Abstract
In this report, a balloon-expandable, biodegradable, drug-eluting bifurcation stent (DEBS) that provides a sustainable release of anti-proliferative sirolimus was developed. Biodegradable bifurcation stents, made of polycaprolactone, were first manufactured by injection molding and hot spot welding techniques. Various properties of the fabricated stents, including compression strengths, collapse pressures, and flow pattern in a circulation test, were characterized. The experimental results showed that biodegradable bifurcation stents exhibited comparable mechanical properties with those of metallic stents and superior flow behavior to that of metallic bifurcation stents deployed via the T And small Protrusion approach. Polylactide-polyglycolide (PLGA) copolymer and sirolimus were then dissolved in acetonitrile and coated onto the surface of the stents by a spray coating device. An elution method and a high performance liquid chromatography analysis were utilized to examine the in vitro release characteristics of sirolimus. Biodegradable bifurcation stents released high concentrations of sirolimus for more than 6 weeks, and the total period of drug release could be prolonged by increasing the drug loading of the PLGA/sirolimus coating layers. In addition, the eluted drug could effectively inhibit the proliferation of smooth muscle cells. The developed DEBS in this study may provide a promising strategy for the treatment of cardiovascular bifurcation lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous coronary intervention (PCI) of bifurcation disease remains a challenge in terms of procedural success rate, as well as long term major cardiac events, target lesion revascularization, restenosis, and stent thrombosis.2,26,40 The use of metallic drug-eluting stents (DESs), coated with anti-proliferative drugs, either sirolimus or paclitaxel, during percutaneous transluminal coronary angioplasty, has been a common practice for the treatment of bifurcation lesions.4,16,20,35 Side branch (SB) ostial restenosis, however, remains a problem in 10–20% of bifurcation lesion cases, due to limited strut coverage, with consequently inadequate drug distribution to the stented artery.8,22,28,30,34 Therefore, the provisional approach of implanting one stent on the main branch has become the default approach to most bifurcation lesions. Bifurcation PCI, however, still remains technically challenging,6 especially when two-stent strategies are required.
Metallic bifurcation stents using multiple stents25 present an exciting innovation as they show an attempt to find a technological solution for a specific subset of coronary lesions. They, however, may also induce various long-term side effects, such as the prevention of the lumen expansion, associated with late favorable remodeling, and the preclusion of surgical revascularization, which may become necessary at a later time.7 In addition, the bifurcation stents may be produced by coating a layer of non-degradable polymers containing anti-proliferative drugs onto the surface of stainless steel stents. It has been reported that metallic drug eluting stents may be a cause of systemic and intra-stent hypersensitivity reactions that are associated with late thrombosis and death.29 On the other hand, Guerin et al.14 analyzed the consequences of the post-dilatation, by using simultaneous, kissing balloon inflation, and observed over-inflation in the deployed stents. The over-inflation reduced the ratio of potential metal to artery, as well as the ratio of potential drug application to area in these over-expanded segments, which in turn impaired the anti-proliferative effect of the drug-eluting stents. Furthermore, the coating damage observed on the ostial struts might also reduce drug delivery and increase the restenosis risk. A biodegradable drug-eluting stent that provides a sustainable release of anti-proliferative pharmaceuticals and resolves gradually after serving its purpose is thus highly desired.
Biodegradable bifurcation stents that integrate the main vessel and the two branches into one part provide the advantages of not needing a second stent, delivering adequate drug concentrations to the coronary artery, especially at the SB ostial to prevent restenosis, and being biodegradable so that the long-term side effects can be minimized.9,10,42 Presently, various groups have focused on the development of biodegradable drug-eluting stents.3,5,15,19,24,27,36,38 None, however, have developed a biodegradable, bifurcation, drug-eluting stent for the treatment of bifurcation lesions.
This study aims to develop biodegradable drug-eluting bifurcation stents (DEBS) that provide a sustained release of anti-proliferative sirolimus (rapamycin). To prepare the DEBS, biodegradable bifurcation stents made of poly(ε-caprolactone) (PCL) were first manufactured using injection molding and hot spot welding techniques. PCL17 is a non-toxic and tissue-compatible material and can be eventually resorbed in the vital organs. It undergoes a two-stage degradation process: first, the ester group is cleaved due to the non-enzymatic hydrolysis; and second, the polymer is seen to undergo intracellular degradation when it is more highly crystallized and of low molecular weight (less than 3000 Da).39 Polycaprolactone degrades at a slower pace than other biodegradable polymers, such as polylactide (PLA), polyglycolide (PGA), and their copolymers, and can therefore be used in drug delivery devices that remain active for over a year. Polycaprolactone is also a semi-crystalline polymer with a low melting point (59–64 °C) and exhibits good flexibility at room temperature and at 37 °C. This would avoid the occurrence of stent fragmentation,18 which may cause the obstruction of arteries. Furthermore, stents with good flexibility are easier to manipulate and deploy with minimal invasion and are more likely to adapt to the bifurcation area.
Sirolimus and polylactide-polyglycolide (PLGA) copolymer were then dissolved in acetonitrile and coated onto the surface of the stents by a spray coating device. Sirolimus is a macrolide compound that acts by selectively blocking the transcriptional activation of cytokines, thereby inhibiting cytokine production. It can inhibit arterial smooth muscle cell proliferation and migration and prevent neointima formation after balloon angioplasty. The anti-proliferative effect of sirolimus has also been used in conjunction with metallic coronary stents (Cypher, Cordis Corp.) to prevent restenosis in coronary arteries following balloon angioplasty. Pires et al.31 showed that sirolimus exhibited more efficiency when compared to paclitaxel, as an anti-restenotic agent in an animal model. Song et al.33 compared the long-term outcomes of patients treated with sirolimus-eluting stents and paclitaxel-eluting stents and reported that the use of the former resulted in better outcomes in patients with bifurcation lesions.
Polylactide-polyglycolide is one of the most widely used polymers used to achieve a sustained release of pharmaceuticals. It is non-toxic, elicits a minimal inflammatory response, and can be eventually absorbed without any accumulation in the vital organs. Under environmental as well physiological conditions, PLGA is hydrolytically degraded into glycolic acid or lactic acid. The degradation rate depends on the molecular weight, surface quality, and composition of the polymers. The complete degradation of PLGA copolymers takes a few months.41
After fabrication, various properties, including compression strengths, collapse pressures, and flow patterns in a circulation test, of the fabricated biodegradable bifurcation stents were characterized. An in vitro elution method and a high performance liquid chromatography (HPLC) analysis were employed to characterize the release behaviors of sirolimus from the DEBS. The effectiveness of eluted sirolimus on inhibiting the proliferation of smooth muscle cells was also examined.
Materials and Methods
Materials
The polymer used for the fabrication of biodegradable stents was PCL, with a molecular weight (M n) of 80,000 Da (Sigma-Aldrich, USA). The materials used for the spray coating of the stents were PLGA with a ratio of 50:50 and a molecular weight of 24,000–38,000 Da (RG 503, Boeringer Ingelheim, Germany), and sirolimus (R0395, Sigma-Aldrich, USA).
Fabrication of Biodegradable Bifurcation Stents
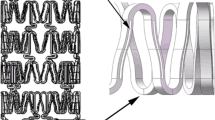
The biodegradable bifurcation stent developed in this study consisted of a main vessel and two branches. To prepare the stent, a mesh-type stent component made of polycaprolactone, which consists of twelve ring-ellipse-ring units (Fig. 1), was first molded by a lab-made injection molding machine.23 The melt temperature and mold temperature used were 100 and 60 °C, respectively. To assemble the main vessel, three stent components were interconnected with the top six ring-ellipse-ring units (resulting in a total of four components), the assembly was rolled into a mesh tube, and the final connecting points were welded by hot spot welding. Each component was interconnected with every bottom six ring-ellipse-ring unit (a total of two components) to make the branches. Figure 2 shows the assembly process. A biodegradable bifurcation stent with external diameters of 2 and 1.4 mm for the main vessel and the two branches, respectively, was obtained. The fabricated stent was passed over two commercially available 2.0 × 15 mm Maverick balloons (Boston Scientific/Scimed, Inc., Maple Grove, Minnesota, USA) for expansion purposes. During balloon inflation, the stents expanded and the rings slid over the central ellipses to the sides, causing an expansion of both the main vessel and two branches of the stent. Figure 3 shows the photograph of the expanded biodegradable stent. The same experiments were also performed for DESs (TAXUS Liberté 3.0 × 12 mm, Boston Scientific Corp., Natick, MA) for comparison purposes. Three stents (N = 3) were used for each test.
Mechanical Strengths
A compression test of the fabricated stents was conducted on a tensiometer. The stents were compressed along their radial direction during testing. Deformations caused by various loads were recorded. Comparisons were made between the biodegradable bifurcation stents and the commercially available DES. The testing was repeated three times.
The collapse pressure of the stent was measured using a lab-made pressure chamber similar to that reported by previous researchers.36,43 The system consisted of a pressure chamber, flow loop, and circulating water at 37 °C. The test stent was mounted in a flexible Tygon tube, connected to the flow loop within the pressure chamber. The volumetric flow rate of water and the luminal pressure were set to be 10 L/min and 76 mmHg, respectively. Three stents (N = 3) were used for each test.
Circulation Test
A flow visualization circulation system, which consisted of a servo-motor driven reciprocating pump, water tank with a temperature moderator, flow rate control, and Y shaped bifurcation glass tubes, was designed and built in our lab. Figure 4a shows the setup for the flow visualization experiment. The hydraulic reciprocating pump is driven by a servo motor and has a pumping frequency of 72 pulses per minute and a flow rate of 90 mL/min. The fluid used for the experiments was a water/glycerin (Sigma-Aldrich, Saint Louis, MO, USA) blended solution, with a viscosity of 3.6 centipoises and a density of 1.13 g/cm3. To better visualize the flow patterns of the fluid during circulation, polyethersulfone (PES) particles with a diameter of 0.4 mm and density of 1.38 g/cm3 were added into the fluid.
Y-shaped glass tubes, with a main vessel (inlet of the fluid) of 3.0 mm in diameter and two branches of 1.8 mm in diameter, were used for the experiments. The angle set between the two branches was 70° (Fig. 4b). A comparison was made between the flow pattern of biodegradable bifurcation stents and commercially available DESs, using the T And small Protrusion (TAP) technique25 during the circulation test. The glass tubes were glued onto the surface of a light box, and the PES particle flow during the circulation process was recorded by a high-speed video camera that records 500 frames per second. The camera was connected to a personal computer for data acquisition. The total observation period of flow visualization was 30 min.
Coating of Sirolimus onto the Surface of the Stents
Sirolimus was coated onto the surface of biodegradable bifurcation stents using a spray coating method.24 PLGA copolymers and sirolimus (a total weight of 5 mg) at predetermined ratios (90/10, 80/20, 50/50 w/w) were first dissolved in 0.4 mL of acetonitrile (J.T. Baker, USA). The mixture was then delivered by the spray coater with a volumetric flow rate of 4 mL/h. After the initial coating, coated stents were kept in a vacuum oven for 24 h for drying. Another drug-loaded layer was spray coated onto the stents. The process was repeated until a total of five layers of PLGA/sirolimus were coated onto the bifurcation stents. All spray coating experiments were completed at room temperature.
Release Characteristics of Sirolimus
An in vitro elution method was employed to determine the release characteristics of sirolimus from the DEBSs. A phosphate buffer saline, 0.15 mol/L (pH 7.4), was used as the dissolution medium. The stents were placed in glass test tubes with 1 mL of phosphate buffer. All tubes were incubated at 37 °C. The dissolution medium was collected at 24 h intervals. Fresh phosphate buffer (1 mL) was added at the beginning of each interval for 6 weeks.
The sirolimus concentrations in buffer for the elution studies were determined by a HPLC assay standard curve for sirolimus. The HPLC analyses were conducted on a Hitachi L2400 UV–VIS System. The column used for the separation of the sirolimus was a Hibar, C-18, 5 μm, 4.6 × 250 mm HPLC column. The mobile phase contained acetonitrile, methanol, and distilled water (45/40/15, v/v/v). The absorbency was monitored at 278 nm and the flow rate was 1.2 mL/min. A calibration curve was made for each set of measurements (correlation coefficient >0.99). All experiments were repeated three times (N = 3) and the sample dilutions were performed to bring the unknown concentrations into the range of the assay standard curve.
Smooth Muscle Cell Cultures
The biodegradable stents with various sirolimus loadings (i.e., 10, 30, and 50%) were placed onto 24-well culture plates. Plates without stents were used as the control. Commercially available rat smooth muscle cells of the thoracic aorta, from Rattus norvegicus (Rat) (A7r5, DB1X, Bioresource Collection and Research Center at the Food Industry Research and Development Institute, Taiwan), were seeded at 5 × 103 cells per plate and cultured at 37 °C in 5% CO2. The culture medium was Eagle’s Minimal Essential Medium (DMEM), with 4 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate and 4.5 g/L glucose + 10% fetal bovine serum. Cell viability in the plates was monitored at 1, 3, 7, 14, 21, and 28 days by MTT assays and quantified using an ELISA (R&D Systems, Minneapolis, MN, USA).
Statistics and Data Analysis
Data were collected from triplicate samples and were analyzed by a one-way analysis of variance (ANOVA) using the drug-loaded groups as a between-subject factor. Bonferroni post hoc analysis was performed when the F test of ANOVA revealed statistical significance. Differences were considered statistically significant for p values <0.05.
Results
Development of Biodegradable Bifurcation Stents
The DEBS developed in this study, which consisted of a main vessel and two branches, was manufactured using injection molding and hot spot welding techniques. The fabricated stent was passed over two commercial available balloons for expansion. During balloon inflation, the stents expanded and the rings slid over the central ellipses to the sides, causing an expansion of both the main vessel and two branches of the stent. Figure 5 shows the variation of the stents’ external diameters with the balloon pressure. The main vessel and the branches could be completely expanded to 3.4 and 1.85 mm, respectively, with a balloon pressure of 2.5 kg/cm2 (bar). With the release of the balloon pressure, both stents retracted to an extent, but were self-locked soon after. With the release of the balloon pressure, a biodegradable bifurcation stent with final external diameters of 3.1 and 1.8 mm, for the main vessel and the branches, respectively, was obtained. Due to the geometric constrains of the central ellipses, once the stent expanded, the rings were not able to slide backward, exhibiting self-locking characteristics.
The compression strengths of the stents43 were measured by a tensile tester and compared to those of commercially available DESs. Figure 6 shows the measured results. For compression strains <10%, the polymeric bifurcation stents, either the main vessel or the branch, show comparable compression strengths to that of DESs.
The collapse pressures36 of both DESs and polymeric stents were conducted in the chamber, with a flow loop imitating the physiological flow within a human coronary artery. While the collapse pressure of the metallic stent was 2.54 ± 0.12 kg/cm2, the collapse pressures of the main vessel and branch of biodegradable bifurcation stents were measured to be 1.38 ± 0.12 and 1.53 ± 0.16 kg/cm2, respectively.
Circulation Test Results
The flow pattern of fluids in the biodegradable bifurcation stents and commercially available stent was tested using a flow visualization circulation system, which consisted of a servo-motor driven reciprocating pump, water tank with a temperature control, flow rate control, and a Y-shaped glass tube (Fig. 4b). A comparison of the flow patterns of glass tubes stented by metallic bifurcation stents technique via TAP and biodegradable bifurcation stents (Fig. 7) was completed. The circulation process took approximately 30 min. During the test, the polymeric particle-filled fluid flowed smoothly through both the DEBS stented tube and the TAP stented tube. Nevertheless, some particles aggregated at the ostial sites of both stented tubes, as shown in Fig. 8. The results matched the clinical observation that most of the restenosis occurs at the ostium. Furthermore, more particles aggregated at the ostrial sites of metallic stents (Fig. 8a) than at those of the biodegradable stents (Fig. 8b). Despite the fact that the glass tubes used in the circulation test were much stiffer and exhibited less flexibility than those of human arteries, the preliminary results of the circulation test showed that the biodegradable drug-eluting stents exhibited superior flow patterns for the water/glycerin fluid, compared with that of commercially available bifurcation stents.
Release of Anti-Proliferative Sirolimus
Three different sirolimus weight loadings (10, 30, and 50% w/w, respectively) were used in this study. Five layers of PLGA/sirolimus were coated onto the surface of the stents. Figures 9a and 9b show, respectively, the daily and accumulated release of sirolimus from the DEBS. All stents show a burst of release of the drug during the first 3 days, followed by a second substantial drug release at 3 weeks. The results in Fig. 9 show that the total release period of the stents increases with the loading percentage of sirolimus. Approximately 40 and 36% of the drugs were released for the 10 and 30% sirolimus loaded stents, respectively, while the 50% drug loaded DEBS released only 15% of the drug at 6 weeks.
The effectiveness of the released sirolimus from the DEBS was examined by a MTT assay (Roche, Germany) of smooth muscle cell viability at 1, 3, 7, 14, 21, and 28 days. The data was analyzed by a ANOVA. The results in Fig. 10 show that the smooth muscle cells in the control group proliferated with time, up to day 21, while it began to degrade at day 28. One possible explanation for the reduction of cell proliferation is that the commercially available smooth muscle cells reached their life span during culturing.13 Cell proliferation decreased accordingly. Furthermore, the smooth muscle cells were cultured alone in the experiments. When there is no co-culture with endothelium, the proliferation of smooth muscle cells can also be limited.11 A more detailed culture experiment should be done in the future to further investigate this phenomenon. Nevertheless, the results in Fig. 10 suggest that the released sirolimus from the DEBS could effectively inhibit the proliferation of smooth muscle cells at different days, compared with the control group (p < 0.01). This further proves the effectiveness of the DEBS developed in this study.
Discussion
Polycaprolactone has been one of the most promising biodegradable bio-materials. It is a semi-crystalline polymer with a low melting point39 and can be easily processed by commonly used polymer processing methods, such as injection molding or extrusion. PCL is also non-toxic and tissue-compatible, exhibits good flexibility at room temperature and at 37 °C, and can be eventually resorbed in the vital organs. The total degradation time of PCL is approximately 1–2 years. Furthermore, the results of our previous study23 showed that no significant mechanical property change and weight loss of the stents were observed after being submerged in phosphate buffer saline for 12 weeks. All these make PCL an ideal material for the backbone of the biodegradable, drug-eluting stents.
The release mechanism of sirolimus from the DEBSs is important to consider. Drugs formulated in polymeric devices are usually released by diffusion through the polymer barrier, by degradation of the polymer materials, or a combination of both mechanisms. Generally, the cumulative release of sirolimus from biodegradable stents with multi-layer coatings, in this study, shows three different stages of release kinetics, namely an initial burst, a diffusion-controlled release, and a degradation-controlled release. After the spray coating process, although most particles are dispersed in the bulk of the PLGA layer, some drug particles may possibly be located on the surface of the coated layer1 and are dissolved quickly. This in turn would cause the initial burst release of the drugs.
Figure 9 shows that the total release period of the stents increases with the loading percentage of sirolimus. This can be explained by the fact that the degradation rate of polymers and the daily release of sirolimus from stents of various drug-loadings were comparable. A stent with a higher drug loading, based on the same release rate, can thus release the drug for a longer time. Following the initial burst release, the cumulative release of sirolimus from the DEBSs exhibits two different stages of release kinetics, namely a diffusion-controlled release at days 3–20 and a degradation-dominated release after day 20.21,32 For the first stage of drug release (days 3–20), the slow degradation of PLGA materials makes diffusion through the stents the only possible mechanism for drug release. As the loading of sirolimus was low, the stents did not release a high concentration until after 20 days, during the elution process. Beginning at day 20, the stents showed an accelerated release of sirolimus. This might be due to the fact that polymers degrade during the elution process. When the polymer molecular weight decreases sufficiently, loss of the polymer begins. The polymer matrix may break to form openings for sirolimus release. Sirolimus is then released along with this polymer and the release rate accelerates accordingly.
It has been reported12,37 that DESs that elute sirolimus over 30 days were more effective in suppressing neointimal hyperplasia and reducing major adverse cardiovascular events than stents that elute drugs over a shorter period of time. Drug elution beyond 30 days, however, could also be theoretically detrimental, as this may delay endothelialisation of the stent, with the potential risk of stent thrombosis. A significant advantage of the DEBSs developed in this study is that the local sirolimus concentrations are high at the target site, especially at the branch ostium, mainly due to the integration of the main vessel and branches into one piece and only one stent being used during deployment. Controlled drug release can be achieved using polymeric materials, within which the active agent is stored in reservoirs. By employing micro-injection molding and multi-layer spray coating techniques, the fabrication of DEBSs that integrate the main vessel and the branches into one stent is possible, releasing a high concentration of sirolimus for more than 6 weeks. This would eliminate the problem associated with the use of multi-stents for the treatment of bifurcation lesions, as well as provide advantages in terms of sustained drug release for cardiovascular applications.
Conclusions
In this study, we successfully developed balloon-expandable, biodegradable DEBSs that provide a sustainable release of anti-proliferative sirolimus. Biodegradable bifurcation stents made of PCL were manufactured by injection molding and hot spot welding techniques. The experimental results showed that DEBSs exhibited comparable mechanical properties with those of commercial stents, and superior flow behavior to that of metallic bifurcation stents via TAP technique. Furthermore, biodegradable bifurcation stents released high concentrations of sirolimus for more than 6 weeks, and the total period of drug release could be prolonged by increasing the drug loading of the PLGA/sirolimus coating layers. The eluted drug could effectively inhibit the proliferation of smooth muscle cells. By employing micro-injection molding and multi-layer spray coating techniques, the fabrication of DEBSs that integrate the main vessel and the branches into one stent is possible, thus eliminating the problem associated with the use of multi-stents for the treatment of bifurcation lesions, as well as providing advantages in terms of sustained drug release for cardiovascular applications. The DEBS may provide a promising strategy for the treatment of cardiovascular bifurcation lesions.
References
Acharya, G., and K. Park. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 58:387–401, 2006.
Al Suwaidi, J., W. Yeh, H. A. Cohen, K. M. Detre, D. O. Williams, and D. R. Holmes, Jr. Immediate and one-year outcome in patients with coronary bifurcation lesions in the modern era (NHLBI dynamic registry). Am. J. Cardiol. 87:1139–1144, 2001.
Alexis, F., S. S. Venkatraman, S. K. Rath, and F. Boey. In vitro study of release mechanisms of paclitaxel and rapamycin from drug-incorporated biodegradable stent matrices. J. Control. Release 98:67–74, 2004.
Burt, H. M., and W. L. Hunter. Drug-eluting stents: a multidisciplinary success story. Adv. Drug Deliv. Rev. 58:350–357, 2006.
Chen, M. C., Y. Chang, C. T. Liu, W. Y. Lai, S. F. Peng, Y. W. Hung, H. W. Tsai, and H. W. Sung. The characteristics and in vivo suppression of neointimal formation with sirolimus-eluting polymeric stents. Biomaterials 30:79–88, 2009.
Cho, G. Y., C. W. Lee, M. K. Hong, J. J. Kim, S. W. Park, and S. J. Park. Effects of stent design on side branch occlusion after coronary stent placement. Catheter Cardiovasc. Interv. 52:18–23, 2001.
Colombo, A., and E. Karvouni. Biodegradable stents: fulfilling the mission and stepping away. Circulation 102(4):371–373, 2000.
Colombo, A., J. W. Moses, M. C. Morice, J. Ludwig, D. R. Holmes, Jr., V. Spanos, et al. Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation 109:1244–1249, 2004.
Daemon, J., and P. W. Serruys. Drug-eluting stent update 2007 Part I: a survey of current and future generation drug-eluting stents: meaningful advances or more of the same? Circulation 116:316–328, 2007.
Daemon, J., and P. W. Serruys. Drug-eluting stent update 2007 Part II: unsettled issues. Circulation 116:961–968, 2007.
Fillinger, M. F., L. N. Sampson, J. L. Cronenwett, R. J. Powell, and R. J. Wagner. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J. Surg. Res. 67:169–178, 1997.
Garg, S., and P. W. Serruys. Coronary stents: current status. J. Am. Coll. Cardiol. 56(2):1–42, 2010.
Gospodarowicz, D., K. Hirabayashi, L. Giguere, and J. P. Tauber. Factors controlling the proliferative rate, final cell density, and life span of bovine vascular smooth muscle cells in culture. J. Cell Biol. 89:568–578, 1981.
Guérin, P., P. Pilet, G. Finet, Y. Gouëffic, J. M. N’Guyen, D. Crochet, I. Tijou, P. Pacaud, and G. Loirand. Drug-eluting stents in bifurcations: bench study of strut deformation and coating lesions. Catheter Cardiovasc. Interv. 3:120–126, 2010.
Hietala, E. M., P. Maasilta, T. Välimaa, A. L. J. Harjula, P. Törmälä, U. S. Salminen, and R. Lassila. Platelet responses and coagulation activation on polylactide and heparin-polycaprolactone-l-lactide-coated polylactide stent struts. J. Biomed. Mater. Res. 67A:785–791, 2003.
Htay, T., and M. W. Liu. Drug-eluting stent: a review and update. Vascul. Health Risk Manag. 1(4):263–276, 2005.
Jiang, S., X. Ji, L. An, and B. Jiang. Crystallization behavior of PCL in hybrid confined environment. Polymer 42:3901–3907, 2001.
Korpela, A., P. Aarnio, H. Sariola, P. Tormala, and A. Harjula. Bioabsorbable self-reinforced poly-l-lactide, metallic, and silicone stents in the management of experimental tracheal stenosis. Chest 115:490–495, 1999.
Kraitzer, A., L. Ofek, R. Schreiber, and M. Zilberman. Long-term in vitro study of paclitaxel-eluting bioresorbable core/shell fiber structures. J. Contr. Release 126:139–148, 2008.
Laarman, G., M. J. Suttorp, M. T. Dirksen, L. Van Heerebeek, F. Kiemeneij, T. Slagboom, L. R. Van der Wieken, J. G. P. Tijssen, B. Rensing, and M. Patterson. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N. Engl. J. Med. 355:1105–1113, 2006.
Lao, L. L., S. S. Venkatraman, and N. A. Peppas. Modeling of drug release from biodegradable polymer blends. Eur. J. Pharm. Biopharm. 70:796–803, 2008.
Lefevre, T., Y. Louvard, M. C. Morice, P. Dumas, C. Loubeyre, A. Benslimane, et al. Stenting of bifurcation lesions: classification, treatments, and results. Catheter Cardiovasc. Interv. 49:274–283, 2000.
Liu, S. J., F. J. Chiang, C. Y. Hsiao, Y. C. Kau, and K. S. Liu. Fabrication of balloon-expandable self-lock drug-eluting polycaprolactone stents using micro-injection molding and spray coating techniques. Ann. Biomed. Eng. 38:3185–3194, 2010.
Liu, S. J., C. Y. Hsiao, J. K. Chen, K. S. Liu, and C. H. Lee. In vitro release of anti-proliferative paclitaxel from novel balloon-expandable polycaprolactone stents. Mater. Sci. Eng. C: Mater. Biol. Appl. 31:1129–1135, 2011.
Louvard, Y., M. Thomas, V. Dzavik, D. Hildich-Smith, A. R. Galassi, M. Pan, et al. Classification of coronary artery bifurcation lesions and treatment: time for a consensus. Catheter Cardiovasc. Interv. 71:175–183, 2008.
Meier, B., A. R. Gruentzig, S. B. King, III, J. S. Douglas, Jr., J. Hollman, T. Ischinger, et al. Risk of side branch occlusion during coronary angioplasty. Am. J. Cardiol. 53:10–14, 1984.
Meng, B., J. Wang, N. Zhu, Q. Y. Meng, F. Z. Cui, and Y. X. Xu. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J. Mater. Sci. Mater. Med. 17:611–617, 2006.
Murasato, Y. Impact of three-dimensional characteristics of the left main coronary artery bifurcation on outcome of crush stenting. Catheter Cardiovasc. Interv. 69:248–256, 2007.
Nakazawa, G., A. V. Finn, M. Joner, E. Ladich, R. Kutys, E. K. Mont, H. K. Gold, A. P. Burke, F. D. Kolodgie, and R. Virmani. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118:1138–1145, 2008.
Pan, M., J. Suarez de Lezo, A. Medina, M. Romero, A. Delgado, J. Segura, et al. Drug-eluting stents for the treatment of bifurcation lesions: a randomized comparison between paclitaxel and sirolimus stents. Am. Heart J. 153(15):e1–e7, 2007.
Pires, N. M. M., B. L. Van der Hoeven, M. R. De Vries, L. M. Havekes, B. J. Van Vlijmen, W. E. Hennink, P. H. A. Quax, and J. W. Jukema. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly(ε-caprolactone) stent cuff. Biomaterials 26:5386–5394, 2005.
Serruys, P. W., J. Aoki, D. McClean, M. Pieper, and E. Sousa. The effect of variable dose and release kinetics on neointimal hyperplasia using a novel paclitaxel-eluting stent platform. J. Am. Coll. Cardiol. 46(2):253–260, 2005.
Song, P. S., D. R. Ryu, Y. B. Song, J.-Y. Hahn, J.-H. Choi, S. H. Lee, et al. Long-term outcomes of sirolimus-eluting stents vs paclitaxel-eluting stents in unprotected left main coronary artery bifurcation lesions. Clin. Cardiol. 34:378–383, 2011.
Steigen, T. K., M. Maeng, R. Wiseth, A. Erglis, I. Kumsars, I. Narbute, et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation 114:1955–1961, 2006.
Van de Werf, F. Drug-eluting stents in acute myocardial infarction. N. Engl. J. Med. 355:1169–1170, 2006.
Venkatraman, S. S., T. L. Poh, T. Vinalia, K. H. Mak, and F. Boey. Collapse pressures of biodegradable stents. Biomaterials 24:2105–2111, 2003.
Virmani, R., G. Guagliumi, A. Farb, G. Musumeci, N. Grieco, T. Motta, L. Mihalcsik, M. Tespili, O. Valsecchi, and F. D. Kolodgie. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109:701–705, 2004.
Wang, X., S. S. Venkatraman, F. Boey, J. S. C. Loo, and L. P. Tan. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials 27:5588–5595, 2006.
Woodruff, M. A., and D. W. Hutmacher. The return of a forgotten polymer-polycaprolactone in the 21st century. Prog. Polym. Sci. 35:1217–1256, 2010.
Yamashita, T., T. Nishida, M. G. Adamian, C. Briguori, M. Vaghetti, N. Corvaja, et al. Bifurcation lesions: two stents versus one stent-immediate and follow-up results. J. Am. Coll. Cardiol. 35:1145–1151, 2000.
Yang, S., K.-F. Leong, Z. Du, and C.-K. Chua. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 7:679–689, 2001.
Zidar, J. P., M. A. Lincoff, and R. S. Stack. Biodegradable stents. In: Textbook of Interventional Cardiology, 2nd ed., edited by E. J. Topol. New York: Saunders, 1994.
Zilberman, M., K. D. Nelson, and R. C. Eberhart. Mechanical properties and in vitro degradation of bioresorbable fibers and expandable fiber-based stents. J. Biomed. Mater. Res. B: Appl. Biomater. 74(2):792–799, 2005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Lee, CH., Chen, CJ., Liu, SJ. et al. The Development of Novel Biodegradable Bifurcation Stents for the Sustainable Release of Anti-Proliferative Sirolimus. Ann Biomed Eng 40, 1961–1970 (2012). https://doi.org/10.1007/s10439-012-0556-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0556-x