Abstract
Human mesenchymal stem cells (hMSCs) are multipotent cells appropriate for a variety of tissue engineering and cell therapy applications. Mechanical properties of hMSCs during differentiation are associated with their particular metabolic activity and regulate cell function due to alternations in cytoskeleton and structural elements. The objective of this study is to evaluate elastic and viscoelastic properties of hMSCs during long term cultivation in control and transforming growth factor-β1 treatment groups using micropipette aspiration technique. The mean Young’s modulus (E) of the control samples remained nearly unchanged during 6 days of cultivation, but that of the test samples showed an initial reduction compared to its relevant control sample after 2 days of treatment by biological growth factor, followed by a significant rise after 4 and 6 days. The viscoelastic creep tests showed that both instantaneous and equilibrium moduli significantly increased with the treatment time and reached to maximum values of 622.9 ± 114.2 and 144.3 ± 11.6 Pa at the sixth day, respectively, while increase in apparent viscosity was not statistically significant. Such change of mechanical properties of hMSCs during specific lineage commitment contributes to regenerative medicine as well as stem-cell-based therapy in which biophysical signals regulate stem cell fate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone marrow is one of the most abundant sources of stromal cells which are appropriate cell sources for tissue engineering and regenerative medicine. Bone marrow-derived mesenchymal stem cells (MSCs) have been reported to be potent cells capable of in vitro expansion and differentiation into a variety of cell types including adipocytes, osteoblasts, chondrocytes, skeletal muscle,1,17 and smooth muscle cells (SMCs) in response to different micro-environmental factors such as chemical or mechanical stimuli.11,15
Transforming growth factor-β1 (TGF-β1) is known to be a multifunctional protein in regulation of cellular growth, differentiation, motility and production of extracellular matrix.3,11,15 It has been indicated that TGF-β1 acts as an inhibitory agent for SMC proliferation while enhancing synthesis of extracellular matrix.21 Some studies suggested that TGF-β1 as a chemical growth factor is capable of inducing expression of specific SMC markers such as h1-calponin, SM22α and alpha smooth muscle actin (ASMA) in MSCs.10,15 Moreover, TGF-β1 has been widely used for differentiation of MSCs into SMC lineage, possibly in combination with other factors such as mechanical stimuli.7,11
Published results of Proteomic analysis of MSCs upon long term treatment by TGF-β1 have shown that this biological growth factor reduced expression of gelsolin while no significant alteration of α-actin was observed. The reduction in the expression of gelsolin, as an actin-severing protein, promoted the assembly of α-actin and actin filaments without considerable influence on total amount of actin fibers.28 During osteogenic differentiation of human mesenchymal stem cells (hMSCs), considerable alterations in morphological and cytoskeletal organization were reported while gradually thick stress fibers were replaced by a thinner actin filament network.18,25 The cytoskeletal reorganization during cell differentiation affects mechanical properties of cells.25,30 Hence cell mechanics plays a crucial role in differentiation and cell fate through cytoskeleton organization, cell adhesion, cell viscoelasticity, and membrane tension.4,14,19
Better understanding of the interaction between cell mechanics and cell differentiation enables more control on differentiation of stem cells.31 Furthermore, it is necessary to match mechanical properties between target cells and those finally differentiated in order to obtain functional target cells in tissue engineering and regenerative medicine as well as cell therapy.4,25
Yu et al. investigated the correlation between cytoskeleton development and cell morphology with cytoskeleton gene regulation during adipogenic and osteogenic differentiations, and quantified the alterations in mechanical properties of MSCs by micropipette aspiration technique which is widely used in the measurement of mechanical properties of whole cell body.8,31 The results indicated stiffening of MSCs during osteogenic differentiation, while initial stiffening followed by softening was observed during adipogenic differentiation.31 The micropipette aspiration technique seems to be more reliable in representing the whole mechanical properties of a cell compared to other techniques in cell mechanics, since micropipette suction produces a deformation of entire suspended cell and eliminates undesired effects of cell–matrix interaction.13,31 To our knowledge, no other study has been ever performed to evaluate mechanical properties of MSCs during differentiation to SMC linage. This study deals with the use of micropipette aspiration technique to measure elastic modulus and viscoelastic parameters of hMSCs during smooth muscle differentiation in response to TGF-β1. Furthermore, our results can be linked to those of Wang et al. on proteomic profiling of MSCs upon TGF-β1 stimulation, and show how enhanced assembly of smooth muscle α-actin and actin filaments by reduced gelsolin expression is associated with alterations in mechanical behavior of hMSCs during long term treatment by TGF-β1.
Materials and Methods
Cell Culture and TGF-β1 Stimulation
Human bone marrow MSCs were provided from Royan Stem Cell Bank (RSCB, Iran), extracted from a 39 years-old male donor with no abnormal conditions and informed consent. Cells of second to sixth passages were used in our experiments. Dulbecco’s modified Eagle medium (DMEM low glucose medium) with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin and 1% fungizone (all from Sigma-Aldrich, Germany) was used as the basal medium for cell culture and expansion. Since TGF-β is capable of inducing both smooth muscle and chondrogenic differentiations, the chosen medium is an appropriate suspension for combining with TGF-β1 in order to solely induce smooth muscle differentiation.11,15,28 Cells were divided into control and test groups. The test group samples were treated by smooth muscle differentiating agent of 10 ng/mL TGF-β1 (Sigma-Aldrich, Germany). The TGF-β1 concentration (i.e., 10 ng/mL) has been used in various studies as a proper concentration for chemical stimulation of MSCs to differentiate into SMCs.15,28 Cells were maintained in a humidified incubator at 37 °C with 5% CO2 for 6 days cultivation in each medium. The culture medium was changed every 2 days. The initial density of cell seeding was 1000 cells per cm2 for each well of 6-well plates. After 24 h of initial cell seeding, the culture medium was replaced by a proper medium for each sample (named day 0). The measurement of the mechanical properties of MSCs was performed in days 2, 4 and 6 both in control group and TGF-β1 treated group.
Micropipette Aspiration and Theoretical Modeling
At selected time points, elastic and viscoelastic properties of MSCs were investigated using micropipette aspiration technique described in details by other researchers.5,6,24 Briefly, a controlled suction pressure is exerted on the cell surface through the pipette, and the cell body is aspirated into a small glass tube while the leading edge of cell surface is monitored.8 Micropipettes with internal diameters ranging from 6 to 10 μm were produced from borosilicate glass capillaries (Sutter Instrument, USA) by heating, pulling, and quick fracturing. The prepared pipettes were coated by Sigmacote (Sigma-Aldrich, Germany) to prevent from cell adhesion.5 For each experiment, according to the suggested procedure, MSCs were detached from their substrate by 0.25% trypsin–EDTA treatment in less than 4 min. Application of such time limit has been suggested to prevent cytoskeleton from disruption and bleb formation which might influence cell mechanical properties.31 The 4 min treatment or less would cause no harm to the cytoskeletal elements specially F-actins which are major determinants of cell mechanical properties.31 In each experimental session, 30 cells were tested (15 for each elastic and viscoelastic characterization). To ensure the reliability, only discoid cells without blebs were selected for aspiration within the limited time of 1 h after trypsinization. Since measurements of elastic properties and viscoelastic parameters were taken at a random orientation on each cell, it was assumed that they represent average properties of the cells.9 All experiments were performed at biological temperature of 37 °C considering the fact that temperature influences cell mechanical properties.23
For testing the creep behavior of each cell, a tare pressure of 10 Pa was firstly applied on cells for 1 min to allow cells to reach equilibrium (Fig. 1a). By exerting this pressure before starting each aspiration, cells were neither aspirated nor pushed away by the pipette and a sealing was formed between the micropipette and the cells in order to minimize errors occurred by any drift to the measurement of pressure. A specific constant pressure was then applied until the hemispherical projection formed in the pipette (Fig. 1b), since hMSCs behave as solid-like materials.23 By further increase in suction pressure, cells did not flow into the pipette but extended into it to a new equilibrium position through increased aspiration length (Fig. 1c). To measure viscoelastic property, constant step pressures ranged in 235-900 Pa were applied due to considerable variation in cell stiffness among test groups. The whole aspiration of the cell was recorded for 360 s by video microscopy. The aspirated lengths of cells were measured using AxioVision software Version 4.8 (Zeiss, Germany) and then changes in the aspiration length were plotted vs. their time intervals (Fig. 1d).
Micropipette aspiration test to determine elastic modulus and viscoelastic parameters of cell body, (a) application of tare pressure (b) incremental pressure reached to critical level and hemispherical projection was formed (c) cell projections in the micropipette reached to maximum level (d) typical creep behavior of hMSCs, fit with nonlinear regression, (e) and typical normalized equilibrium length (L/a) vs. applied series of stepwise increases in aspiration pressure (Δp), fit with linear regression. The slop of the line defines the Young’s modulus of the sample. Scale bars indicate 10 μm
To determine viscoelastic coefficients of MSCs, theoretical modeling—considering linear viscoelastic three-parameter solid model—was utilized.24 Briefly, in this model, a spring with elastic constant k 1 is connected in parallel with a spring (elastic constant k 2) and a dashpot (apparent viscosity μ) in series. The k 1 factor is defined as the equilibrium modulus and k 1 + k 2 as the instantaneous one. The aspirated length as a function of time, i.e., L(t), and apparent viscosity (μ) were defined by following equations5,24:
where t indicates the time, a defines the inner radius of the micropipette, Δp describes the applied suction pressure, τ is a time constant, and φ is defined as the wall function which depends on the ratio of the thickness of the pipette wall to the radius of the micropipette. In this study, φ was assumed to be 2.1 based on the punch model.24 The viscoelastic parameters were determined by solving Eq. (1) using nonlinear regression of the experimental plot of the change in aspiration length vs. time for the given pressure.
Some deviations from typical viscoelastic pattern were observed during analysis of the experimental data of the creep tests. These deviations were described in details by other researchers and are generally categorized as: 1. abrupt increase in aspirated length after reaching the equilibrium state of viscoelastic pattern (generally after 120 S) due to disruption of cytoskeleton; 2. fluctuation of data prior to reaching equilibrium due to stepwise interaction between large deformation and contraction of cell body; 3. a considerable peak before reaching the equilibrium length possibly caused by rearrangement of cytoskeletal fibers as the response of the cell to aspiration.23,26 We eliminated the results which derived from the deviatory patterns as previously suggested.23
To evaluate elastic property, Young’s Modulus of elasticity (E) of the cells was measured. After MSCs reached the stable condition due to applied tare pressure, sucking pressure in six increasing steps from 70 to 800 Pa was then exerted on aspirated samples. A resting period of 60 s was considered between two consecutive steps to allow cells reach their new equilibrium. At the end of each pressure increment, the aspirated length of the cell inside the pipette was measured and plotted vs. the respected time interval (Fig. 1e). According to the theoretical model formulated by Theret et al.,24 the following equation was used to calculate the Young’s modulus of elasticity from the slope of the normalized equilibrium length of cell projection (L/a) vs. the applied suction pressure (Δp), considering linear regression.
where E is the Young’s modulus of elasticity of the infinite homogeneous half-space solid.
RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
The expression of mRNA of SMC-specific markers (including SM22α, ASMA, and h1-calponin) was quantified by quantitative real-time reverse transcription and polymerase reaction (RT-PCR). The total RNA was extracted using RNA isolation kit (Qiagen, USA), and the cDNA was then synthesized using reverse-transcription kit (Qiagen, USA). Quantitative PCR analysis was performed using ABI StepOne Real Time-PCR instrument and SYBR® Green Master Mix (both from Applied Biosystems, USA) as recommended by manufacturer. The primers for the genes of interest are all listed in Table 1. The gene expression level of each sample was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from the same sample, and results of the test groups were presented by normalization of expression level to that of respective control group.
Statistical Analysis
For each time point of each treatment condition, at least three different experiments were performed and the resultant parameters presented as the mean ± standard deviation (SD). The t test analysis was performed to compare results of test group with those of control group in each day of experiment; and ANOVA to examine the differences among samples of each group at different time points considering p values of <0.05 as significant.
Results
Up-Regulation of Smooth Muscle Genes
When treating hMSCs by TGF-β1 (10 ng/mL), cells promoted the expression of the early stage of SM contractile markers (such as ASMA and SM22α), and up-regulated the expression of h1-calponin as an intermediate marker of smooth muscle differentiation. After 6 days of treatment by TGF-β1, the expression levels of smooth muscle specific markers were noticeably higher than those of the relevant control group. At this time point, the relative gene expression of h1-calponin was 2.84 times higher than that of the control group. For ASMA and SM22α markers, the relative expressions reached 2.34 and 1.83, respectively (Fig. 2).
Expression levels of SMC-specific genes in response to chemical (TGF-β1: 10 ng/mL) stimulation for 6 days. The quantified expression level of each gene was normalized to that of GAPDH in the same sample, and then the relative gene expression was normalized to the respective expression of control for each gene. Parameter * describes statistical significance (p < 0.05), comparison between test and control groups
Cell Elastic Modulus
When determining the mean Young’s modulus of hMSCs, the aspirated lengths of cells were less than twice the inner radius of the micropipette. The linear regression of the normalized equilibrium aspirated length (L/a) vs. the applied suction pressure (Δp) is presented in Fig. 3. The average correlation coefficient is R 2 = 0.96 ± 0.016 describing a proper fitness of data.
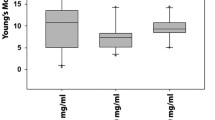
The results described statistically significant change of the mean Young’s modulus of hMSCs at selected time points during differentiation into SMCs (p < 0.0002). According to Eq. (3), by calculating the slop of the lines in Fig. 3, the mean Young’s modulus of elasticity (E) for hMSCs in 2, 4 and 6 days of cultivation with TGF-β1 were obtained as 25 ± 4.8, 90.5 ± 9.1 and 133.2 ± 11.8 Pa respectively, considering that the control samples did not show any significant change in elastic modulus during the period of experiment (p > 0.05) with the average E value of 50.1 ± 7.8 Pa.
The treatment of hMSCs by TGF-β1 for 2 days significantly reduced the E value almost half of that of the control group in the same day (p < 0.03); however, the E value of the treated samples significantly increased in days 4 and 6 up to 1.8 and 2.7 times higher than those of the control group respectively (p < 0.01) (Fig. 3).
Cell Viscoelasticity
Upon exerting specific suction pressure on hMSCs through the micropipette, aspirated length into the pipette exhibited initial instantaneous jump and then the rate of aspirated length monotonically decreased and eventually reached its equilibrium length within almost 120 s. When fitting the normalized aspiration length (L/a) vs. time for all experiments with a standard viscoelastic solid model (Fig. 1d), the correlation coefficients of R 2 > 0.97 were obtained. The high value of correlation coefficient confirmed the time-dependent creep behavior of hMSCs in both control and test groups.
In creep experiments, cells did not completely enter the micropipette and the values of normalized aspiration lengths (L/a) were more than 3. The analysis of experimental data described that viscoelastic parameters in control group did not show any statistically significant change during experiments (p > 0.05). However within the test group (induction of smooth muscle differentiation), viscoelastic parameters including equilibrium modulus (k 1) and instantaneous modulus (k 1 + k 2) significantly increased during time of treatment at selected time points (p < 0.001), and in day 6 reached their maximum level, while in day 2 their minimum values. On the contrary, the time constant (τ) was reduced on selected time points during induced differentiation, but the reduction was not statistically significant (p > 0.05) (Fig 4). Our results indicated no statistically significant increase in the apparent viscosity during differentiation (p > 0.05).
Alteration of viscoelastic parameters of hMSCs during smooth muscle differentiation in response to TGF-β1 stimulation, data in each day were normalized to those of control group in the same day. Dotted line represents ratio of 1.0 (no change in viscoelastic parameters compared to the control samples), parameter * describes statistical significance in t test analysis, and parameter # indicates significance in ANOVA
Compared to control groups, after 2 days treatment of hMSCs by TGF-β1, the instantaneous modulus significantly decreased and reached the value of 123.3 ± 8.4 Pa (p < 0.05). However, the decrease of equilibrium modulus of test samples from that of control samples was not statistically significant reaching the value of 47.65 ± 9.85 Pa (p > 0.05). These parameters were then elevated significantly in days 4 and 6 of cultivation compared to control conditions (p < 0.02) and reached the maximum values of 622.87 ± 114.2 and 144.28 ± 11.6 Pa at day 6 for instantaneous and equilibrium moduli respectively. The apparent viscosity did not show any significant change during treatment by TGF-β1 (p > 0.05). After initial reduction of the apparent viscosity in day 2 to the value of 1285.8 ± 236.8 Pa s, this parameter reached its maximum value of 2597.5 ± 548.6 Pa s at day 6.
Discussion
Cell mechanics plays a main role in biological function of cells. Mechanical properties of cells (such as elasticity and viscoelasticity) influence cell behavior including motility, proliferation, adhesion and differentiation.4,29 Cytoskeleton as the framework of cell determines mechanical behavior and structural integrity of cells.27 Dynamic structure of cytoskeleton is capable of reorganization depending on cell type, stage of development and micro-environmental cues such as chemical stimulation.25,30 Hence, it might have regulatory effects on cell lineage commitment,14 especially in muscle cells, in which the function of cells depends directly on cytoskeletal elements. For example, during long term treatment of MSCs by TGF-β1 stimulation, the number of the actin filaments significantly increased and eventually cell morphology changed to a myoblast-like shape.28
Therefore, one of the main goals of cellular biomechanics is to investigate the relationship between mechanical behavior of cells and alteration in up-regulation of cytoskeletal genes and proteins during the process by which cells choose their fate.
In stem cell-based tissue engineering, functionality of engineered cells depends on the process by which differentiation is induced. Both chemical and mechanical stimuli and their combination contribute to the process of induction. For SMC induction, hMSCs have been treated by chemical agents such as ascorbic acid and TGF-β1,15 and mechanical loadings such as uniaxial strain.11 Such algorithms define the pathway in which cell differentiation is conducted and functional cells are obtained. The cytoskeletal rearrangement is correlated with the biological events in which differentiation is accomplished.14 Analysis of cell mechanical properties defines alterations of cytoskeleton and can be useful in the study of cellular events including the mechanisms in which differentiation is guided.4,30
The function of SMCs is correlated with contractility which highly depends on a proper assembly of cytoskeletal elements especially actin fibers.2 Hence, smooth muscle induction of stem cells requires an appropriate evaluation of cell cytoskeleton (and consequently cell mechanical properties) while monitoring up-regulation of SM specific markers. In this study, we used TGF-β1 as a chemical factor for inducing differentiation of hMSCs into SMCs. Our objective was to quantify alterations in mechanical properties of hMSCs (as the descriptor of cell cytoskeleton) during differentiation to SMCs through up-regulation of smooth muscle cytoskeletal proteins. Results confirmed enhanced expression of SMC specific genes compared to that of control samples after 6 days of treatment by TGF-β1 (Fig. 2). ASMA, SM22α and h1-calponin are known as cytoskeleton genes that are practical markers for assessing SMC maturation.16 It has been reported that ASMA protein contributes to stress fiber formation and acts as the regulator of SMC contraction.20 This marker is essential for cell spreading and its incorporation into stress fiber formation relates with the strength of cell–matrix adhesion.16 The role of SM22α is generally related to formation of stress fibers and development of cell–matrix adhesion. SM22α co-localizes with ASMA and may be influential in remodeling of actin filaments, but its function is not entirely clear yet.16,20 In addition, the function of h1-calponin is related to regulation of SMC contraction; however, the inherent mechanism also remains unknown.16 Considering the function of these markers, differences among the gene expression levels between two groups after 6 days of cultivation may arise from different reorganization of cytoskeleton filament during differentiation. Since gene expression is transient and only gives information at transcriptional level of gene regulation, measuring protein expression would be supportive. Immunoblotting analysis of hMSCs has revealed that TGF-β1 significantly reduced the expression level of gelsolin after 4 and 6 days of treatment, whereas the total amount of α-actin remained nearly unchanged during 6 days.28 It is known that gelsolin enables remodeling of actin structure and regulation of cell motility and apoptosis.22 The decrease of gelsolin, a multifunctional actin regulatory protein, enhances the assembly of actin filament and α-actin, which results in stiffening of cells in time of treatment.28 These confirm the results of current study on evaluation of cell mechanical properties through significant elevation of elastic modulus and viscoelastic parameters including instantaneous and equilibrium moduli during treatment by growth factor potentially by enhanced assembly of actin filaments. The results exhibited unexpected decrease in cell stiffness in day 2 which may arise from stress fiber disorganization due to a change in cell phenotype, which leads to softening of the cell body.
The results of this study may contribute to regenerative medicine and cell therapy, when in vitro expansion and manipulation of stem cells are required. The knowledge of correlation between mechanical properties of MSCs and expression of cytoskeleton genes enables more control on differentiation and provides groundwork for stem cell-based tissue engineering in order to optimize culture conditions and effective usage of external physical cues as well as substrate properties as the regulatory mechanisms of differentiation to functional target cells.12
References
Bianco, P., M. Riminucci, S. Granthos, and P. G. Robey. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180–192, 2001.
Chou, R., M. Stromer, R. Robson, and T. Huiatt. Assembly of contractile and cytoskeletal elements in developing smooth muscle cells. Dev. Biol. 149:339–348, 1992.
Derynck, R., and Y. Zhang. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature 425:577–584, 2003.
Gonzalez-Cruz, R. D., V. C. Fonseca, and E. M. Darling. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. PNAS 109:1523–1529, 2012.
Guilak, F., J. Tedrow, and R. Burgkart. Viscoelastic properties of cell nucleus. Biochem. Biophys. Res. Commun. 269:781–786, 2000.
Guilak, F., L. G. Alexopoulos, M. A. Haider, H. P. Ting-Beall, and L. A. Setton. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons Isolated by Cartilage Homogenization. Ann. Biomed. Eng. 33:1312–1318, 2005.
Guo, X., and S. Chen. Transforming growth factor-β and smooth muscle differentiation. World J. Biol. Chem. 3:41–52, 2012.
Hochmuth, R. M. Micropipette aspiration of living cells. J. Biomech. 33:15–22, 2000.
Jones, W. R., H. P. Ting-Beall, G. M. Lee, S. S. Kelley, R. M. Hochmuth, and F. Guilak. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 32:119–127, 1999.
Kurpinski, K., H. Lam, J. Chu, A. Wang, A. Kim, E. Tsay, S. Agrawal, D. V. Schaffer, and S. Li. Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28:734–742, 2010.
Kurpinski, K., J. Chu, D. Wang, and S. Li. Proteomic profiling of mesenchymal stem cells responses to mechanical strain and TGF-β1. Cell. Mol. Bioeng. 2:606–614, 2009.
Li, D., J. Zhou, F. Chowdhury, J. Cheng, N. Wang, and F. Wang. Role of mechanical factors in fate decisions of stem cells. Regen. Med. 6:229–240, 2011.
Lim, C. T., E. H. Zhou, and S. T. Quek. Mechanical models for living cells: a review. J. Biomech. 39:195–216, 2006.
McBeath, R., D. Pirone, C. Nelson, K. Bhadiraju, and C. S. Chen. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6:483–495, 2004.
Narita, Y., A. Yamawaki, H. Kagami, M. Ueda, and Y. Udea. Effects of transforming growth factor-beta1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 333:449–459, 2008.
Owens, G. K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 75:487–517, 1995.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshakl. Multilineage potential of adult human mesenchymal stem cells. Science 284:147–151, 1999.
Rodriguez, J. P., M. Gonzalez, S. Rios, and V. Cambiazo. Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 93:721–731, 2004.
Settleman, J. Tension precedes commitment-even for a stem cell. Mol. Cell 14:148–150, 2004.
Shi, Z. D., G. Abraham, and J. M. Tarbell. Shear stress modulation of smooth muscle cell marker genes in 2-D and 3-D depends on mechanotransduction by heparin sulfate proteoglycans and ERK1/2. PLoS ONE 5:12196, 2010.
Stegemann, J. P., and R. M. Nerem. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann. Biomed. Eng. 31:391–402, 2003.
Sun, H., M. Yamamoto, M. Mejillano, and H. Yin. Gelsolin, a Multifunctional Actin Regulatory Protein. J. Biol. Chem. 274:33179–33182, 1999.
Tan, S. C., W. X. Pan, G. Ma, N. Cai, K. W. Leong, and K. Liao. Viscoelastic behavior of human mesenchymal stem cells. BMC Cell Biol. 9:40, 2008.
Theret, D. P., M. J. Levesque, M. Sato, R. M. Nerem, and L. T. Wheeler. The application of a homogeneous half-space model in the analysis of endothelial-cell micropipette measurements. J. Biomech. Eng. 110:190–199, 1988.
Titushkin, I., and M. Cho. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophys. J. 93:3693–3702, 2007.
Trickey, W. R., T. P. Vail, and F. Guilak. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J. Orthop. Res. 22:131–139, 2004.
Volokh, K. Y. Cytoskeletal architecture and mechanical behavior of living cells. Biorheology 40:213–220, 2003.
Wang, D., J. Park, J. Chu, A. Krakowski, K. Luo, D. Chen, and S. Li. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor β1 stimulation. J. Biol. Chem. 279:43725–43734, 2004.
Wang, J. H., and B. P. Thampatty. An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 5:1–16, 2006.
Yourek, G., M. Hussain, and J. Mao. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 53:219–228, 2007.
Yu, H., C. Y. Tay, W. S. Leong, S. C. Tan, K. Liao, and L. P. Tan. Mechanical behavior of human mesenchymal stem cells during adipogenic and osteogenic differentiation. Biochem. Biophys. Res. Commun. 393:150–155, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Khani, MM., Tafazzoli-Shadpour, M., Rostami, M. et al. Evaluation of Mechanical Properties of Human Mesenchymal Stem Cells During Differentiation to Smooth Muscle Cells. Ann Biomed Eng 42, 1373–1380 (2014). https://doi.org/10.1007/s10439-013-0889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0889-0