Abstract
The aim of this study was to quantify the effect of chemically induced diabetes mellitus (DM) on the mechanical properties of the Achilles tendon of rats and correlate it with metabolic and biomechanical findings. Adult rats were selected randomly and assigned to two groups, the diabetic group consisted of animals receiving a dose of streptozotocin to induce type I diabetes and the control group. The animals were placed in metabolic cages for analysis of metabolism. Ten weeks after diabetes induction, the Achilles tendon of both groups were collected and submitted to a traction test in a conventional testing machine. The measurements of mechanical properties indicated that the elastic modulus (MPa) was significantly higher in the control group (p < 0.01). In Maximum tension (MPa), the groups did not have differences (p > 0.01). Energy/tendon area (N mm/mm2), specific strain (%) and maximum specific strain (mm) were higher in tendon tests of the diabetic group (p < 0.01). We observed that the mechanical properties of tendons have correlations with metabolic properties of the diabetic animals. These results showed that induced DM in rats have an important negative effect on the mechanical properties of the Achilles tendon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In studying complications of chronic diabetes, retinopathy and nephropathy have naturally been the focus of much evaluation and publication due to the gravity of these complications. However, little attention has been paid to alterations in the muscular–skeletal system, which can contribute to a decline in the general state of health of diabetic people.1,10 Details about the relationship between diabetes and tendinopathy still remain unclear,1,2,5,14 nevertheless, case reports and some epidemiological studies frequently emphasize the possible connection between diabetes mellitus (DM) and alterations to tendons in various parts of the body.12,25 There is now evidence that diabetes may alter Achilles tendon stiffness and thickening, predisposing the patient to foot ulceration.24
Specifically, the effect of DM on collagen structure of tendons is still not well established.1,2,5,14 Among preliminary work on this matter, a study realized by Monnier et al. 20 found collagen alterations in diabetic individuals, speculating that diabetes could promote an effect similar to aging on the musculoskeletal system.
To evaluate possible tendon alterations in diabetic patients, some researchers have investigated the increase of thickness, fiber organization and presence of calcification in tendons of patients with DM using diagnostic imaging methods, most commonly the musculoskeletal ultrasound,1,2,6,13,31 followed by computed tomography10 and magnetic resonance imaging.24
Only one study14 used electron microscopy to evaluate samples of the Achilles tendon in diabetic patients. Several morphological alterations were observed, such as increased density, irregularity and smaller fibril diameter and collagen disorganization with an abnormal configuration.
Besides the occurrence of changes in tendon structure in diabetic individuals, some studies have examined the alterations in the biomechanical operation of the connective tissue. However, these studies do not refer directly to the diabetes state, but to the effect of reducing sugars, glucose and ribose, reacting with collagen to produce non-enzymatic glycation on the tendon, which leads to biochemical and biomechanical impairment.26
The mechanical properties of the Achilles tendon influence the function and performance of the musculoskeletal system during locomotion.7–9,27 The elasticity of tendons increases the efficiency of muscle during activity cycles such as gait that involves stretching and contraction of the muscle–tendon complex. Given this, the mechanical properties of the Achilles tendon reduce the work required of the muscle. This makes it possible for the gesture of locomotion, which would be mechanically unfavorable for muscle alone, to be performed using less energy.7,15,27
Several studies have evaluated the mechanical properties of tendons and documented their plasticity in response to different situations, such as immobilization and/or suspension,3,22 vibration strength training,16 tensile strength,32 training strength,19,28,29 and endurance training.11,16,29,32 In the specific case of diabetes, there are few studies on the mechanical properties of tendons.
This study is based on the hypothesis that the Achilles tendon of diabetic rats with chronic hyperglycemia and metabolic dysfunction undergoes changes in mechanical properties that can be assessed using a tensile test machine of conventional mechanical design.
Despite this, no studies have been found concerning the effect of chemically induced DM on the mechanical properties of the Achilles tendon in animals. Therefore, the aim of this study was to quantify the effect of chemically induced DM on mechanical properties of the Achilles tendon in rats and correlate it to clinical and metabolic findings.
Materials and Methods
Animals
For this study, we used 22 male albino rats from the Wistar lineage, maintained in the vivarium of the Anatomy Department of the Federal University of Pernambuco. These animals were kept in an environment of 23 ± 1 °C, in an inverted cycle of light/darkness (12 h) and they were offered a maintenance diet (Labina®, Purina) and water ad libitum in the vivarium. When they reached the age of 70 days, these animals were randomly distributed into two groups: control group—CG (n = 11), consisting of healthy rats (not diabetic) and diabetic group—DG (n = 11). However, due to complications of DM, four animals were excluded from the study.
Type I diabetes was induced when the rats reached an age of 70 days and mass of 323.11 ± 19.38 g through a single intraperitoneal injection of a streptozotocin solution (Sigma Chemical Co, USA), after fasting for 12 h. The streptozotocin (STZ) was diluted in a sodium citrate buffering solution (10 mM) at pH 4.5 and administered in a single dose of 60 mg/kg of the animal’s weight, carefully measured in a precision digital balance (Model BS3000A Bioprecisa, BR.). The non-diabetic animals received the same equivalent doses of a citrate sodium buffering solution, and 30 min after treatment the animals in both groups were fed, according to the protocol to induce diabetes described by Szkudelski.30 The protocol was approved by the Ethics Committee of Animal Experimentation of Federal University of Pernambuco, UFPE.
Laboratory Evaluation
Five animals were randomly selected from each experimental group, and placed in individual metabolic cages (TECNIPLAST 3701m081) for a 72 h period, registering with their weight (g), water intake (mL/24 h), food intake (g/24 h), diuresis (mL/24 h) and collecting blood and urine, for dosages of blood glucose (mg/dL) and urinary volume (mL), respectively, every 24 h. The methods for collection were similar to the study of Lerco et al. 17
The metabolic analyses were performed on three occasions: (1) when the animals were 63 days old (1 week before the induction of DM); (2) 7 days after the induction of DM; (3) 10 weeks after DM induction. The analyses were applied at the same times to the control group.
Tissue Preparation
In the 10th week, after the final analysis in the metabolic cage, the rats of both groups were anesthetized with a solution of Xylazine (Rompum®, Bayer) (10 mg/kg) and Ketamine Chloridrate (Ketalar®) (25 mg/kg), 0.10 mL per 100 g weight. An incision was made along the posterior surface of the right leg, followed by the detachment of the Achilles tendon. This complex was dissected from the soft tissue proximally above the muscle–tendon junction, and distally, the tendon attachment to the calcaneus bone was preserved. After the removal of the samples, the animals were killed.
The Achilles tendons were transported in a thermal container, covered with gauze slightly moistened with a saline solution 9% and sent for analysis of conventional mechanical testing immediately after its removal.
Mechanical Testing
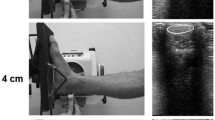
The tendon–bone complex was attached to metal connectors (2.5 × 3.5 cm) with the serrated surface to the outside. This was secured to a conventional mechanical testing machine (EMIC, DL 500 model, Brazil) through a self-locking system by leverage, Fig. 1. To reinforce the interface between the metal connectors and the muscle–tendon junction (proximally) and the tendon–bone complex (distally) we used cyanoacrylate. During this procedure, we were very careful to not let the gel spread over the tendon.
The tendons were tensioned to the point of failure of the specimen, at a speed of 0.1 mm/s, and the strength was constantly measured by a load cell of 500 N; the dislocation of the specimen was registered automatically by the software TESC (Test Script) for automatic testing, compatible to the microprocessor testing machines, an EMIC product. The parameters studied were the elastic modulus (MPa), maximum tension (MPa), maximum specific strain (mm), energy/tendon area that corresponds to the area below the stress–strain curve (N mm/mm2) and cross-sectional area (mm2). Data were recorded automatically by the software TESC—Test Script Automation testing, consistent with the testing machine microprocessor EMIC.
The cross-sectional area (CSA) of the unloaded tendon for the conventional mechanical testing was estimated according to the formula for determining the area of an ellipse: \( {\text{CSA}} = \left( {{\frac{D \cdot T}{4}}} \right)\pi , \) where D = width of the middle third of the tendon, T = thickness of the middle third of the tendon. This geometric shape is the closest to the observed transversal sections of the tendon. The measurements of thickness and width of the tendon were obtained using a caliper.
Statistical Analysis
To describe the sample characteristics we used descriptive measurements, such as measure of central tendency (mean) and dispersion (standard deviation). To compare the mean variables between the various treatments we used the Student t test for the independent sample analysis to compare the control group and the diabetic group and the Pearson Correlation Coefficient for the analysis between the metabolic variables and the mechanical property variables. Data were analyzed with the SPSS software (Statistical Package for Social Sciences). We accepted 1% as the significance level.
Results
On the day of biomechanical testing, the animal’s body weight was 416 ± 38.73 g in the control group and 272 ± 48.73 g in the diabetes group, the latter showing a reduction of 34% of the body weight (p < 0.01).
To minimize the influence of body weight in each group on the results of water intake (mL/24 h), food intake (g/24 h), and diuresis (mL/24 h), we present and discuss these results normalized to 100 g of the animal’s weight.
In the first metabolic evaluation, water intake (mL/100 g), food intake (g/100 g), diuresis (mL/100 g), as well as blood glucose and body weight, did not show statistical differences between the study groups. However, in the second and third analyses these parameters were significantly increased (p < 0.01) in diabetic group (Table 1).
Biomechanical tests showed statistical differences in biomechanical properties of the Achilles tendon between control and diabetic groups.
The typical stress–strain curve of the rat Achilles tendon is represented in Fig. 2, where it is shown as a classic non-linear shape and it is divided into two regions. The initial region is characterized by low stiffness of the tendon and with the collagen fibers stretching to approximately 4 to 15%. Then the second region starts. This is considered linear, because with the increment in traction, the tendon offers resistance and the slope stress–strain curve becomes almost constant. An additional increment in maximum strain results in tissue failure which is associated with a drastic tension decrease.3
The measurements of mechanical properties showed that the elastic modulus (MPa) was significantly higher in the control group (p < 0.01). However, when we analyzed maximum tension (MPa) the groups did not show statistical significance (p > 0.01). Specific strain (%), maximum tension strain (mm), and energy/tendon area (N mm/mm2) were higher in the tendon tests of diabetic group (p < 0.01). The cross-sectional area (mm2) of the tendon was greater in the control group when compared to the diabetic group (p < 0.01) (Table 2).
Achilles tendon mechanical properties were correlated with the results of the metabolic and clinical results, before the euthanasia of the animals. We observed that maximum tension strain has a strong correlation with the water intake of the CG (r = 0.975; p = 0.005) and shows, as well, a substantial correlation with the diuresis of the diabetic group (r = 0.975; p = 0.037). The cross-sectional area showed a strong correlation with the diabetic animals’ weight (r = 0.90; p = 0.037) and with diuresis (r = 0.99; p = 0.001). Other mechanical properties did not show correlations with the metabolic and clinical variables.
Discussion
We chose the Achilles tendon due to its function and superficial location. Furthermore, some research with humans showed that DM can change the thickness and integrity of this tendon 2,6,14,31 and another study describes that morphological alterations in the structure of the Achilles tendon can predispose patients to develop “diabetic foot”.13
The method of analysis of mechanical properties in Achilles tendon is similar to the studies of Almeida-Silveira et al. and Reddy et al. 3,26 Moreover, it is known that mechanical properties of biological tissues can change according to age and the mammalian species evaluated, and therefore this study aims to compare the results of mechanical tests between the groups we studied rather than the values published in the literature.
Our study produced distinct results in the mechanical properties of the Achilles tendon in the groups we examined. When compared to the control group, DM was found to result in significant decreases of 63.3% in elastic modulus and of 45.8% in the cross-sectional area and increases of 75.1% in specific strain properties, of 74.8% in the maximum tension strain and of 180% in energy/area.
The elastic modulus is taken to be the slope of the best-fit straight line through the approximately linear region of the stress–strain curve,26 however, in biological materials it refers not only to elasticity, but also to visco-elasticity and failure (rupture) results.3,23,33 Nevertheless, the reduction of elastic modulus in DM Achilles tendon was observed, and this corroborates with the results of Reddy et al.,26 who described, when they studied the mechanical properties of the Achilles tendon with non-enzymatic glycation, a process that happens in DM, that tendons are stiffer and the elastic and visco-elastic properties have alterations.
We showed that the differences in cross-sectional area cannot be attributed only to the alterations in collagen components.18 The results of this study corroborate with these findings, since the cross-sectional area of tendons has a strong correlation with the weight and diuresis of diabetic animals.
According to Muller et al.,21 with the reduction of elastic and visco-elastic properties, the capacity of the tendon to resist strain when stressed will be diminished, which agrees with the results of specific strain and maximum tension strain of this study. This research showed that on diabetic group, the stress–strain curve has a lower slope (elastic modulus) when compared to the control group. This indicates more rigidity on the early traction and therefore less mechanical efficiency of the Achilles tendon to resist the stress (Fig. 3).
The energy/area tendon is the resource that the tissue has to absorb the energy in the cross-sectional area of the tendon, indicating that if the energy absorption is higher this could lead to a lower efficiency of the structure. The collagen in connective tissue has organized fibers in parallel matrices to facilitate transmission of the energy (forces) of the muscle to the bone. In the presence of DM and non-enzymatic glycolization, manifestations could occur in physical properties of the tendon leading to a disorganization of fibers and consequently greater energy absorption and lower resistance of the tissue; this abnormality has been observed in other experimental studies of animal skin and tendon.4,21,26
Grant et al. 14 demonstrated that the collagen fibers of the Achilles tendon in DM patients have a smaller diameter, are denser, stiffer and have morphological changes when compared to the collagen fibers in tendons of non-diabetic patients. Other authors correlate the alterations in visco-elastic capacity and, consequently, a lower capacity to dissipate energy, of the tendon with stress fractures and with skin injuries and feet collapse.14,26,34
Although we cannot explain the causal relationship between metabolic aspects with mechanical properties, this study shows important correlations between normal hydration of the animal with strain characteristics, also normalized. Furthermore, metabolic alterations due to DM, such as the great volume of diuresis, have strong correlations to the detectable increase in strain (r = 0.975; p = 0.037). Possibly, the hyperglycemic state can be a confounding variable.
In the diabetic patient, dysfunctional, mechanical alterations or a ruptured Achilles tendon will predictably lead to further morbidity. The inability of the foot to clear the ground during the stance phase of gait, increased plantar pressures and eventual attenuation of the tibialis anterior tendon, are all likely consequences of an absent, ruptured, or dysfunctional Achilles tendon. Restoration of the function of Achilles tendon may resolve these pathologies and restore normal function to the foot and reduce biomechanical complications, and this may be particularly important in diabetic patients.
To analyze the mechanical properties on Achilles tendons we used a conventional mechanical traction performed in a single strain, therefore, the visco-elastic components of the tendons could not be separately quantified. The vitro analysis does not reflect important aspects such as the tipping point of the tendon insertion into calcaneus bone and the lack of activity of the triceps surae muscle. Other researches should be realized, describing the causality of these mechanical alterations on Achilles tendons.
Conclusions
The mechanical properties in tendons of animals chemically induced to DM have significant alterations when compared to a control group. The mechanical properties alterations of tendons can reduce the threshold of energy transmission to the periphery and predispose the tendon to premature rupture due to stress. This study may be useful to better understand the complications of musculoskeletal system caused by DM.
References
Akturk, M., et al. Thickness of the supraspinatus and biceps tendons in diabetic patients. Diabetes Care 25(2):408, 2002.
Akturk, M., et al. Evaluation of Achilles tendon thickening in type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 115(2):92–96, 2007.
Almeida-Silveira, M. I., et al. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur. J. Appl. Physiol. 81(3):252–257, 2000.
Andreassen, T. T., H. Oxlund, and C. C. Danielsen. The influence of non-enzymatic glycosylation and formation of fluorescent reaction products on the mechanical properties of rat tail tendons. Connect. Tissue Res. 17(1):1–9, 1988.
Aydeniz, A., S. Gursoy, and E. Guney. Which musculoskeletal complications are most frequently seen in type 2 diabetes mellitus? J. Int. Med. Res. 36(3):505–511, 2008.
Batista, F., et al. Achilles tendinopathy in diabetes mellitus. Foot Ankle Int. 29(5):498–501, 2008.
Biewener, A. A., and T. J. Roberts. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc. Sport Sci. Rev. 28(3):99–107, 2000.
Biewener, A. A., et al. Muscle mechanical advantage of human walking and running: implications for energy cost. J. Appl. Physiol. 97(6):2266–2274, 2004.
Bobbert, M. F. Dependence of human squat jump performance on the series elastic compliance of the triceps surae: a simulation study. J. Exp. Biol. 204(Pt 3):533–542, 2001.
Bolton, N. R., et al. Computed tomography to visualize and quantify the plantar aponeurosis and flexor hallucis longus tendon in the diabetic foot. Clin. Biomech. (Bristol, Avon) 20(5):540–546, 2005.
Buchanan, C. I., and R. L. Marsh. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J. Appl. Physiol. 90(1):164–171, 2001.
DiDomenico, L. A., K. Williams, and A. F. Petrolla. Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J. Foot Ankle Surg. 47(5):463–467, 2008.
Giacomozzi, C., et al. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin. Biomech. (Bristol, Avon) 20(5):532–539, 2005.
Grant, W. P., et al. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J. Foot Ankle Surg. 36(4):272–278, 1997; (discussion 330).
Hof, A. L., J. P. Van Zandwijk, and M. F. Bobbert. Mechanics of human triceps surae muscle in walking, running and jumping. Acta Physiol. Scand. 174(1):17–30, 2002.
Legerlotz, K., et al. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J. Appl. Physiol. 102(2):564–572, 2007.
Lerco, M. M., et al. Caracterização de um modelo experimental de Diabetes Mellitus, induzido pela aloxana em ratos: estudo clínico e laboratorial. Acta Cir. Brasil. 18:132–142, 2003.
Loren, G. J., and R. L. Lieber. Tendon biomechanical properties enhance human wrist muscle specialization. J. Biomech. 28(7):791–799, 1995.
Magnusson, S. P., P. Hansen, and M. Kjaer. Tendon properties in relation to muscular activity and physical training. Scand. J. Med. Sci. Sports 13(4):211–223, 2003.
Monnier, V. M., R. R. Kohn, and A. Cerami. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA 81(2):583–587, 1984.
Müller, S. S., et al. Análise comparativa das propriedades mecânicas do ligamento da patela e do tendão calcâneo. Acta Ortop. Brasil. 12:134–140, 2004.
Natali, L. H., et al. Efeitos da corrida em esteira em músculos sóleos de ratos encurtados por imobilização. Rev. Bras. Med. Esporte. 14(6):490–493, 2008.
Oxlund, H., R. Manthorpe, and A. Viidik. The biochemical properties of connective tissue in rabbits as influenced by short-term glucocorticoid treatment. J. Biomech. 14(3):129–133, 1981.
Papanas, N., et al. Achilles tendon volume in type 2 diabetic patients with or without peripheral neuropathy: MRI study. Exp. Clin. Endocrinol. Diabetes 117(10):645–648, 2009.
Ramirez, L. C., and P. Raskin. Diabetic foot tendinopathy: abnormalities in the flexor plantar tendons in patients with diabetes mellitus. J. Diabetes Complicat. 12(6):337–339, 1998.
Reddy, G. K., L. Stehno-Bittel, and C. S. Enwemeka. Glycation-induced matrix stability in the rabbit Achilles tendon. Arch. Biochem. Biophys. 399(2):174–180, 2002.
Roberts, T. J., and R. L. Marsh. Probing the limits to muscle-powered accelerations: lessons from jumping bullfrogs. J. Exp. Biol. 206(Pt 15):2567–2580, 2003.
Seynnes, O., et al. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J. Appl. Physiol. 107(2):523, 2009.
Simonsen, E. B., H. Klitgaard, and F. Bojsen-Moller. The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. J. Sports Sci. 13(4):291–295, 1995.
Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 50(6):537–546, 2001.
Unlu, Z., et al. Ultrasonographic evaluation of pes anserinus tendino-bursitis in patients with type 2 diabetes mellitus. J. Rheumatol. 30(2):352–354, 2003.
Viidik, A. The effect of training on the tensile strength of isolated rabbit tendons. Scand. J. Plast. Reconstr. Surg. 1(2):141–147, 1967.
Viidik, A., C. C. Danielson, and H. Oxlund. On fundamental and phenomenological models, structure and mechanical properties of collagen, elastin and glycosaminoglycan complexes. Biorheology 19(3):437–451, 1982.
Vlassara, H., R. Bucala, and L. Striker. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 70(2):138–151, 1994.
Acknowledgments
All authors provided concept, idea, research design, writing, and data analysis. We acknowledge the financial support provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 477096/2008-5) and fellowship from the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) to Mr. Oliveira.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. Torry oversaw the review of this article.
Rights and permissions
About this article
Cite this article
de Oliveira, R.R., de Lira, K.D.S., de Castro Silveira, P.V. et al. Mechanical Properties of Achilles Tendon in Rats Induced to Experimental Diabetes. Ann Biomed Eng 39, 1528–1534 (2011). https://doi.org/10.1007/s10439-011-0247-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0247-z