Abstract
Numerical models of the mitral valve have been used to elucidate mitral valve function and mechanics. These models have evolved from simple two-dimensional approximations to complex three-dimensional fully coupled fluid structure interaction models. However, to date these models lack direct one-to-one experimental validation. As computational solvers vary considerably, experimental benchmark data are critically important to ensure model accuracy. In this study, a novel left heart simulator was designed specifically for the validation of numerical mitral valve models. Several distinct experimental techniques were collectively performed to resolve mitral valve geometry and hemodynamics. In particular, micro-computed tomography was used to obtain accurate and high-resolution (39 μm voxel) native valvular anatomy, which included the mitral leaflets, chordae tendinae, and papillary muscles. Three-dimensional echocardiography was used to obtain systolic leaflet geometry. Stereoscopic digital particle image velocimetry provided all three components of fluid velocity through the mitral valve, resolved every 25 ms in the cardiac cycle. A strong central filling jet (V ~ 0.6 m/s) was observed during peak systole with minimal out-of-plane velocities. In addition, physiologic hemodynamic boundary conditions were defined and all data were synchronously acquired through a central trigger. Finally, the simulator is a precisely controlled environment, in which flow conditions and geometry can be systematically prescribed and resultant valvular function and hemodynamics assessed. Thus, this work represents the first comprehensive database of high fidelity experimental data, critical for extensive validation of mitral valve fluid structure interaction simulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disruption of the delicate geometric and mechanical balance between the components of the mitral valve, including the annulus, leaflets, chordae tendineae, and papillary muscles, often results in mitral regurgitation.14,19,30 Surgical repair is the preferred medical therapy as it is considered superior to replacement with reduced operative mortality and thromboembolic complications, while exhibiting greater survival and better preservation of left ventricular function.6,39 However, surgical repair remains more challenging and the current national repair rate is only 40%.2 Recent practices to improve pre-operative planning, through the use of real time three-dimensional echocardiography and predictive computational modeling, are expected to help increase repair rates and improve the outcomes.1 These tools can provide better visualization and understanding of pre-operative mitral valve geometry, lesion indication, and potential post-operative function.
Numerical models of the mitral valve have evolved from simple, two-dimensional mathematical approximations to three-dimensional patient specific models with fluid–structure interaction.23,24,28 These evolving methods can provide a unique and non-invasive analysis of mitral valve function and biomechanics, and evaluate fluid flow characteristics pre- and post-operatively.24–26,41 More recently, medical imaging has been employed to extract patient specific mitral valve anatomy for direct surgical applications, such as patient specific annuloplasty ring design, MitraClip implantation and closure, and predictive virtual modification of the annulus and chordae.3,13,16,27,33,41,43,46
However, as numerical mitral valve models have grown in complexity, from utilizing patient specific leaflet profiles, non-uniform thickness, to prescribed macrostructure, most still lack direct one-to-one experimental validation. Typically, the computed endpoints of the numerical solvers are compared to previously published data, for which only mean values are available, and often result in mismatches in valve geometry and boundary conditions. As computational solvers can vary considerably (from execution with commercially available packages to in-house custom codes), experimental benchmark data are important to ensure model accuracy.42 Without such validation against benchmark data, the computational model is of “questionable” value in its clinical utility. As rightly pointed out by Einstein and colleagues, “…three-dimensional in vitro flow, deformation and strain data with a native mitral valve are sorely needed”.11
Herein, we present a novel left heart simulator designed specifically for the validation of mitral valve fluid structure interaction models. Several distinct experimental techniques were collectively performed to resolve mitral valve geometry and hemodynamics. In particular, micro-computed tomography and three-dimensional echocardiography were used to obtain high-resolution valvular anatomy and systolic leaflet geometry, while stereoscopic digital particle image velocimetry provided detailed characterization of the mitral flow field. In addition, physiologic hemodynamic boundary conditions were defined and all data were synchronously acquired through a central trigger.
Materials and Methods
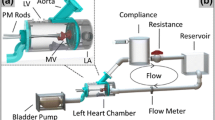
A novel left heart simulator was designed and manufactured for the comprehensive experimental validation of numerical mitral valve models.14,19,30 In particular, the modular design was optimized to allow for micro-computed tomography (micro-CT) and digital particle image velocimetry (DPIV) techniques (Fig. 1). The valve-mounting chamber was designed such that the same valve could be investigated using multiple experimental methods, without requiring disassembly of the ventricular chamber, or agitation to the mitral valve geometry. The left heart consists of a thin walled acrylic chamber (t = 0.635 cm) with a cylindrical cross section (ϕ = 8.89 cm), thus making it accessible to micro-CT imaging with quick removal of the aortic and atrial chambers. The cylindrical design results in uniform scattering and X-ray absorption, which yields a cleaner valve segmentation. The acrylic thin wall minimizes both X-ray energy absorption and optical refractive index disturbances. The aortic chamber was designed at a 135° angle from the mitral valve to preserve their physiologic spatial orientation.44 The aortic chamber houses a 25 mm bi-leaflet mechanical heart valve (Medtronic Parallel; Minneapolis, MN). A cloth covered silicon and acrylic annulus plate allows for mounting native mitral valves. A spring and metal wire drawstring annulus17 was not used as the metal deleteriously scatters the X-rays creating large image artifacts and poor micro-CT reconstruction. The papillary muscles were attached to mechanical holders, which allow for three-dimensional positioning (apical–basal, septal–lateral, anterior–posterior) of the native papillary muscles. The papillary muscles and annulus were fixed in space for multiple experimental techniques. The experimental platform closely simulated the physiologic hemodynamic environment of the mitral valve, which was controlled using lumped compliances and resistances. The annulus and papillary muscles holders were also capable of being dynamically adjusted during experimentation to mimic various disease conditions, without requiring any disassembly or stopping of the experiment.
Mitral Valve Selection and Preparation
Fresh ovine hearts were procured from a local abattoir. Mitral valves were sized using a Carpentier-Edwards Physio II annuloplasty ring sizer (Edwards Lifesciences; Irving, CA); size 28 mitral valves were selected for this study.
The mitral apparatus—annulus, posterior and anterior leaflets, chordae tendineae, and papillary muscles—were excised from the explanted heart. The mitral annulus was sutured to an annulus plate and the papillary muscles were attached to mechanical rods. The native valve was mounted in the left ventricle of the simulator and the mitral annulus and papillary muscles were set to their normal positions as previously described.18 The resulting valvular annular geometry and papillary muscle positions were retained throughout each experimental measurement technique. The simulator was filled with 0.9% saline solution and leaflet dynamics and coaptation geometry were studied at room temperature under physiologic hemodynamic conditions (120 mmHg peak left ventricular pressure, 4.8 L/min average cardiac output at a heart rate of 70 beats per minute) to ensure proper valvular function.
Atrial, ventricular, and aortic pressures were measured using pressure transducers (Deltran® DPT-200, Utah Medical Products, Inc.; Midvale, UT). An inline electromagnetic flow probe (600 series, Carolina Medical Electronics; East Bend, NC) mounted upstream of the left atrium was used to measure transmitral mitral flow. Data were acquired for 15 cardiac cycles at 1 kHz using a custom LabView Virtual Instrument (v.9.0, 2009, National Instruments Corporation; Austin, TX). A central trigger was used to acquire synchronous data for all modalities.
Three-Dimensional Echocardiography
3D echocardiography was acquired using a Phillips Intera machine (Phillips iE33 system, Phillips Healthcare; Andover, MA), with an X7-2 pediatric probe. Full volume images were acquired with good temporal resolution (~50 Hz). A validated in-house MATLAB code was used to extract the mitral leaflet geometry for comparison with in vitro and in silico leaflet kinematics (Icenogle et al. 2012 in preparation). In short, J-splines were used for semi-automatic segmentation of the posterior and anterior mitral leaflets during peak systole. 18 long axis apical–basal slices across the valve were used for segmentation (slice thickness ~2.8 mm) and data were interpolated using an algorithm which was based on the weighted average of the positions of the surrounding points (Butterfly mesh refinement).10 This scheme smoothly interpolates new points in any triangular mesh without changing the position of the original vertices. This ensured the user-selected segmentation was not modified during mesh interpolation.
Stereoscopic Digital Particle Image Velocimetry
Following geometry and mechanical characterization of the mitral valve, the Left Heart Simulator was adapted for DPIV. The working fluid was 36% glycerin in water, which was used to match the kinematic viscosity of blood (3.5 cSt). The ventricular chamber was immersed in an external rectangular chamber containing the same working fluid to minimize optical distortions resulting from the curved cross-section. Neutrally buoyant florescent seeding particles (PMMA with RhB dye, 1–20 μm, Dantec Dynamics; Denmark) were imaged using a laser light source (Nd:YAG lasers, 17 mJ/pulse, 532 nm, ESI Inc.; Portland, OR). The laser beam was adapted with appropriate optics to create a laser sheet (1 mm thickness) bisecting the mitral valve parallel to the septal–lateral long axis plane (Fig. 2). Due to the presence and obstruction of the papillary muscles in the field of view, the angle of the camera was adjusted (~30°) to increase the portion of the flow field that could be imaged. However, the non-orthogonal viewing angle of the camera resulted in velocity bias due to contamination with the out-of-plane velocity. In order to resolve this bias, as well as to capture the three-dimensional velocity field through the valve, stereoscopic PIV was performed. Two CCD cameras (Imager Pro, 1600 × 1200 px, LaVision; Germany) with a Nikon Micro-Nikkor 60 mm lens were arranged in a stereoscopic configuration on either side of the ventricular chamber, at an angle to optimize optical access to mitral flow.
The cameras were mounted with Schiempflug adapters to ensure the entire field of view of particles was in focus. An orange filter eliminated reflected laser light (cut-off wavelength = 560 nm, Hoya Optics, Santa Clara, CA) and only captured fluorescent scattering from the seeding particles.
To enable stereoscopic reconstruction of the full velocity vector in the plane of interest, a calibration procedure was performed. A three-dimensional two-level target was positioned in the laser plane and imaged from both cameras. Using the Calibration function in DaVis 7.2 (LaVision; Germany), a polynomial mapping function of the image plane was created.40 This mapping function was utilized in DaVis 7.2 to reconstruct the three components of velocity in the imaging plane.
Data were acquired at room temperature for adult pulsatile hemodynamic conditions of 4.8 L/min cardiac output, 120 mmHg transmitral pressure at 70 bpm heart rate. 50 phase locked image pairs were acquired every 25 ms in the cardiac cycle. The time difference (dt: 200–1000 μs) between image pairs was optimized for each point in the cardiac cycle to capture high fidelity velocity vectors (particle displacement 5–8 pixels).
Particle cross correlation was performed on the images using multi-pass interrogation with decreasing window size (64 × 64 to 32 × 32 pixels) in DaVis 7.2. A single mask was created based on the combined calibrated camera images, such that regions of the imaging plane obstructed from either camera were masked out. Consequently, vectors were only calculated in regions where flow was visible from both cameras, improving data accuracy and minimizing the calculations of erroneous vectors. Since the leaflets of the valve moved during different points in the cardiac cycle, different masks were created for each time point. The resulting vector fields were ensemble averaged and three dimensional velocity fields across the cardiac cycle were obtained.
Micro-computed Tomography
The atrial chamber and aortic section were removed from the left heart simulator, and the left ventricle was fixtured to the micro-CT gantry using a custom adaptor plate. The mitral valve geometry was retained from the earlier hemodynamic characterization. The mitral valve was scanned in air under ambient pressure (~1 atm, mitral leaflets were open) using a vivaCT 40 system (Scanco Medical AG; Brüttisellen, Switzerland). The geometry was acquired at 39 μm voxel size (~600 image slices) using scanning parameters optimized for low density soft tissues (55 keV energy, 109 μA intensity, and 300 ms integration time).
A Gaussian filter (σ = 1.2, support = 2) was applied to the image data to remove signal noise and improve edge enhancement. Slice by slice user initialized contouring was performed to define the valve anatomy to be included for segmentation. Gray scale images were filtered and converted to binary images using upper and lower bound thresholds.7 An in-house MATLAB (R2010a, The MathWorks; Natick, MA) program was used to segment mitral valve anatomy through canny edge detection. GeoMagic Studio (12; Morrisville, NC) was used to mesh, smooth, and visualize resultant mitral valve geometry.
Results
Hemodynamic Characterization
Representative mitral flow and atrial, ventricular, and aortic pressure are shown in Fig. 3. As the flow waveforms were tuned to the same parameters for both saline and glycerin blood analogs, only a single representative waveform is shown (taken from the DPIV experiments). The ensemble averaged mitral flow was 4.8 ± 0.1 L/min, corresponding to a stroke volume of 69.0 ± 2.1 mL; the closing volume was 6.5 ± 0.6 mL. Peak mitral flow rate was 13.2 L/min. Left ventricular pressure peaked at 125.6 ± 1.7 mmHg, and the rate of LVP (dLVP/dt) increase was 1506 ± 52.8 mmHg/s. Aortic pressure was 122 ± 1.8/52 ± 0.9 mmHg. Diastole lasted approximately 560 ms (as denoted by positive flow through the mitral valve); the remaining 300 ms comprised systole. The mitral valve closed in approximately 100 ms (as observed in the retrograde flow) and remained closed for 200 ms (zero flow). The results were presented as mean ± 1 standard deviation.
Geometric Characterization
Peak systolic anterior and posterior leaflet geometries segmented from three-dimensional echocardiography are shown in Fig. 4. The leaflets demonstrate good closure curvature and functional redundancy creating a competent seal. Annular area was 4.5 cm2. Coaptation length at the center of the valve was 5.0 mm; this decreased considerably to 2.0 mm near the commissure. The point of coaptation was 9.3 mm from the anterior annulus and occurred 2.3 mm from the annular plane. Different time points in the cardiac cycle have been similarly segmented for distinct comparison of leaflet motion with numerical simulation across the cardiac cycle.
MicroCT derived geometry is shown in Fig. 5. Imaging the valve in air provided excellent contrast for reconstruction. The left ventricular chamber had minimal interference, which allowed a high quality segmentation to be performed. Two defined leaflets with small commissural scallops were observed. Chordae tendineae, derived from each papillary muscle, were inserted into the mitral leaflets. The chordal structures were well defined and show continuous insertions into the free edge, belly, and base of the mitral leaflets. Leaflets and chordal structure were asymmetric and have varying lengths and thicknesses. Comparatively, numerical simulations have begun to apply such geometric features.24,45 Additionally, unlike present numerical approximations, the chordae do not originate from a singular point on the papillary muscles; chordal insertions vary along the body of each papillary muscle. Chordae also appear to branch or diffuse radially into the mitral leaflet, which is typically not represented numerically. The reconstructed annulus and papillary muscles can be registered to the CAD model of the left heart simulator for use in computational modeling.
Stereoscopic Particle Image Velocimetry (SPIV)
Figure 6 shows the ensemble-averaged vectors fields at four time points in the cardiac cycle. During early systole, the flow field was mostly quiescent when both the mitral and aortic valves were closed (Fig. 6a). During early diastole, the mitral valve opens and flow begins to accelerate through the mitral valve. This is indicated by the velocity vectors into the ventricle originating from the mitral orifice. Flow is primarily oriented perpendicular to the mitral annular plane, with some entrainment of fluid from the quiescent portions of the ventricle, particularly near the top and bottom of the image shown here (Fig. 6b). The peak velocity of flow through the valve is about 0.4 m/s in the center of the filling jet.
Stereoscopic flow fields through the native mitral valve. Contour plots of the velocity magnitude with superimposed velocity vectors are shown at four points in the cardiac cycle. (a) Early systole. (b) Early diastole. (c) Peak diastole. (d) End diastole. The overlaid flow curve is used to indicate the time point in the cardiac cycle where the data were acquired. Image convention for all panels of this figure and Fig. 7 are shown denoted in (c)
At peak diastole, a strong central filling jet was observed (Fig. 6c). The peak velocity observed in this jet was about 0.6 m/s, which is similar to the velocities observed in flow through the mitral valve in clinical studies using echocardiography and in in vitro studies on native mitral valves.9,22,35 The mitral filling jet appears anteriorly oriented, as indicated by the velocity vectors overlaid on the contour plot. This is indicative of the relative mobility of the two leaflets, with the anterior leaflet having slightly higher mobility resulting in the jet skewing slightly towards the anterior side of the valve. This is similar to jets observed in clinical studies using phase contrast magnetic resonance imaging (PC-MRI) and echo-PIV.15,20,21 As suggested by Kilner et al.,21 the dominant direction of flow through the mitral valve is observed to be towards the anterior mitral leaflet free edge. This diastolic jet also causes some entrainment of fluid from the quiescent region near the aortic outflow, indicated by the redirection of flow towards the central jet. The Reynolds number of the flow through the mitral valve, based on the cardiac output at peak diastole (15 L/min) and mitral annular area (4.5 cm2) is about 3370.
During end diastole, ventricular pressure increases, slowing and reversing flow through the mitral valve and ultimately forcing it shut. This is indicated by the velocity vectors oriented towards the mitral annulus (Fig. 6d). There is minimal flow in the remaining portions of the ventricular chamber during this time point in the cardiac cycle. The aortic outflow is out of the desired field of view for this experiment. No flow was observed when the mitral valve was closed (not shown) as the valve was competent.
Figure 7a shows contours of the out-of-plane velocity (W) at peak diastole. The low magnitude of W through the entire field of view indicates that the flow is predominantly two dimensional through the native mitral valve, which can be expected in the central plane of the normal valve. However, it is expected that mitral flow velocity and direction may be affected with abnormal valve geometry, as with leaflet tethering caused by papillary muscle displacement or with edge-to-edge repair. Similar low out-of-plane velocities were observed at all points in the cardiac cycle that were studied. Figure 7b shows the out-of-plane vorticity during peak diastolic filling through the mitral valve. As expected, the highest magnitudes of vorticity are observed at the edge of the central filling jet. The region of clockwise fluid rotation, indicated by the negative vorticity values, appears stronger and more coherent compared to the positive vorticity observed near the papillary muscle shown.
Discussion
Surgical strategies and outcomes of mitral valve repairs significantly depend on surgical skill and experience currently.5 The repair strategy is typically determined based on a 2-D/3-D echocardiographic assessment of the mitral valve and the left ventricle.8,12 However, in addition to echocardiographic assessment of the valve, it is important to understand and characterize the hemodynamic alterations in the flow through the mitral valve as a result of disease as well as repair. Numerical models provide the ideal test bed for such a “surgical planning” platform, with their ability to mimic complex repair procedures preoperatively.3,13,16,27,33,41,43,46 However, high fidelity experimental data are necessary to validate these fluid structure interaction models, before they can be put into widespread clinical use. Unfortunately, the complex geometric and hemodynamic environment associated with the native mitral valve is an extremely challenging platform to obtain high-resolution fluid and geometric data. The current study represents a novel platform to provide these data for one-to-one validation with FSI models.
A novel left heart simulator was designed specifically for the direct experimental validation of computational mitral valve models. Specifically, the modular design allowed multiple experimental techniques to be performed for a single mitral valve without perturbing the geometry. The experimental platform closely simulated the physiologic hemodynamic environment of the mitral valve. The atrial, ventricular, and aortic pressures and the mitral flow were stable and well simulated.4 Additionally, the waveforms were easily tunable and can simulate various pathologies such as hypertension or congestive heart failure.
Geometric characterization was performed using two complementary state of the art medical imaging modalities. Micro-CT imaging was performed because the high spatial resolution was necessary to accurately resolve the detailed and intricate chordal branching. In particular, no other modality is currently capably of reliably imaging the marginal chordae, which control leaflet coaptation, and are thus critical to numerical modeling accuracy. Three-dimensional echocardiography was used because it can non-invasively capture dynamic leaflet motion with sufficient spatial and temporal resolution. Moreover, leaflet geometry can be quantified at any point in the cardiac cycle. Since peak systolic and peak diastolic phases are most often assessed in mitral valve computational models and in clinical diagnosis. Consequently, the segmented leaflet geometry can be used for direct numerical comparison.
For the first time, stereoscopic digital particle image velocimetry was performed on a native mitral valve with intact subvalvular apparatus to quantify the fluid fields through and proximal to the mitral valve. This well-established technique is capable of measuring velocity vectors with high spatial resolution at any point in the cardiac cycle, which is necessary for the direct quantitative validation of computation fluid dynamics.
The current simulator incorporates many aspects of flow characterization which have not been addressed by previous studies—(1) The full three-dimensional velocity vector was resolved using stereoscopic PIV (2) The entire sub-valvular apparatus was preserved to ensure proper valve opening and closing dynamics (3) The flow through the valve was pulsatile, ensuring the correct dynamic motion of the valve leaflets. Steady flow models can only study one specific time point during the cardiac cycle. (4) Registration of the flow fields to the valve geometry obtained from 3D echo and micro CT is straightforward since all measurements are made on the same valve.
Finally, the simulator presents a precisely tunable environment—bulk hemodynamics and mitral valve geometry can be prescribed. Fluid dynamics proximal and through the mitral valve can be systematically studied under a myriad of carefully tuned conditions. Such studies can provide insight into the relationship between mitral valve geometry and left ventricular filling, and consequently the effects on systolic ejection.
Limitations and Future Studies
While the mechanical and hemodynamic environment of the mitral valve was simulated, some limitations existed. The rigid ventricle used in this study did not mimic native dynamic annular34 and ventricular motion. However, previous studies in complimentary experimental and in vivo studies have shown that leaflet dynamics compare favorably, in spite of this simplification.38 Further, the ventricular chamber was not shaped like a native ventricle, hence this study focused only on flow immediately distal to the mitral annulus. However, the relative orientation of the mitral inflow and left ventricular outflow tract was maintained by using an angled aortic section (Fig. 1). Only passive mitral filling was simulated in this study. Late diastolic flow as a result of atrial contraction will be simulated in the future using a positive displacement pump upstream of the mitral valve. Different working fluids were used for stereoscopic PIV (36% glycerin) and all other modalities (0.9% saline). Water-glycerin was necessary to (1) minimize optical distortions between the acrylic and fluid, (2) match the kinematic viscosity of blood for more accurate velocity measurements. Since the pressure field across the valve do not change between saline and glycerin, although the Reynolds number is different, the geometry captured in echocardiography should closely match that in the DPIV experiments.47 This was done because prolonged exposure to glycerin alters valvular material properties. The subvalvular apparatus obstructed portions of the field of view, however this was due to the need for optical access for PIV, which was exacerbated by the need for good viewing windows for two cameras. Future studies could explore the use of echocardiographic PIV to eliminate the need for optical access, however the spatial resolution of that technique might be a limiting factor. The use of non-metallic components could also enable phase contrast magnetic resonance imaging acquisitions on the same valve model.
It should be noted that the work presented in this manuscript represents an n = 1. The geometric data presented is that of a singular valve, for which the subsequent experimental conditions were performed. This strategically allows for the direct experimental to computational comparisons. More data on normal, pathological, and repaired mitral valves will be collected as these methods have now been carefully developed and demonstrated. Finally, the valves used in this study (4.5 cm2) were smaller than previously published ovine studies (~7 cm2),29,37 as limited by the micro-CT field of view; this made direct comparison difficult. However, basic mitral valve geometrical relations were respected when the valve was sutured to the annulus plate and tested. Mainly, the inter-commissural distance was conserved from the explanted heart, while the anterior leaflet comprised two-thirds of the coaptation orifice. Also, the papillary muscles were carefully positioned to result in physiologic coaptation.
In the future, these newly established methods would be combined with the serial acquisition of leaflet strain and chordal forces to characterize mitral valve tissue mechanics.19,30,32,36 The current work was focused solely on establishing the methods for fluid flow validation, which has previously never been done with a native valve. Additionally, valvular disease, due to abnormal valvular geometry, and subsequent surgical repair will be simulated.14,31 In particular, mitral regurgitation will be simulated by dilating the mitral annulus and displacing the papillary muscles. Mitral annuloplasty, edge-to-edge repair, and leaflet augmentation techniques could be performed as targeted surgical repairs. Expanded use of echocardiography will provide clinically relevant measures like color Doppler and proximal isovelocity surface area (PISA). Collectively, these studies will improve the surgical planning capabilities of numerical models.
References
Adams, D. H., and A. C. Anyanwu. The cardiologist’s role in increasing the rate of mitral valve repair in degenerative disease. Curr. Opin. Cardiol. 23:105–110, 2008.
Adams, D., A. Anyanwu, L. Sugeng, and R. Lang. Degenerative mitral valve regurgitation: surgical echocardiography. Curr. Cardiol. Rep. 10:226–232, 2008.
Arnoldi, A., A. Invernizzi, R. Ponzini, E. Votta, E. G. Caiani, and A. Redaelli. Mitral valve models reconstructor: a Python based GUI software in a HPC environment for patient-specific FEM structural analysis. In: Innovations and Advances in Computer Sciences and Engineering, edited by T. Sobh. Netherlands: Springer, 2010, pp. 215–219.
Bargiggia, G., C. Bertucci, F. Recusani, et al. A new method for estimating left ventricular dP/dt by continuous wave Doppler-echocardiography. Validation studies at cardiac catheterization. Circulation 80:1287–1292, 1989.
Bolling, S. F., S. Li, S. M. O’Brien, J. M. Brennan, R. L. Prager, and J. S. Gammie. Predictors of mitral valve repair: clinical and surgeon factors. Ann. Thorac. Surg. 90:1904–1912, 2010.
Bonow, R. O., B. A. Carabello, K. Chatterjee, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 114:e84–e231, 2006.
Bouxsein, M. L., S. K. Boyd, B. A. Christiansen, R. E. Guldberg, K. J. Jepsen, and R. Müller. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25:1468–1486, 2010.
Chikwe, J., D. H. Adams, K. N. Su, et al. Can three-dimensional echocardiography accurately predict complexity of mitral valve repair? Eur. J. Cardiothorac. Surg. 41:518–524, 2012.
Domenichini, F., G. Pedrizzetti, and B. Baccani. Three-dimensional filling flow into a model left ventricle. J. Fluid Mech. 539:179–198, 2005.
Dyn, N., D. Levin, and J. Gregory. Butterfly subdivision scheme for surface interpolation with tension control. ACM Trans. Graph. 9:160–169, 1990.
Einstein, D. R., F. Del Pin, X. Jiao, et al. Fluid–structure interactions of the mitral valve and left heart: comprehensive strategies, past, present and future. Int. J. Numer. Methods Biomed. Eng. 26:348–380, 2010.
Fischer, G. W., A. C. Anyanwu, and D. H. Adams. Change in surgical management as a consequence of real-time 3D TEE: assessment of left ventricular function. Semin. Cardiothorac. Vasc. Anesthesia 13:238–240, 2009.
Hammer, P., P. del Nido, and R. Howe. Anisotropic mass-spring method accurately simulates mitral valve closure from image-based models. In: Functional Imaging and Modeling of the Heart, edited by D. Metaxas, and L. Axel. Berlin: Springer, 2011, pp. 233–240.
He, S., J. H. Jimenez, Z. He, and A. P. Yoganathan. Mitral leaflet geometry perturbations with papillary muscle displacement and annular dilation: an in vitro study of ischemic mitral regurgitation. J. Heart Valve Disease 12:300–307, 2003.
Hong, G.-R., G. Pedrizzetti, G. Tonti, et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc. Imaging 1:705–717, 2008.
Ionasec, R. I., I. Voigt, B. Georgescu, et al. Patient-specific modeling and quantification of the aortic and mitral valves from 4-D cardiac CT and TEE. IEEE Trans. Med. Imaging 29:1636–1651, 2010.
Jimenez, J. H., S. W. Liou, M. Padala, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J. Thorac. Cardiovasc. Surg. 134:1562–1568, 2007.
Jimenez, J. H., D. D. Soerensen, Z. He, S. He, and A. P. Yoganathan. Effects of a saddle shaped annulus on mitral valve function and chordal force distribution: an in vitro study. Ann. Biomed. Eng. 31:1171–1181, 2003.
Jimenez, J., D. D. Soerensen, Z. He, J. Ritchie, and A. P. Yoganathan. Effects of papillary muscle position on chordal force distribution: an in vitro study. J. Heart Valve Disease 14:295–302, 2005.
Kheradvar, A., H. Houle, G. Pedrizzetti, et al. Echocardiographic particle image velocimetry: a novel technique for quantification of left ventricular blood vorticity pattern. J. Am. Soc. Echocardiogr. 23:86–94, 2010.
Kilner, P. J., G.-Z. Yang, A. J. Wilkes, R. H. Mohiaddin, D. N. Firmin, and M. H. Yacoub. Asymmetric redirection of flow through the heart. Nature 404:759–761, 2000.
Kim, W. Y., P. G. Walker, E. M. Pedersen, et al. Left ventricular blood flow patterns in normal subjects: a quantitative analysis by three-dimensional magnetic resonance velocity mapping. J. Am. Coll. Cardiol. 26:224–238, 1995.
Kunzelman, K. S., R. P. Cochran, C. Chuong, W. Ring, E. Verrier, and R. Eberhart. Finite element analysis of the mitral valve. J. Heart Valve Disease 2:326–340, 1993.
Kunzelman, K. S., D. R. Einstein, and R. P. Cochran. Fluid–structure interaction models of the mitral valve: function in normal and pathological states. Philos. Trans. R. Soc. B Biol. Sci. 362:1393–1406, 2007.
Kunzelman, K. S., M. S. Reimink, and R. P. Cochran. Annular dilatation increases stress in the mitral valve and delays coaptation: a finite element computer model. Cardiovasc. Surg. 5:427–434, 1997.
Lau, K. D., V. Díaz-Zuccarini, P. Scambler, and G. Burriesci. Fluid–structure interaction study of the edge-to-edge repair technique on the mitral valve. J. Biomech. 44:2409–2417, 2011.
Mansi, T., I. Voigt, E. Mengue, et al. Towards patient-specific finite-element simulation of MitralClip procedure. In: Medical Image Computing and Computer-Assisted Intervention, edited by G. Fichtinger, A. Martel, and T. Peters. Berlin: Springer, 2011, pp. 452–459.
McQueen, D. M., C. S. Peskin, and E. L. Yellin. Fluid dynamics of the mitral valve: physiological aspects of a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 242:H1095–H1110, 1982.
Nguyen, T. C., A. Itoh, C. J. Carlhäll, et al. The effect of pure mitral regurgitation on mitral annular geometry and three-dimensional saddle shape. J. Thorac. Cardiovasc. Surg. 136:557–565, 2008.
Padala, M., R. A. Hutchison, L. R. Croft, et al. Saddle shape of the mitral annulus reduces systolic strains on the P2 segment of the posterior mitral leaflet. Ann. Thorac. Surg. 88:1499–1504, 2009.
Padala, M., S. N. Powell, L. R. Croft, V. H. Thourani, A. P. Yoganathan, and D. H. Adams. Mitral valve hemodynamics after repair of acute posterior leaflet prolapse: quadrangular resection versus triangular resection versus neochordoplasty. J. Thorac. Cardiovasc. Surg. 138:309–315, 2009.
Padala, M., M. S. Sacks, S. W. Liou, K. Balachandran, Z. He, and A. P. Yoganathan. Mechanics of the mitral valve strut chordae insertion region. J. Biomech. Eng. 132:081004, 2010.
Pouch, A. M., C. Xu, P. A. Yushkevich, et al. Semi-automated mitral valve morphometry and computational stress analysis using 3D ultrasound. J. Biomech. 45:903–907, 2012.
Rausch, M., W. Bothe, J.-P. Kvitting, et al. Characterization of mitral valve annular dynamics in the beating heart. Ann. Biomed. Eng. 90:1–13, 2011.
Reul, H., N. Talukder, and E. W. Müller. Fluid mechanics of the natural mitral valve. J. Biomech. 14:361–372, 1981.
Ritchie, J., J. Jimenez, Z. He, M. S. Sacks, and A. P. Yoganathan. The material properties of the native porcine mitral valve chordae tendineae: an in vitro investigation. J. Biomech. 39:1129–1135, 2006.
Ryan, L., B. Jackson, L. Parish, et al. Quantification and localization of mitral valve tenting in ischemic mitral regurgitation using real-time three-dimensional echocardiography. Eur. J. Cardiothorac. Surg. 31:839–844, 2007.
Sacks, M. S., Y. Enomoto, J. R. Graybill, et al. In vivo dynamic deformation of the mitral valve anterior leaflet. Ann. Thorac. Surg. 82:1369–1377, 2006.
Shuhaiber, J., and R. J. Anderson. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur. J. Cardiothorac. Surg. 31:267–275, 2007.
Soloff, S. M., R. J. Adrian, and Z.-C. Liu. Distortion compensation for generalized stereoscopic particle image velocimetry. Meas. Sci. Tech. 8:1441–1454, 1997.
Stevanella, M., F. Maffessanti, C. Conti, et al. Mitral valve patient-specific finite element modeling from cardiac MRI: application to an annuloplasty procedure. Cardiovasc. Eng. Technol. 2:66–76, 2011.
Stewart, S., E. Paterson, G. Burgreen, et al. Assessment of CFD performance in simulations of an idealized medical device: results of FDA’s first computational interlaboratory study. Cardiovasc. Eng. Technol. 1–22:2012, 2012.
Tenenholtz, N. A., P. E. Hammer, R. J. Schneider, N. V. Vasilyev, and R. D. Howe. On the Design of an Interactive. California, USA: Patient-Specific Surgical Simulator for Mitral Valve Repair. IEEE/RSJ International Conference on Intelligent Robots and Systems. San Francisco, 2011.
Veronesi, F., C. Corsi, L. Sugeng, et al. A study of functional anatomy of aortic-mitral valve coupling using 3D matrix transesophageal echocardiography/clinical perspective. Circ. Cardiovasc. Imaging 2:24–31, 2009.
Votta, E., E. G. Caiani, F. Veronesi, M. Sonicini, F. Montevecchi, and A. Redaelli. Mitral valve finite element modelling from ultrasound data: pilot study for a new approach to understand mitral function and clinical scenarios. Philos. Trans. A Math. Phys. Eng. Sci. 366:3411–3424, 2008.
Wong, V. M., J. F. Wenk, Z. Zhang, et al. The effect of mitral annuloplasty shape in ischemic mitral regurgitation: a finite element simulation. Ann. Thorac. Surg. 93:776–782, 2012.
Yoganathan, A. P., W. H. Corcoran, and E. C. Harrison. Pressure drops across prosthetic aortic heart valves under steady and pulsatile flow—in vitro measurements. J. Biomech. 12:153–164, 1979.
Acknowledgments
This study was funded by a grant from the National Heart Lung and Blood Institute (R01HL092926). The authors would also like to thank Angela Lin for assisting with microCT imaging and reconstruction, JM Machining and Mark McJunkin for manufacturing of flow loop components, and Roy Tweedy’s for specimen donation.
Conflict of interest
There were no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Rabbah, JP., Saikrishnan, N. & Yoganathan, A.P. A Novel Left Heart Simulator for the Multi-modality Characterization of Native Mitral Valve Geometry and Fluid Mechanics. Ann Biomed Eng 41, 305–315 (2013). https://doi.org/10.1007/s10439-012-0651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0651-z