. In this experimental set-up and during the short stimulation and culturing time, stimulation at 13.7 V/m did cause an electrochemical reaction at the electrodes. Fluorescent intensity ratios were determined and analyzed using the Olympus Fluoview software. Briefly, straight-line regions of interest (ROI) were drawn along selected neurites as well as across the soma, and fluorescent intensity vs. time data was collected.18 The intensity values were normalized by the starting intensity values from the same ROI, and then plotted. Applied EFs were measured again for the calcium tracking stimulation chamber. Voltage was measured in the media, and divided by the distance between the measuring electrodes. Current was calculated, using the resistivity of L-15 media, as 0.70 μA for 0.04 V/m, 3.02 μA for 0.2 V/m, and 0.25 mA for 13.7 V/m.
Statistical Analysis
Neurite length was tested for normal distribution using the Shapiro–Wilk test. It was determined that the data was non-normal, therefore, neurite lengths were transformed using a log transformation as follows: transformed length = log10(length + 1). Factors included stimulation levels of (i) 0.53 V/m and (ii) 1.83 V/m or (iii) no stimulation, as well as growth conditions, either under (i) EGF/FGF or (ii) IFN-γ. JMP 10.0 software was utilized to run a two-factor ANOVA model assessing the interaction between these stimulation levels and growth conditions. An α level of 0.05 was used to determine significance. Data is shown as a mean ± standard deviation (SD). Statistical analysis of neurite directionality was performed in Excel using the Rayleigh Z test. Angular data was doubled since it was bimodal, and the test was performed also using an α level of 0.05 to establish significance. Metabolic activity fold change data was analyzed in JMP 10.0 using a two-factor ANOVA model (factors and levels listed above) and an α level of 0.05.
Results
NSPCs Length, Polarity and Differentiation Analysis
Neurite length data was not normally distributed; therefore, a log transformation was used to fit the data to a proper distribution for statistical analysis as described above. A two-way ANOVA on the transformed lengths showed the interaction between voltage and media conditions to be significantly different (p = 0.0071). Therefore a post hoc, Tukey’s HSD (honestly significant difference) test was used to analyze significance between groups. Both 0.53 V/m and 1.83 V/m groups containing IFN-γ had significantly longer extensions (112.0 ± 88.8 μm, 105.4 ± 58.0 μm), compared to the no ES control group (p value <0.0001) (Figs. 1, 2). The maximum single neurite length seen in stimulated samples containing IFN-γ was 580.0 μm at 0.53 V/m, while a maximum length of 280.5 μm was measured at 1.83 V/m for the EGF/FGF stimulated group. The length of each neurite and angle was determined by measuring from the soma to the end of each process, or measured to the termination of a neurite at a neighboring cell. Control groups, receiving no ES, averaged lengths of 21.2 ± 8.3 μm and 36.2 ± 32.7 μm with the addition of INF-γ and EGF/FGF, respectively. The maximum single neurite length seen in all controls was 141.9 μm with the addition of IFN-γ. In preliminary experiments a select number of neurites reached lengths up to 600 μm with addition of IFN-γ (Fig. 2c). We originally hypothesized that ES would affect polarity of neurite length, but statistical analysis rejected this hypothesis revealing that neurites did not extend significantly in a specific orientation or alignment to the field (p > 0.05).
Average neurite lengths after 3 days of culture (with 10 min/ days ES on day 1 and 2) transformed by log10(x + 1) for linear statistical analysis. Untransformed values are included for each group inside each bar (μm). Both media conditions (IFN-γ or EGF/FGF) and varying EF stimulation resulted in a significant difference of NSPC length between groups (p < 0.0001). Letters denote significance by two-factor ANOVA with post hoc Tukey HSD analyses. Transformed and untransformed values presented as mean ± SD, with n ranging from 50 to 150 neurites.
(a, b) Polar plots presenting neurite lengths of NSPCs stimulated in vitro at 0 (control), 0.53, and 1.83 V/m with addition of (a) IFN-γ or (b) EGF/FGF. No directional bias was detected using radial statistical analyses (p > 0.05). Angles are in degrees and lengths are shown in μm. (c) Long neurites were especially noticeable in EF stimulated NSPC treatments. Scale bar = 200 μm.
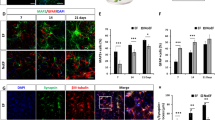
Qualitatively studying the maturity of NSPCs with ES in both medium conditions demonstrated that groups stimulated at 0.53 V/m and 1.83 V/m had superior positive neuronal staining compared to controls (Figs. 3, 4). More specifically, the neuronal stains, β-III tubulin and NeuN, indicated differentiation and maturation of NSPCs and was apparent at both 0.53 V/m and 1.83 V/m (Figs. 3, 4e, 4i, 4h, 4l). However, nestin-labeling indicated that NSPCs did not completely differentiate after 3 days in culture (Figs. 3, 4c, 4g, 4k, 4o). The presence of f-actin was more visible and better organized in the ES groups compared to controls (Figs. 3, 4f, 4j). Phase-contrast images of NSPCs after ES also reveal morphological differences and extensive lengths as compared to controls (Fig. 5). Additionally, mature cytoskeletal growth cone structures with actin filopodia-like projections were seen in these same two ES groups as well (Fig. 6).
NSPCs after 3 days in IFN-γ differentiation medium. (a–d) Control (no ES) NSPCs display neuronal and cytoskeletal structures but not as prominent as 0.53 V/m (e–h) and 1.83 V/m (i–l), which show longer interconnected neurites and more mature neuronal staining (NeuN). All nuclei are stained with Hoechst 33342. Scale bar = 100 µm.
NSPCs after 3 days in EGF/FGF growth medium. (a–d) NSPCs that did not undergo ES have very few neurites and little NeuN staining. (e–h) Cells stimulated at 0.53 V/m show increased neurite outgrowth and mature neuronal staining (e and h). (i–l) The 1.83 V/m group showed very high number of neurites with mature looking cell processes. All nuclei are stained with Hoechst 33342. Scale bar = 100 µm.
Representative 60× images of NSPC growth cones at day 3. (a) After no stimulation, tubulin structure is most noticeable. (b–c) After DC ES regime at 0.53 or 1.83 V/m, respectively, f-actin is prominently displayed as the finger-like projections that are characteristic of growth cones as tubulin is present in the shaft of the axon and center of the growth cone. Scale bar = 5 µm.
Live Time-Lapse Imaging
Live imaging of NSPCs showed that short time (<2 h) responses were varied. We observed a response showing initial retraction upon the application of an electric field followed by elongation of neurites post stimulation (Suppl. Vid. 1). Supplemental video 2 shows that ES can result in an almost immediate response and can sometimes result in neurite retraction. Overall the NSPC neurites seemed to be very active during and directly after ES especially compared to controls (Suppl. Vid. 3).
Cell Metabolism Assay
NSPC metabolic activity was tested before and after stimulation using the PrestoBlue assay so that the fold change in cell activity could be determined; these data are shown in Supplemental Fig. 3. Results revealed that cells cultured in IFN-γ are less metabolically active after the 3 days culture period whether or not they were stimulated; while NSPCs cultured in EGF/FGF were significantly more active than the IFN-γ groups (p < 0.0001). The largest decrease in metabolic activity occurred in the 0.53 V/m group cultured in IFN-γ with a decrease of 0.48 ± 0.07 fold compared to day 0 (before differentiation factor treatment and electrical stimulation), followed by the 1.83 V/m group that decreased by 0.51 ± 0.03 fold compared to day 0.
Calcium Tracking Experiments
Calcium tracking experiments show that free intracellular calcium in both the soma and the neurite can increase during ES at 0.04 V/m as compared to periods where no ES is present (Fig. 7, Suppl. Vid. 4). In a representative stimulated neurite at 0.04 V/m, the maximum normalized Ca2+ intensity was seen 68 s after the voltage was applied, and then began to wane. Turning off the ES at 180 s did not appreciably change the rate of decrease as it proceeded back to initial levels. In the soma, a different response was observed, and the normalized Ca2+ intensity reached a maximum at 110 s and did not decrease. This observation is partially due to the fact that more calcium was present in the soma to begin with and the laser setting was selected such that we could observe changes in the neurites. In separate experiments ES was also increased in a step-wise fashion from 0.04 to 13.7 V/m (Fig. 7, Suppl. Vid. 5). In the soma of NSPCs stimulated with increasing ES, there was no measureable increase of Ca2+ intensity at 10 s with 0.04 V/m, but Ca2+did increase at 110 s with 0.2 V/m. Most noticeably, we found that when ES was increased to 13.7 V/m at 210 s, there was a sharp decline in Ca2+. A similar response in the neurite of the same cell was also seen, but to a lesser extent. One explanation for this reaction is due to the electrochemical response that was observed at the electrodes when the EF was increased to 13.7 V/m. This drastic change is believed to be directly tied to the relatively high current (0.25 mA) that was present at this time point or could be a result of depletion of calcium stores.
Ca2+ tracking experiments in DC ES of NSPCs. (a) Graphs and images of Ca2+ recordings in the soma and a neurite of a single selected NSPC (cultured in IFN-γ for 3 days) stimulated at 0.04 V/m (b) Graphs and images of Ca2+ recordings from soma and neurite of a selected NSPC cultured in IFN-γ for 3 days stimulated at 0.04, 0.2, and 13.7 V/m in a ramping step-wise fashion. Videos of each experiment (a, b) are included as supplemental data (Suppl. Vids. 4, 5).
Discussion
With our experimental set-up, the stimulation of NPSCs with direct current EFs of 0.53 or 1.83 V/m produced mature neurons with extensions almost 3 times greater than controls after a period of only days. Maximum neurite lengths for stimulated cells were up to nearly 600 μm (for 0.53 V/m) with maximum control lengths of no greater than 200 μm (Figs. 1, 2).
ES did not significantly affect cell activity in our study (p > 0.05, Suppl. Fig. 3). Groups cultured with EGF/FGF resulted in a significant increase in metabolic activity, as compared to IFN-γ treated groups, which demonstrated comparably decreased metabolic activity (p < 0.0001). These results suggest that cells cultured in differentiation media could be more focused on neuronal differentiation and neurite extension than multiplying in cell number. It would be necessary to test NSPCs over a longer time period to see if the viability gap between these groups becomes larger or significant between electrically stimulated cells.
Other groups have shown that short duration DC ES (10–60 min/day) result in longer neurite outgrowth in different cell types, such as chick embryo dorsal root ganglia, possibly due to changes in regulation of growth factors.46,49 Combining our results with those of short duration stimulation regimes, it appears that short duration ES in the range of 0.53–1.83 V/m approximately 10–60 min/day, is all that is required to provide significant benefit in terms of neurite extension as well as neuronal differentiation and maturation of NSPCs. Furthermore, stimulating for too long or at higher field strengths may result in negative effects such as a reduction in neurite length or unorganized morphology.
There are several postulated mechanisms for neurite outgrowth as a result of ES. Patel and Poo suggest that the promotion of growth cone formation is a result of changes in the distribution of adhesion receptors, cell membrane growth factors, neurotrophins, and cytoskeletal proteins that could result in the increased elongation seen after application of ES.37,42 Koppes et al. found that a 1 h stimulation regime resulted in an increase of growth associated protein 43 (GAP-43) and brain-derived neurotrophic factor (BDNF) in adult rats, resulting in significant neurite outgrowth of DRG.22 It would be in the interest of future experiments to observe and trace the production of other small molecules and proteins after ES of NSPCs, especially since the root cause of NSPC elongation may be driven by increased secretion of these signaling molecules. Another mechanism suggested in the literature is that gradients of ions and molecules in the culture media caused by the ES can directly encourage neurite elongation.42,45 From our Ca2+ recorded calcium experiments, we show a change in the intracellular Ca2+ levels in NSPCs upon stimulation. Rate of outgrowth has been linked to increased voltage gated Ca2+ activity within the filopodia of growth cones with addition of an applied field, as supported in the literature by Freeman et al. 15 Cohan et al. demonstrated that evoked action potentials resulted in an instant rise of intracellular free Ca2+, as we noted in our experiments as well.9 This further promotes the idea that Ca2+ plays an important role in neurite outgrowth of NSPCs, and could take part in the yet to be identified pathways involved.
In this study, one of our goals was to determine if applied short duration DC ES acts as a differentiation factor, thus we compared EF stimulation of NSPCs exposed to a known neuronal differentiation factor (IFN-γ) to cells maintained in expansion medium (EGF/FGF). Without ES, we have previously shown that IFN-γ stimulation of SVZ-derived NSPCs generated a population containing greater than 60% neurons, whereas culture in EGF/FGF yielded a low percentage of neurons after 8 days.28 The experiments in this study showed that ES encouraged mature neuronal staining in both differentiation and growth media, particularly at 0.53 V/m and 1.83 V/m (Figs. 3, 4). These results suggest that ES combined with appropriate growth factors can be used to both enhance and accelerate the actions of known neuronal differentiation factors, such as IFN-γ. Furthermore, ES alone may provide a non-chemical means of inducing differentiation as shown by others.7,33 Our finding of upregulated positive neuronal staining in EGF/FGF media plus 0.53 or 1.83 V/m show that DC ES can help to override mitogen signaling (Fig. 4). This is especially interesting when contrasting our findings with those of a recent study where mouse NSPCs in mitogens were stimulated with a salt-bridge at 250 V/m (current density not reported) for 8 h. Babona-Pilipos et al.4 found that NSPCs maintained progenitor markers in growth conditions regardless of ES, and preferred to migrate toward the cathode in the presence of ES. The mechanisms involved in ES-enhanced cell differentiation is uncertain and could involve signaling pathways that regulate Ca2+ or modification of proteins.12,41 One of the most striking responses we observed in these experiments was the morphological difference between control groups compared to 0.53 V/m and 1.83 V/m groups (Figs. 3, 4, 5, and 6). The emergence of prominent growth cones in our studies (Fig. 6) after only 20 min of ES over 2 days (3 days total differentiation) may be indicative of more mature cells generated from adult NSPCs in a relatively short period of time. Growth cones play an important role in the function of the neuron by guiding it to its synaptic target. Since we know that electrical fields occur endogenously within the body,36 it is quite possible that this may serve as an initiation cue for the growth cone. As of yet, our findings are inconclusive as to whether the ES acted as a differentiation factor which then pre-disposed the cells to extend longer neurites or if it simply caused the stem cells to grow longer processes. The development of better tools is required to uncouple these responses allowing real-time quantification of the differentiation state of stem cells alongside neurite length and constitution.
Also of interest to us in this study was if DC ES would encourage alignment of NSPC neurites. Many cell types are known to preferentially migrate towards the cathode during long duration ES such as in human keratinocytes,43 HUVEC cells,53 bovine aortic endothelial cells,30 and hippocampal-derived NSPCs.3 Subependymal NSPCs and human embryonic derived NPSCs both exhibited directed migration toward the cathode when DC ES was applied for 2.5 and 1 h, respectively, and galvanotaxis immediately ceased when stimulation was removed.4,13 Others have shown alignment where more neurites were directed toward the anode, although less often, such as seen with human granulocytes39 and PC-12 cells.9 Our results (Fig. 2) agreed with similar studies where DRG do not show any conclusive evidence of alignment, particularly after short durations of ES.3,23,49 Further, reported differences among alignment preference between cell types could indicate that different pathways responsible for galvanotaxis vary according to the cells under consideration.
To better understand the mechanisms by which cells use endogenous electrical fields in development and injury models, the intervals at which retraction and growth occur in neurites must be revealed in future studies to formulate optimal timing of ES. We have found that within the first minutes of ES, cells exposed to DC ES can undergo retraction; however, post-stimulation cells extend neurites rapidly (Suppl. Vid. 2). This result has been confirmed in studies with developing amphibians where neural crest cells show retraction within seconds of ES followed by accelerated growth.36 In future live imaging ES experiments, it would be instructional to monitor the entire culture period to better understand the dynamics and timing of cellular responses. Overall, the observed response to EF stimulation from 0.53 to 1.83 V/m is positive based on the extreme lengths and mature neuronal phenotypes that this ES encourages after 3 days (Figs. 1, 2, 3, 4, 5, 6, and 7).
To date, we are unaware of the exact cause of why ES is beneficial to growing neurons, but there are many suggested mechanisms by which extension of neurites occur in response to EFs. More specifically, mobility of the growth cone and extension/retraction of neurites is strongly linked to the flow of Ca2+ into the cell, caused by depolarization.8 Increased depolarization by DC ES increased intracellular calcium, as we show in our system (Fig. 7). The observed Ca2+ fluctuations are possibly caused by changes due to either Ca2+ leaving and entering the cell or the result of Ca2+ induced Ca2+ release. Even though Ca2+ changes were variable and sometimes small in magnitude in some parts of the cell, these variations are still important as Ca2+ fluctuations are known to activate several important downstream cellular mechanisms including neuron extension, differentiation and plasticity.2,17 For example, Ca2+ is known to be partially responsible for cadherin-based cell attachment, transport of organelles, and vesicle fusion, which all can dramatically affect the outgrowth of neurites and formation of growth cones.25
In our experiments, we were interested to find if differentiating NSPCs would exhibit Ca2+ signaling in response to DC ES as would be expected from primary neurons. Overall the observed Ca2+ fluctuations of stimulated cells demonstrated electrical functionality (Fig. 7, Suppl. Vids. 4–5). Generation of an action potential in cells has been shown to be created by an applied extracellular current.20 We saw that NSPCs demonstrated immediate Ca2+ increases when ES was applied (Fig. 7), with the largest change in intensity occurring in the neurite at 0.04 V/m. The changes in Ca2+ levels are similar in both the soma and the neurite when the EF was increased from 0.04 to 0.2 V/m. Previous work has revealed that neuronal somas contain higher concentrations of Ca2+ channels relative to concentrations found in axons.5 In addition, in other work the filopodia guiding growth cone movements have been shown to retract initially from an increase in intracellular Ca2+, followed by a recovery period where normalization of Ca2+ levels by cellular mechanisms occurs.40 This agrees with what we have seen with initial periods of growth cone retraction (Suppl. Vid. 2) followed by neurite extension (Suppl. Vid. 1). With the addition of an outside stimulus, i.e., ES of 0.53 and 1.83 V/m it is possible that intracellular Ca2+ was increased to more preferred levels for neurite extension. As is explained by Kater and Mills, an optimal level of intracellular Ca2+ is needed for growth cone function and promotion of neurite extension.21 In our ramped stimulation experiment, increasing the applied voltage from 0.04 to 13.7 V/m resulted in increasing Ca2+ levels until reaching 13.7 V/m, where an almost immediate drop of Ca2+ was noted (Fig. 7). In compendium, the application of greater than 1.83 V/m resulted in decreased f-actin and tubulin organization, and shorter neurite lengths, suggesting that higher voltage results in cellular damage and could interference with the cell’s ability to properly regulate Ca2+, which is vital to many neuronal signal transduction pathways and processes.5,20
There is great debate over the effectiveness of ES as a regenerative strategy, largely due to a lack of agreed upon stimulation type (DC/AC) and regime (exposure time, voltage, etc.). Thus significant work remains to be completed to better understand the phenomena to translate these findings. In our study, we have found that short duration (<10 min/ days) EFs of 0.53 and 1.83 V/m are beneficial to NSPCs in vitro as seen by an increase in neurite extension and better neuronal differentiation, which could be valuable for nervous system tissue engineering type approaches incorporating stem cells that require axonal extension.
Beyond the role of ES in NSPC lineage commitment, the primary emphasis of our manuscript, as compared to other similar studies, was on the short duration ES we chose to employ. Not only did we employ short duration ES, but we also observed the effects of EGF/FGF and IFN-γ in combination with the application of ES. We have not seen in the literature that other groups have utilized IFN-γ as a differentiation factor in combination with ES. Our results varied from other studies primarily in that the lengths of NSPCs neurites, which were much longer than other studies that have been completed. We believe that this finding alone could be a beneficial aspect to integrate into tissue engineering approaches or regenerative medicine for future therapeutic tools in brain and spinal cord injuries, where large gaps are currently impossible to bridge.
Conclusions
The effects of short duration DC ES of NSPCs in vitro in combination with EGF/FGF or IFN-γ produced morphologically mature neurons with longer neurite lengths in a relatively short time period (<10 min/ days for 2 days) compared to no stimulation. We have found that DC ES of neuronally-differentiated NSPCs caused an elevated level of Ca2+ in the cell within seconds, and that there appears to be a threshold for which these cells can endure. Live imaging during ES revealed that stimulation caused retraction before elongation, but cells remain viable and active during this stimulation regime. This result along with the appearance of mature markers, growth-cone characteristics, and rapid extension of NSPCs suggests that using ES could provide a potential new solution to CNS regeneration. We hope to apply these results to neural regenerative strategies in the near future.
References
National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2013. https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202013.pdf.
Anglister, L., I. Farber, C. Shahar, and A. Grinvald. Localization of voltage-sensitive calcium channels along developing neurites: their possible role in regulating neurite elongation. Dev. Biol. 94(2):351–365, 1982.
Ariza, C. A., A. T. Fleury, C. J. Tormos, V. Petruk, S. Chawla, J. Oh, D. S. Sakaguchi, and S. K. Mallapragada. The influence of electric fields on hippocamapal neural progenitor cells. Stem Rev. Repair 6:585–600, 2010.
Babona-Pilipos, R., I. A. Droujinine, M. R. Popovic, and C. M. Morshead. Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS ONE 6(8), 2011.
Bolsover, S., and I. Spector. Measurements of calcium transients in the soma, neurite, and growth cone of single cultured neurons. J. Neurosci. 6(7):1934–1940, 1986.
Bovolenta, P., F. Wandosell, and M. Nieto-Sampedro. CNS glial scar tissue—a source of molecules which inhibit central neurite outgrowth. Prog. Brain Res. 94:367−379, 1992.
Chang, K. A., J. W. Kim, J. A. Kim, S. E. Lee, S. Kim, W. H. Suh, H. S. Kim, S. Kwon, S. J. Kim, and Y. H. Suh. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS ONE 6(4):e18738, 2011.
Connor, J. A. Digital imaging of free calcium changes and of spatial gradients in growing processes in single, mammalian central nervous system cells. Proc. Natl Acad. Sci. 83(16):6179–6183, 1986.
Cork, R. J., M. E. McGinnis, J. Tsai, and K. R. Robinson. The growth of Pc-12 neurites is biased towards the anode of an applied electrical field. Dev. Neurobiol. 25(12):1509–1516, 1994.
Craig, C., V. Tropepe, C. Morshead, B. Reynolds, S. Weiss, and Dvd Kooy. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 16(8):2649–2658, 1996.
Fawcett, J., and R. Asher. The glial scar and central nervous system repair. Brain Res. Bull. 49(6):377–391, 1999.
Felaco, M., M. Reale, A. Grilli, M. A. De Lutiis, R. C. Barbacane, S. Di Luzio, and P. Conti. Impact of extremely low frequency electromagnetic fields on Cd4 expression in peripheral blood mononuclear cells. Mol. Cell. Biochem. 201(1–2):49–55, 1999.
Feng, J.-F., J. Liu, X.-Z. Zhang, L. Zhang, J.-Y. Jiang, J. Nolta, and M. Zhao. Brief report: guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 30(2):349–355, 2012.
Fitch, M., C. Doller, C. Combos, G. Landreth, and J. Silver. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 19(19), 1999.
Freeman, J., P. Manis, G. Snipes, B. Mayes, P. Samson, J. J. Wikswo, and D. Freeman. Steady growth cone currents revealed by a novel circularly vibrating probe: a possibly mechanism underlying neurite growth. J. Neurosci. Res. 13(1–2):257–283, 1985.
Frohlich, F., and D. A. McCormick. Endogenous electric fields may guide neocortical network activity. Neuron 67(1):129–143, 2010.
Ghosh, A., and M. E. Greenberg. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science 268:239–247, 1995.
Haugland, R. P. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies, Invitrogen Corp., p. 1126.
Hotary, K. B., and K. R. Robinson. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev. Biol. 140(1):149–160, 1990.
Jimbo, Y., H. P. C. Robinson, and A. Kawana. Simultaneous measurement of intracellular calcium and electrical activity from patterned neural networks in culture. IEEE Trans. Biomed. Eng. 40(8):804–810, 1993.
Kater, S. B., and L. R. Mills. Regulation of growth cone behavior by calcium. J. Neurosci. 11(4):891–899, 1991.
Koppes, A. N., A. M. Seggio, and D. M. Thompson. Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J. Neural Eng. 8(4):13, 2011.
Koppes, A. N., A. M. Seggio, and D. M. Thompson. Neurite outgrowth is significantly increased by the simultaneous presen.tation of schwann cells and moderate exogneous electric fields. J. Neural Eng. 8(4), 2011.
Kuhn, H. G., J. Winkler, G. Kempermann, L. J. Thai, and F. H. Gage. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 17(15):5820–5829, 1997.
Lankford, K. L., and P. C. Letourneau. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J. Cell Biol. 109(3):1229–1243, 1969.
Leipzig, N. D., C. Xu, T. Zahir, and M. S. Shoichet. Functional immobilization of interferon-gamma induces neuronal differentiation of neural stem cells. J. Biomed. Mater. Res., Part A 93A(2):625–633, 2010.
Leipzig, N. D., C. XU, T. Zahir, and M. S. Shoichet. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials 32(1):57–64, 2011.
Li, H., A. Wijekoon, and N. D. Leipzig. 3d differentiation of neural stem cells in macroporous photopolymerizable hydrogel scaffolds. PLoS ONE 7(11):e48824, 2012.
Li, L., and J. Jiang. Stem cell niches and endogenous electric fields in tissue repair. Front. Med. 5(1):40–44, 2011.
Li, X., and J. Kolega. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J. Vasc. Res. 39(5):391–404, 2001.
Lipton, S. A. Blockade of electrical activity promotes the death of mammalian retinal ganglion cells in culture. Neurobiology 83(24):9774–9778, 1986.
Lois, C., and A. Alvarezbuylla. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl Acad. Sci. U.S.A. 90(5):2074–2077, 1993.
Matos, M. A., and M. T. Cicerone. Alternating current electric field effects on neural stem cell viability and differentiation. Biotechnol. Prog. 26(3):664–670, 2010.
McCaig, C. D., and A. M. Rajnicek. Electrical fields, nerve growth and nerve regeneration. Exp. Physiol. 76(4):473–494, 1991.
McCaig, C. D., B. Song, and A. M. Rajicek. Electrical dimensions in cell science. J. Cell Sci. 122:4267–4276, 2009.
Mycielska, M. E., and M. B. A. Djamgoz. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 117(Pt. 9):1631–1639, 2004.
Patel, N., and M. Poo. Orientation of neurite growth by extracellular electric fields. J. Neurosci. 2(4):483–496, 1982.
Pullar, C. E. The Physiology of Bioelectricity in Development, Tissue Regeneration, and Cancer. Boca Raton, FL: CRC Press, 2011.
Rapp, B., A. de Boisfleury-Chevance., and Gruler, H. Galvanotaxis of human granulocytes. Eur. Biophys. J. 16(5):313−319, 1988.
Rehder, V., and S. B. Kater. Regulation of neuronal growth cone filopodia by intracellular calcium. J. Neurosci. 12(8):3175–3186, 1992.
Rollwitz, J., M. Lupke, and M. Simko. Fifty-Hertz magnetic fields induce free radical formation in mouse bone marrow-derived promonocytes and macrophages. Biochim. Biophys. Acta 1674(3):231–238, 2004.
Schmidt, C. E., V. R. Shastri, J. P. Vacanti, and R. Langer. Stimulation of neurite outgrowth using an electrically conducting polymer. PNAS 94(17):8948–8953, 1997.
Sheridan, D. M., R. R. Isseroff, and R. Nuccitelli. Imposition of a physiologic dc electric field alters the migratory response of human keratinocytes on extracellular matrix molecules. J. Investig. Derm. 106(4):642–646, 1996.
Shi, R., and R. B. Borgens. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev. Dyn. 202:101–114, 1995.
Sisken, B. F., M. Kanje, G. Lundborg, E. Herbst, and W. Kurtz. Stimulation of rat sciatic nerve regeneration with pulsed electromagnetic fields. Brain Res. 485(2):309–316, 1989.
Wan, L., R. Xia, and W. Ding. Low-frequency electrical stimulation improves neurite outgrowth of dorsal root ganglion neurons in vitro via upregulating Ca2+-mediated brain-derived neurotrophic factor expression. Neural Regen. Res. 5(16):1256–1260, 2010.
Whittemore, S. R., D. J. Morassutti, W. M. Walters, R. H. Liu, and D. S. K. Magnuson. Mitogen and substrate differentially affect the lineage restriction of adult rat subventricular zone neural precursor cell populations. Exp. Cell Res. 252(1):75–95, 1999.
Wilkinson, A. E., A. M. McCormick, and N. D. Leipzig. Central nervous system tissue engineering: current considerations and strategies. Synth. Lect. Tissue Eng. 8:1–112, 2011.
Wood, M., and R. Kuntz Willits. Short-duration, dc electrical stimulation increases chick embryo DRG neurite outgrowth. Bioelectromagnetics 27:328–331, 2006.
Yang, G., H. Long, J. Wu, and H. Huang. A Novel Electrical Field Bioreactor for Wound Healing Study. International Conference on BioMedical Engineering and Informatics, 2008.
Yiu, G., and Z. He. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7(8):617–627, 2006.
Zahir, T., H. Nomura, X. Guo, H. Kim, C. Tator, C. Morsehead, and M. Shoichet. Bioengineering neural stem/progenitor cell-coated tubes for spinal cord injury and repair. Cell Transplant. 17:245–254, 2008.
Zhao, M., H. Bai, E. Wang, J. V. Forrester, and C. D. McCaig. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J. Cell Sci. 117(Pt. 3):397–405, 2003.
Acknowledgments
We would like to thank The University of Akron for the funding that supported this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Eric M. Darling oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10439_2014_1058_MOESM1_ESM.mov
Supplemental Video 1. Live imaging videos of cells stimulated at 0.53 V/m with addition of IFN-γ (2 days culture). Video shows initial retraction seen in some cells at start of stimulation. Video time is 30 min, with voltage turned on at 10 min and turned off at 20 min. (MOV 5042 kb)
10439_2014_1058_MOESM2_ESM.mov
Supplemental Video 2. Live imaging videos of cells stimulated at 0.53 V/m under addition of IFN-γ. Video shows elongation of neurite following stimulation for 10 min. Video time is 30 min, with voltage turned on at 10 min and turned off at 20 min (MOV 18073 kb)
10439_2014_1058_MOESM3_ESM.mov
Supplemental Video 3. Live imaging videos of cells with no ES with addition of IFN-γ (2 days culture). Video time is 30 min, with voltage turned on at 10 min and turned off at 20 min. (MOV 8628 kb)
10439_2014_1058_MOESM4_ESM.mov
Supplemental Video 4. Ca2+ videos of cells stimulated at 0.04 V/m as summarized in Fig. 7. Video shows increase in Ca2+ permeability during stimulation and decreasing after saturation and cessation of stimulation. (MOV 72200 kb)
10439_2014_1058_MOESM5_ESM.mov
Supplemental Video 5. Ca2+ videos of cells stimulated at with voltage increased step-wise ramp from 0.04 to 13.7 V/m as summarized in Fig. 7. Videos show stimulation increasing Ca2+ permeability during stimulation then sharply decreasing shortly after 13.7 V/m is initiated. (MOV 22416 kb)
10439_2014_1058_MOESM6_ESM.pdf
Supplemental Figure 1. Average neurite lengths after 3 days of culture (with 10 min/ days ES on day 1 and 2). Untransformed values are included for each group. Stimulated cells (0.8, 1.2, or 1.4 applied V) showed significance from control groups (*p < 0.0001) by single factor ANOVA. Mean ± SD. (PDF 87 kb)
10439_2014_1058_MOESM7_ESM.pdf
Supplemental Figure 2. Polar plots from neurite lengths of initial experiments with NSPCs stimulated at 0.41, 0.53, 0.53 V/m (labels are given in the form of applied voltage of 0.8, 1.2, and 1.4 V due to similarity) or no stimulation with addition of (A) IFN-γ or (B) EGF/FGF. No directional bias was detected using radial statistical analyses (p > 0.05). (PDF 177 kb)
10439_2014_1058_MOESM8_ESM.pdf
Supplemental Figure 3. Metabolic activity of NSPCs assayed using PrestoBlue reagent presented as fold change from day 0 to day 3. All IFN- γ groups decreased in viability while all EGF/FGF cultured cells increased in metabolic activity, resulting in a significant difference between these growth factor treatments as revealed by ANOVA (p < 0.0001). (PDF 198 kb)
Rights and permissions
About this article
Cite this article
Kobelt, L.J., Wilkinson, A.E., McCormick, A.M. et al. Short Duration Electrical Stimulation to Enhance Neurite Outgrowth and Maturation of Adult Neural Stem Progenitor Cells. Ann Biomed Eng 42, 2164–2176 (2014). https://doi.org/10.1007/s10439-014-1058-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1058-9