Abstract
The objective of our session at the 2008 International Bio-Fluid Symposium and Workshop was to review the state-of-the-art in image-based modeling of blood flow, and identify future directions. Here we summarize progress in the field of image-based modeling of blood flow and vessel wall dynamics from mid-2005 to early 2009. We first describe the tremendous progress made in the application of image-based modeling techniques to elucidate the role of hemodynamics in vascular pathophysiology, plan treatments for congenital and acquired diseases in individual patients, and design and evaluate endovascular devices. We then review the advances that have been made in improving the methodology for modeling blood flow and vessel wall dynamics in image-based models, and consider issues related to extracting hemodynamic parameters and verification and validation. Finally, the strengths and weaknesses of current work in image-based modeling and the opportunities and threats to the field are described. We believe that with a doubling of our efforts toward the clinical application of image-based modeling tools, the next few years could surpass the tremendous gains made in the last few.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As noted in our prior review of the first decade of research in image-based modeling of blood flow,136 while much progress had been made, significant challenges needed to be addressed in the second decade of this nascent field. These included the need for algorithmic improvements related to geometric modeling, boundary conditions, fluid–structure interactions between the blood stream and vessel wall, multiscale modeling, and simulation of vascular adaptation and disease to name a few. In that previous review of the field, we noted that the relative ease of simulating blood flow in image-based models was simultaneously a blessing and a curse. Used appropriately, these methods could provide investigators with powerful new tools, rivaling and even surpassing experimental fluid mechanics methods to investigate mechanisms of disease, and design and evaluation of medical devices and therapeutic interventions. However, we expressed concern that these tools had the potential to fuel a hemodynamic data explosion without concomitant gains in understanding. Finally, we noted the dearth of experimental data for prescribing physiologic input parameters and validating models and methods. In short, the field of image-based modeling had great potential but little kinetic energy.
In the four years since this previous review was published, remarkable progress has been made in many areas of image-based models, with dramatic algorithmic improvements and robust applications to many clinical problems of congenital and acquired cardiovascular diseases. Other areas remained “business as usual”. In this paper, we first review progress made since 2005 in developing and applying methods of image-based modeling of blood flow, and next present areas where little progress has been made. We then summarize and expand upon the strengths and weaknesses arising from this survey, and outline what we perceive as some of the key opportunities and threats for image-based modeling over the next five years and beyond.
Applications of Image-Based Modeling
Hemodynamic Factors in Vascular Pathophysiology
The earliest image-based computational fluid dynamics (CFD) analyses were motivated by the goal to elucidate the role of hemodynamic forces in the development of atherosclerosis at the carotid104 and coronary79 arteries, quantify hemodynamics in abdominal aortic aneurysms,147 , 148 and plan patient-specific surgical protocols.145,146 These vascular territories were chosen for their obvious clinical relevance, but also for the potential for imaging both the lumen and the artery wall: the superficial carotid bifurcation is an ideal target for non-invasive ultrasound (US) and magnetic resonance imaging (MRI); the large abdominal aorta can be particularly well-resolved with MRI or computed tomographic (CT) imaging; and routine catheterization of the coronary arteries under X-ray guidance makes them amenable to invasive intravascular ultrasound (IVUS).
Since our last review, much progress has been made in elucidating the role of hemodynamic forces in coronary artery plaque development, notably the distinct hemodynamic mechanisms associated with the development vs. rupture of vulnerable plaques.129 For example, a recent serial MRI-based case study demonstrated an association between the eventual site of plaque ulceration and elevated wall shear stress (WSS),52 a finding corroborated by an IVUS-based CFD study of 31 plaques that showed a clear association between elevated WSS and elevated strain within the plaque.46 On the other hand, a serial MRI-based study of 21 carotid artery plaques found that plaques tend to progress in regions exposed to low WSS and low wall stresses.144
While the carotid bifurcation has remained a popular target for image-based studies, intended subject-specific comparisons of early wall thickening and hemodynamic factors have been limited by ongoing challenges of resolving the vessel wall non-invasively. In a 3D ultrasound-based study of 14 healthy adults, Augst et al. showed no obvious relationship between intima-media thickness (IMT) and WSS-related parameters.6 However, blood pressure was found to be strongly correlated with carotid bulb IMT, suggesting that a larger sample might be required to discriminate systemic from local factors in such a cross-sectional study. Otherwise, for the most part, studies of the carotid bifurcation have focused on the sensitivity of image-based modeling to acquisition and operator uncertainties47 – 50 , 150 and modeling assumptions.85 , 88 , 107 , 169 What has emerged from these studies is that, despite relatively large quantitative uncertainties or errors, qualitative patterns of disturbed flow are remarkably robust. This point was underlined by Lee et al.,85 who demonstrated that inter-individual variations in patterns of disturbed flow were well above the “noise” threshold of intra-individual uncertainties. This study was also notable for its size (50 cases), and its suggestion that surrogate geometric markers, which are much easier to acquire in the clinic compared to image-based CFD simulations, may prove sufficient for inferring the burden of disturbed flow in large-scale (N > 1000) studies of local risk factors in atherosclerosis currently underway by that group. Similarly, Wood et al. used MRI-based CFD studies of 18 young adults to highlight the potential role of vessel tortuosity and curvature in peripheral artery disease.165
Promising trends emerging from the above-cited recent studies are shifts from individual case studies to sample sizes on the order of tens, and from cross-sectional to serial studies. Nowhere is this more evident than the veritable explosion of studies of hemodynamic factors in cerebral aneurysm development and rupture, fueled by the deployment of 3D angiographic imaging in the clinic.124 Within a year of the first published case studies,55 , 71 , 135 Shojima et al. 128 had demonstrated, using 20 cases, the potential importance of low WSS in aneurysm progression and rupture. One year later, Cebral et al. 19 published a landmark study of 62 cases demonstrating an association between the size and stability of inflow to the aneurysm and its risk of rupture. Notable in both of these studies was an attempt to relate the complex hemodynamic data to simpler geometric factors (in the case of the former, aneurysm aspect ratio), or broad hemodynamic “phenotypes” (in the case of the latter). Owing to the risks associated with aneurysm interventions, unruptured aneurysms are often monitored clinically, providing an ideal “laboratory” for serial image-based studies. For example, Saloner and colleagues demonstrated the role of low WSS and stagnant flow in subsequent aneurysm progression or thrombosis, respectively.14 , 72 , 117
In the last few years there has also been progress in quantifying hemodynamic conditions in the human abdominal aorta for investigations into localization of atherosclerosis and the progression of abdominal aortic aneurysms (AAA). Tang et al. quantified hemodynamic conditions in subject-specific models of the human abdominal aorta constructed from magnetic resonance angiograms (MRA) of five young, healthy subjects.143 Phase contrast magnetic resonance imaging (PC-MRI) techniques were used to acquire velocity data at the supraceliac and infrarenal levels under resting conditions. Simulations were performed under resting and mild exercise pulsatile flow conditions, and provided data to support the hypothesis that exercise provides localized benefits to the cardiovascular system through acute mechanical stimuli that trigger longer-term biological processes leading to protection against the development or progression of atherosclerosis. Image-based modeling techniques may indeed be as valuable in studies related to exercise-induced inhibition and regression of atherosclerosis as they are in initiation and progression. Image-based modeling techniques are also being used to provide hemodynamic data in a clinical study testing the hypothesis that exercise can be employed to slow the progression of small (3–5 cm diameter) abdominal aortic aneurysms.25 , 89 Finally, image-based modeling techniques have been used to compare hemodynamic conditions in the abdominal aorta of patients with spinal cord injury to normal control subjects to determine whether differing hemodynamic conditions might explain why the former have a three-fold greater risk of AAA.25 , 167
Progress in such observational clinical studies of hemodynamic factors has been complemented by progress in the use of image-based models to forge a more direct link between the biomechanics and biological mechanisms. Friedman and colleagues pioneered anatomically-realistic CFD models of porcine iliac arteries to guide the study of the cellular and genetic responses underlying shear-induced endothelial cell dysfunction.42 , 57 , 58 , 83 A recent serial IVUS-based CFD study of cholesterol-fed swine demonstrated a clear association between the type of plaque and the baseline WSS,22 consistent with the findings of Cheng et al. 24 from their CFD-inspired perivascular cuff studies of mouse carotid arteries. For obvious reasons, much recent attention has focused on mouse models of atherosclerosis. Despite appreciable differences in scaling of WSS from mice to men,23 , 51 , 157 Suo et al. 141 observed an association between relatively low WSS levels and the expression of various adhesion molecules on the inner wall of the mouse aortic arch. On the other hand, Feintuch et al. 31 noted reductions in the extremes of WSS at the aortic arch of mice vs. humans that appears to be at odds with the observed patterns of disease in these two species. Finally, recent rabbit and canine studies have highlighted the role of hemodynamic forces in, respectively, the expression of biomarkers associated with vascular remodeling in aneurysms73 and ultrastructural changes at the apices of arterial bifurcations that may precede aneurysm formation.99
Characterization of Hemodynamics for Treatment Planning and Device Design
Since our last review, significant progress has been made in the application of image-based modeling techniques to patient-specific planning of interventions for cardiovascular disease and device design and evaluation. Wilson et al.161 described the development of a new software system for image-based modeling and cardiovascular surgery planning, and demonstrated its application to plan aortofemoral bypass grafting in a patient with lower extremity occlusive disease. Hassan et al. 54 used image-based modeling techniques to simulate and compare the consequences of left and right vertebral artery occlusion in a patient with a giant vertebrobasilar aneurysm. Acevedo-Bolton et al. 1 created an image-based model from MRA data of a patient with an inoperable basilar artery aneurysm. PC-MRI was used to obtain input flow conditions and computational fluid dynamics techniques were used to compute velocity and WSS fields in cases where either the left or right vertebral artery was occluded. These authors noted that changing vertebral flow would alter regions of low WSS in the aneurysm and potentially reduce areas of flow stagnation that might promote further growth. More recently, Rayz et al. 116 performed a study on the effect of vertebral artery flow in four patients with basilar artery aneurysms. Computed flow fields were found to agree well with measurements made using PC-MRI. Subsequently, these authors reported good correspondence between regions of slow flow predicted by CFD at baseline and the deposition of thrombus observed at follow-up of three basilar aneurysm cases.117
In the last several years, a few groups have started to apply image-based modeling techniques to the assessment of surgical procedures for patients with congenital heart diseases. Perhaps the most studied procedure is the total cavopulmonary connection or extra-cardiac Fontan surgery, with a rich history of the application of computational15 , 28 , 101 – 103 and experimental methods26 , 126 , 168 to examine hemodynamic conditions in alternate surgical options. Whereas much of the prior computational analysis was performed with idealized models, there is a trend toward the use of image-based models and patient-specific planning. Yoganathan and colleagues developed image-based modeling tools specifically for the Fontan procedure.27 , 110 , 160 Marsden et al. used image-based modeling techniques to investigate the effects of exercise in Fontan patients97 and design a new surgical variant of the total cavopulmonary connection.95
Prior to 2005, simulations of blood flow in cardiovascular devices were principally in idealized device and arterial models. Nevertheless, considerable insight was gained studying flow through stents in occlusive81 and aneurysmal140 disease and computing fluid forces in stent grafts.78 , 91 In the last four years, image-based modeling techniques have started to be used to evaluate endovascular devices in more realistic models of the circulation. Two areas in particular have received the most attention: stenting and coiling cerebral aneurysm, and stent-grafting of abdominal and thoracic aortic aneurysms.
With regard to stenting and coiling of cerebrovascular aneurysms, a number of groups have made remarkable progress in developing image-based modeling techniques. Notable work is that of Cebral and colleagues5 with adaptive embedded unstructured grids and Ventikos and colleagues74 with a porous media approach. As a testament to progress in applying image-based modeling techniques in modeling blood flow through cerebrovascular stents, a “Virtual Intracranial Stenting Challenge” was held in 2007.114 Six research groups solved a common problem and compared their results including velocity and WSS fields.
Image-based modeling techniques hold great promise for the design and evaluation of aortic stent grafts.170 Howell et al. 63 used image-based modeling techniques to compute pressure and shear forces in models created from computed tomography data in 4 patients who had stent grafts to treat their abdominal aortic aneurysms. More recently, Figueroa et al. used image-based modeling techniques to demonstrate that, because all abdominal and thoracic aortic aneurysms are asymmetric and non-planar and many are tortuous, the dominant forces causing stent graft migration act in the lateral not longitudinal directions.34 , 35 These results provide an explanation for the observed lateral movement of stent grafts into the aneurysm sac in some patients.115
Methods of Image-Based Modeling
Image-Based Geometric Modeling
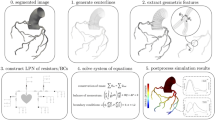
The first and arguably the most important step in the image-based modeling process is extraction of the lumen boundary from images of the vascular anatomy. Much research over the past decade has focused on this process, with early studies relying on manual or computer-assisted segmentation of individual image slices, coupled to any number of 3D reconstruction techniques.134 , 156 Since our last review, improvements in the spatial resolution and quality of medical images, coupled with faster central and graphics processing units, have made it much easier to perform 3D vessel extractions interactively. A favored approach is the level set method, which balances image- and shape-based forces to define the lumen boundary as an implicit function, which can subsequently be discretized.2 , 9
For volumetric images having well-defined lumen-wall boundaries, such as those obtained from CT or contrast-enhanced MRA, 3D segmentations are preferred for their convenience, speed and robustness.10 However, the resulting 3D surfaces are difficult to edit, so 2D segmentation is the preferred option for segmentation of suboptimal images or low contrast structures like vessel walls, thrombus or plaque components.156 This is especially true for IVUS images, which are acquired on a series of non-parallel planes following the vessel axis.130 For non-branching segments, it is straightforward to loft the series of lumen and/or wall “rings” together to form a surface or even a volumetric mesh. Branches present challenges, although not insurmountable ones.45
Perhaps the most significant development in image-based geometric modeling since our last review is the availability of open-source toolkits, both general to image visualization and processing (vtk67 and itk64) and specific to vascular modeling (vmtk2 , 66 and simtk65 , 123). These are making it much easier to devote research efforts to applications rather than tool development, and they facilitate the standardization of vascular model construction and analysis in a way that may promote the sharing of data.
In contrast to surface definition, generation of high quality volumetric meshes from complex surface models remains largely the purview of commercial mesh generators, although there are open-source efforts aimed at democratizing this process. For the most part, volumetric meshes for vascular models still tend to be uniformly resolved or only grossly adapted to the physical length scales of the geometry, with the result that most meshes are over- or under-resolved relative to the requirements of the solution. In light of the need (or at least the desire) to resolve local wall vs. bulk flow quantities, adaptive mesh generation techniques are being pursued. Prakash and Ethier described an adaptive meshing strategy based on uniform refinement to obtain acceptable predictions for the WSS.112 Recently, Antiga and colleagues developed an open-source adaptive solution strategy for image-based modeling of blood flow.12
Simulation of blood flow through medical devices with multi-scale geometric features (e.g. stents or coils) can pose considerable challenges for mesh generators, even ones with adaptive meshing capabilities.140 Cebral and colleagues developed a hybrid approach where the interior of the vessel is discretized with conforming meshes and an adaptive mesh embedding technique is used for the endovascular devices.20 , 92
Since arterial models often have long tubular sections, and blood flow velocity gradients are typically much greater in the radial than the longitudinal directions, adaptive meshing strategies that can generate anisotropic and boundary layer elements are often desirable. Shephard and colleagues developed novel methods for generating finite element meshes of cardiovascular models including anisotropic adaptivity108 , 122 and boundary layer meshing.120 , 121 For these methods, error estimators linked to the Hessian of the flow speed are used to obtain anisotropic size information (in terms of a metric tensor size field). A solution obtained on a preliminary mesh provides an indication of the best alignment for the element and the size of the element in all three directions relative to that alignment. Anisotropic adaptivity and boundary layer meshing techniques can yield mesh-independent solutions for image-based models of blood flow with considerably fewer elements compared to isotropic adaptive mesh refinement methods.
Boundary Conditions
Invariably for image-based models, the domain of interest is truncated and thus taken out of the context of the entire circulatory system. For rigid wall studies, this necessitates the prescription of inflow and outflow boundary conditions. Usually the velocity profile is explicitly prescribed at the inlet, often with a presumed shape (e.g., plug-shaped for the ascending aorta, fully-developed for the carotid bifurcation), but sometimes based on the measured time-varying velocity profiles. There is ongoing debate about whether the fully-developed inflow assumption is reasonable in light of the fact the upstream segments that have been discarded, and which are only nominally straight, may harbor decidedly undeveloped velocity profiles.40 , 155 Nevertheless, it would appear that “memory” of the inlet velocity profile may be effectively lost by the time it confronts the overwhelming influence of the complex downstream domains-of-interest, provided they are truncated sufficiently far upstream; however, this criterion remains vague and problem-specific.16 , 107
At their simplest, outflow conditions can be based on the dimensions of the outflow tracts under the assumptions of mass conservation and any number of proposed scaling laws.75 (The same scaling laws may also reasonably be used to infer individual inflow conditions via scaling of a canonical flow waveform shape.85) Imposition of individual inflow and outflow rates based on PC-MRI or US measurements may be preferred; however, for rigid-wall studies, possible phase shifts and attenuation of outflow vs. inflow rates, being typically measured some distance apart, must be corrected.137 While in principle flow waveforms could be applied, uncorrected, in distensible models, in practice the distensible model will invariably have different properties from the true in vivo values, and imposition of flow waveforms becomes problematic. Rather, data on flow waveform attenuations or phase shifts may be most useful for inferring the bulk compliance of the vessels and assigning properties for deformable vessel walls. US and MRI may also be used to provide direct measurements of the time-varying lumen diameter or area, which can be scaled to infer the time-varying pressure waveform.153
In 2005, the vast majority of 3D simulations of blood flow used prescribed inlet velocity profiles and a combination of prescribed outlet velocity profiles and zero normal traction. This approach was adequate for many of the simulations being performed at that time, since they utilized measured or prescribed flow distributions, ignored vessel compliance, and had the goal of computing velocity fields and WSS. Yet, for applications where flow and pressure waveforms were not known a priori, e.g. predicting outcomes of interventions, the limitations of the available boundary conditions were clear. With the advent of methods for including vessel compliance, and an increased emphasis on generating more extensive vascular models and predictive applications, the need for more realistic boundary conditions became acute.
In the last four years, considerable progress has been made in developing multiscale and multidomain models whereby flow rate and pressure on an outflow (or inflow) of the image-based, 3D numerical domain is coupled to the inflow (our outflow) of a reduced-order model, i.e. a one-dimensional network or zero-dimensional (lumped) model.11 , 82 , 100 , 154 Both explicit (staggered) and implicit (fully-coupled) approaches to link the image-based 3D models and reduced-order one-dimensional or zero-dimensional models have been utilized. The latter implicit-coupling approach has the advantages of providing a stable and efficient formulation, but requires modifications to the governing equations at the variational level.154 Methods relying on explicit, staggered data exchange between 3D image-based models and reduced-order models are generally less robust as compared to implicit fully-coupled methods, but can be used to couple disparate codes together and do not require reformulation of the numerical method for the 3D computational algorithms.82 , 100 In either approach, the addition of a reduced-order model for the downstream or upstream domains necessitates parameter specification for these models. Branching topology, length, diameter and material properties of vessel segments need to be assigned for one-dimensional network models and are typically generated using fractal or morphometry-based methods.109 , 131 , 133 For lumped-parameter models, appropriate values of resistance, capacitance, and inductance parameters and elastance functions (for lumped heart models) are assigned to achieve the desired hemodynamic characteristics of the upstream or downstream domain. Numerical optimization methods can be employed to tune the parameter values of the reduced-order models to match measured flow distribution or pressure values in the patient-specific model.131 , 132
Fluid–Structure Interactions
Prior to 2005, with the notable exception of the work of Perktold and colleagues,59 , 90 , 111 , 113 nearly all blood flow simulations were conducted in rigid-walled models. Of the few fluid–structure interaction simulations being performed, the lack of realistic boundary conditions required changes in vessel properties to permit deformation about a constant or zero pressure state, and precluded computation of realistic pressure and flow wave propagation. In the last four years, there have been a number of significant advances in simulation of the fluid–structure interactions between the flowing blood and vessel wall. Traditional fluid–structure interaction techniques based on the arbitrary Lagrangian–Eulerian (ALE) method were improved to meet the unique challenges inherent in modeling blood flow in arteries, including the “added mass” effect that causes instabilities for blood flow fluid–structure interaction problems when loosely coupled time-stepping algorithms are employed.17 , 41 In addition, new, non-ALE methods were introduced in this period to simulate blood flow and vessel wall dynamics in patient-specific models.
A number of groups have successfully solved patient-specific problems using ALE methods including large fluid motion and nonlinear continuum or shell representations of the vessel wall. Gerbeau and colleagues developed a robust partitioned, strongly-coupled ALE method to address the added mass effect and applied it to solve for blood flow and vessel wall dynamics in patient-specific models.32 , 44 Wolters et al. described a patient-specific simulation of blood flow in an AAA model using an ALE-based approach.162 Torii and colleagues utilized a stabilized space-time fluid structure interaction finite element method to study blood flow in image-based models of cerebral aneurysms.149 , 151 Hughes and colleagues used new isogeometric non-uniform rational B-spline (NURBS)-based ALE methods to solve patient-specific fluid–structure interaction blood flow problems.8
Figueroa et al. described an alternative to ALE, the Coupled Momentum Method, for solving highly complex patient-specific, 3D blood flow and vessel wall dynamics problems under the simplifying assumption of small deformations and thin-walled vessels.36 This method, inspired by J.R. Womersley’s analytical solution for pulsatile flow in a semi-infinite circular elastic tube,164 embeds the elastodynamics problem for the deformation of the vessel wall within the variational equation of the fluid dynamics problem. Specifically, the lateral wall of the fluid domain is assigned an unknown traction boundary condition, which is then related to a fictitious body force term in the elastodynamics variational equation. Using a fixed fluid mesh, enforcing the condition that degrees-of-freedom of the fluid and solid domains are identical at the interface, and employing a linear membrane model for the vessel wall results in a robust scheme with minimal computational cost beyond that for a rigid wall.
Extracting Hemodynamic Parameters
A central challenge of image-based modeling is making sense of the enormous amount of data that can—and could—be extracted. Most studies have focused on surface-based quantities, which are thought to be most closely linked to the underlying pathophysiologies. Cycle-averaged WSS magnitude and oscillatory shear index remain the most commonly reported hemodynamic wall parameters. Various WSS-gradient-related parameters have been proposed over the years,77 most recently the gradient oscillatory number ostensibly linked to aneurysm initiation.127 Attention has also recently focused on the harmonic content of the time-varying WSS57 and its relationship to the responsiveness of endothelial cells.56 Nevertheless, sensitivity of the more sophisticated parameters to model uncertainties50 , 150 and, especially in light of this, potential redundancies among them,86 should not be overlooked.
Traditionally, hemodynamic wall parameters have been presented as spatial maps or trends at a particular spatial location. As image-based modeling has moved from individual to multiple cases, several groups have encouraged a more statistical treatment of these quantities. One approach, followed by Friedman’s group in their studies of porcine iliac arteries, is to map each vessel onto a standardized template,58 reminiscent of the cutting and pinning of vascular specimens, which facilitates the averaging of hemodynamic data across cases. Similarly, the tubular nature of coronary artery segments affords simple mapping and “geometry-guided” averaging of data between or within cases.159 Such template-based mapping may be extended to bifurcations and other complex vascular configurations via the decomposition of these vessels into their component branches.4
Alternatively, and also as a consequence of template-based mapping, one may consider the histogram of a particular hemodynamic quantity as a means of converting continuous spatial distributions into categorical ones. This approach was used by Stone et al. 138 to demonstrate an association between regions exposed to low shear at baseline and wall remodeling at six-month follow-up, and by Friedman et al. 42 to anticipate regions of porcine iliac arteries consistently exposed to low, medium or high shear. More recently, Lee et al. 85 proposed the quantification of an individual vessel’s “burden” of disturbed flow as its surface area exposed to a given hemodynamic parameter beyond a threshold value objectively defined from the statistics of the group. More generally, surface-area-based histograms may prove useful for classifying vessels according to their overall hemodynamic characteristics.158
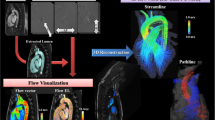
Aside from surface-related quantities, velocity fields or other volumetric parameters also have much to tell. Velocity isocontours are ideal for visualizing or quantifying high-speed jets or regions of slowly recirculating flow in aneurysms,18 , 117 and retrograde flow at bifurcations.155 Flow helicity may also prove useful,106 especially in light of the spiral course of early atherosclerotic lesions.139 Simulated dye visualizations are attractive for visualizing the complexities of pulsatile flows76 but also for facilitating indirect comparisons against in vivo imaging.21 , 39
Verification and Validation
In our 2005 review paper we noted the importance of verification of computational methods and validation of mathematical models—solving the equations right vs. solving the right equations7 , 118—and issued a recommendation specifically calling for more work in this area. Verification has received very little attention thus far and it is unclear how many numerical solutions are actually compared against analytical solutions such as Womersley’s solution for pulsatile flow in rigid vessels.163 There are no published reports comparing fluid–structure interaction solutions against analytical solutions, such as Womersley’s solution for pulsatile flow in elastic vessels.164
Since 2005 there have been new experimental validation studies, but most of them have been using in vitro models and methods. Ku et al. reported an excellent comparison between predicted velocity patterns and experimental data obtained using cine PC-MRI in an in vitro model of a restriction in a main vessel and a bypass around it.80 Hoi et al. developed a validation study of CFD methods with particle-image velocimetry data in an in vitro model of a cerebral aneurysm.60 Ford et al. compared CFD velocity profiles with particle-image velocimetry data in physical models fabricated to match image data sets of a patient with a giant internal carotid artery aneurysm and a basilar tip aneurysm.38 They demonstrated a good agreement between computed and measured velocities including the amplitude and spatial distribution of cycle-to-cycle variations observed despite the imposition of periodic boundary conditions. Good agreement was also reported for both time-averaged and fluctuating velocity components measured by laser Doppler anemometry vs. direct numerical simulations of transitional flow in a realistic arteriovenous graft geometry.87
Validation of numerical methods is considerably more challenging in vivo, where experiments are difficult to control and measurement data are challenging to acquire. Recently, Boussel et al. performed a comparison of CFD results and in vivo MRI measurements of flow in cerebral aneurysms.13 Although initial results are encouraging, in vivo experimental validation studies are preliminary. In addition, it is likely that prediction of flow distribution in multi-branched models, in vivo flow-velocity profiles, and pressure fields will present considerable challenges. Finally, as simulations of unstable or turbulent flows begin to encounter flow features having length scales within an order of magnitude of red blood cells, it will be critical to validate, in vivo, the predictions of models that invariably treat blood as a homogeneous fluid.3
Future Directions
Strengths and Weaknesses
As evidenced by our survey of recent progress, image-based modeling has matured to the point where many groups are now using these techniques, and findings are now routinely published in the clinical and biological research literature. The availability of commercial software and open-source toolkits allows even novices to solve problems that, four years ago, would have been the domain of only a few specialized groups. Advances in computational methods and computing hardware are also making it possible to solve increasingly more challenging problems, notably those involving flow instabilities and/or turbulence associated with a variety of vascular pathologies.38 , 84 , 89 Similarly, it is now possible to carry out realistic in silico testing of devices or vascular interventions in a way that was not possible four years ago.
A downside to the relative ease with which image-based modeling can now be performed is the real potential for uncritical application of these sophisticated technologies. For example, Reynolds-averaged (two-equation) turbulence models, commonly available in most commercial CFD solvers, continue to be applied despite strong evidence that they are unsuitable for pulsatile, relaminarizing flows of interest in cardiovascular research.119 Many papers can also be found that purport to carry out mesh refinement analyses, when in fact the range of spatial resolutions considered is too narrow to draw reliable conclusions. In this regard it is important that clinical and biology research journals solicit expert technical reviews in much the same way that many now insist on expert review of the statistics.
These are reflections of a broader weakness of image-based modeling: the often-pristine appearance of these presented results belies the uncertainties or inaccuracies inherent in the model construction and physical approximations. As noted earlier, there have been some attempts to quantify these implicit error bars, and anyway it seems likely that the gross features predicted by these models are faithful representations of the in vivo hemodynamics, at least in the context of normal physiological variability. Still, as is now being done in the medical imaging community,93 some attempt to visualize and confront these uncertainties is warranted.
While progress has been made in developing more realistic boundary conditions for image-based modeling that enable quantification of pressure fields and prediction, not prescription, of flow distribution, these techniques are not universally employed in cases where they should be considered mandatory, namely modeling fluid–structure interactions and surgical planning. There is a disturbing trend of outflow pressure waveforms being prescribed for fluid–structure interaction simulations despite the fact that, even if such pressures could be measured, these waveforms would be incompatible with the physics assumed in the computational domain. Softer, more natural boundary conditions relating pressure and flow waveforms enable the researcher to employ a boundary condition that models the effect of the upstream or downstream domain, and is minimally influenced by flow and pressure in the numerical domain.
Arguably, we as a community have become too focused on the role of WSS in vascular pathophysiology. In many cases, we neglect the deformation of the vessel wall and argue that wall motion does not affect the velocity and shear stress fields and hence can be ignored. In some vascular beds, it may indeed be true that wall motion may not significantly affect velocity fields, but there are other reasons for considering it. Vascular diseases are diseases of the vessel wall, and internal tissue-level stresses affect vascular biologic phenomena in many cases as much or more than WSS. A prime example is in the initiation, growth and progression of intracranial and aortic aneurysms.69 Within the wall, any number of stress- or strain-based indices can be derived, while interactions between structural and fluid mechanical stresses may also warrant consideration.152 Realistic computation of pressure fields is a prerequisite for any consideration of wall stress. In some cases, this pressure field may be used for decoupled structural analysis. In other cases, fluid–structure coupling will be mandatory. Furthermore, we need to look away from the wall, back into the bloodstream itself, to understand vascular pathophysiology. For example, techniques to track particles142 and describe coherent features of flow separation and stagnation125 should be exploited and extended.
Finally, our simulations are, for all intents and purposes, based on Mechanical, not Biomechanical models. Namely, the only biology they incorporate is that of the overall system physiology. Once again, the vascular system is governed in health and disease by a complex interplay between biological, chemical and physical factors. There is a need to enhance the biological relevance of simulations and couple numerical analyses with mass transport,30 growth and remodeling68 and systems biology70 methods to test mechanistic hypotheses related to vascular adaptation, atherosclerosis initiation, progression and plaque rupture, and aneurysm initiation, progression and rupture.
Opportunities and Threats
Despite learning much about the complexity of blood flow in realistic vascular geometries, image-based models, and knowledge derived from them, have resisted translation to the clinic. The reasons for this are twofold. First, the process of creating a computational model from medical images remains arduous, at least in comparison to diagnostic imaging acquisitions. Second, the dense CFD datasets are difficult to distill into simple, clinically-relevant criteria. This sets up a vicious cycle, whereby the unproven utility of hemodynamic information makes it difficult to rationalize the large trials required to prove their utility.
A promising trend, and one that opens up significant opportunities for image-based modeling to prove itself, is that of identifying broad hemodynamic “phenotypes” into which cases can be grouped.19 Taking this one step further, and in recognition of the fact that local hemodynamics are driven largely by the shape of the vessel, the groups of Steinman and Antiga in particular have focused on the identification of geometric factors that might serve as robust surrogate markers of the “burden” of disturbed flow.37 , 85 Such attempts to abstract image-based models are consistent with the recognition that, as noted earlier, they can be relied upon in a qualitative, but not necessarily quantitative sense. It is also consistent with a clinical mindset that must balance the desire for sensitivity and specificity with practicalities of obtaining it.
By way of example, consider that, from an engineering standpoint, geometric factors like stenosis severity and aneurysm size are crude surrogate markers of the imbalance between biomechanical stress and strength, which is what ultimately determines the rupture of a plaque or aneurysm. Nonetheless, they are widely used in the clinic, not owing to ignorance of what precipitates most heart attacks and strokes, but because they have an intuitive connection to the disease; they are relatively straightforward to measure; and their use is supported by large, evidence-based trials. The wealth of anatomic imaging data coming from cross-sectional or prospective imaging studies offers a powerful opportunity for image-based modeling to identify hemodynamic or geometric phenotypes, and test them for associations with outcome, with samples sizes that would be difficult to justify otherwise.
Having said this, we are in no way diminishing the potential importance of detailed hemodynamic studies, or the clinical or biological impact of hemodynamic factors that cannot be distilled into simple categorical quantities. In this context, however, medical imaging poses a significant “threat”. Within the past few years, 3D cine phase contrast (also called 4D) MRI velocimetry94 has seen rapid improvements in scan time and image quality, to the point where it is now being applied to visualize and quantify flow patterns in large arteries that were previously the sole domain of image-based modeling.53 , 61 , 62 , 98 , 166 It is now feasible to derive wall shear stresses and flow instabilities in vivo,29 , 43 although not without appreciable inaccuracies or uncertainties.13 The speed and/or quality of these acquisitions are poised to improve dramatically within the next few years, given exciting developments in the area of highly undersampled projection techniques.105 In short, and in light of its own sources of error and uncertainty noted above, image-based modeling may soon lose the “competitive advantage” for visualization and quantification of local hemodynamics. On the other hand, direct imaging of in vivo hemodynamics offers a path of least resistance for translating the knowledge and lessons learned from image-based models in a way that might otherwise be stalled by the significant technical and regulatory hurdles to bringing complex engineering models in a clinical setting.
Patient-specific planning of interventions for cardiovascular disease and the design and evaluation of medical devices are applications where image-based modeling has significant advantages over medical imaging methods. Indeed, no matter how advanced medical imaging methods become, they will never be able to predict the future of an intervention or the fate of an implanted medical device. Opportunities related to the development and application of image-based modeling methods for patient-specific treatment planning are numerous. Many are clear now, but others will invariably emerge in the future.
With regard to the development of new methodologies, there is a need for further research into the development of organ-specific outflow boundary conditions, formal and informal optimization methods for tuning boundary conditions to match directly measured (e.g. blood pressure and velocity measurements), indirectly measured (e.g. tissue and organ perfusion data) or prescribed (e.g. scaling laws or literature) data. There is a need to model acute changes in physiologic parameters that might arise immediately after an intervention as well as chronic changes that might occur. Feedback control systems could be used for the former and fluid–solid-growth33 methods for the latter. Image-based methods could be extended to incorporate more realistic tissue properties and external tissue support extracted from IVUS, 4DMRI or cardiac-gated CT imaging using inverse methods. Finally, image-based modeling techniques can and should be coupled to formal optimization methods for surgical planning applications.96
New applications of patient-specific planning of cardiovascular interventions on the horizon include those related to diagnosing and treating coronary, carotid, renal, and peripheral arterial disease. Further advances are also expected in the existing areas of patient-specific planning for treating congenital heart disease and repairing aneurysms. For patient-specific treatment planning applications, there is a pressing need for retrospective and prospective studies to evaluate predictions against measured data and ultimately demonstrate improved clinical outcomes. Without such studies, the clinical impact of these tools will be diminished. Finally, there are opportunities for further application of image-based modeling to evaluate the safety and efficacy of cardiovascular devices. Image-based modeling may be particularly useful in understanding reasons why devices fail in some patients but not in others, for designing devices for less prevalent diseases or particularly vulnerable patients (e.g. pediatric), and for understanding low event-rate failures.
Conclusion
In its first decade, image-based modeling saw significant technical developments that now enable the study of hemodynamic factors in a wide variety of congenital and acquired vascular pathologies, and application to planning patient-specific interventions and designing and evaluating endovascular devices. With a growing appreciation for their relative strengths and weaknesses, we can anticipate a simpler and more clinically-oriented approach to the use of image-based models for blood flow and vessel dynamics in the next few years. This is not to discourage the development of ever more sophisticated biomedical engineering modeling technologies, especially as they relate to providing data on pressure fields, blood-wall interactions, and acute and chronic adaptive and maladaptive changes. In time much will be learned from the fruits of these labors, especially as they couple to emerging models of the vascular biology. What concerns us is that, like the cynical joke about fusion energy, they will always seem to be “twenty years away”. In an effort to forestall this, we encourage using what we have available right now, crude or not, to provide both compelling evidence and the tools necessary to demonstrate (or, if need be, circumscribe) the practical utility of local hemodynamic information in the management and prevention of cardiovascular diseases. If this connection between the scientists and engineers developing and applying image-based modeling tools and the scientists and clinicians focused on understanding and treating vascular disease can be further strengthened, the field of image-based modeling of blood flow and vessel wall dynamics will have a bright future and we will expect rapid growth in the years to come.
Abbreviations
- AAA:
-
Abdominal aortic aneurysms
- ALE:
-
Arbitrary Lagrangian–Eulerian
- CFD:
-
Computational fluid dynamics
- CT:
-
Computed tomography
- IMT:
-
Intima-media thickness
- IUVS:
-
Invasive intravascular ultrasound
- MRA:
-
Magnetic resonance angiograms
- MRI:
-
Magnetic resonance imaging
- PC-MRI:
-
Phase contrast magnetic resonance imaging
- US:
-
Ultrasound
- WSS:
-
Wall shear stress
References
Acevedo-Bolton, G., L. D. Jou, B. P. Dispensa, M. T. Lawton, R. T. Higashida, A. J. Martin, W. L. Young, and D. Saloner. Estimating the hemodynamic impact of interventional treatments of aneurysms: numerical simulation with experimental validation: Technical case report. Neurosurgery 59:E429–E430, 2006; author reply E429–E430.
Antiga, L., M. Piccinelli, L. Botti, B. Ene-Iordache, A. Remuzzi, and D. A. Steinman. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 46:1097–1112, 2008.
Antiga, L., and D. A. Steinman. Rethinking turbulence in blood. Biorheology 46:77–81, 2009.
Antiga, L., and D. A. Steinman. Robust and objective decomposition and mapping of bifurcating vessels. IEEE Trans. Med. Imaging 23:704–713, 2004.
Appanaboyina, S., F. Mut, R. Lohner, C. A. Putman, and J. R. Cebral. Computational fluid dynamics of stented intracranial aneurysms using adaptive embedded unstructured grids. Int. J. Numer. Meth. Fluids 57:475–493, 2008.
Augst, A. D., B. Ariff, G. T. S. A. Mc, X. Y. Xu, and A. D. Hughes. Analysis of complex flow and the relationship between blood pressure, wall shear stress, and intima-media thickness in the human carotid artery. Am. J. Physiol. Heart Circ. Physiol. 293:H1031–H1037, 2007.
Babuska, I., and J. T. Oden. Verification and validation in computational engineering and science: basic concepts. Comput. Meth. Appl. Mech. Eng. 193:4057–4066, 2004.
Bazilevs, Y., V. M. Calo, Y. Zhang, and T. J. R. Hughes. Isogeometric fluid–structure interaction analysis with applications to arterial blood flow. Comput. Mech. 38:310–322, 2006.
Bekkers, E. J., and C. A. Taylor. Multiscale vascular surface model generation from medical imaging data using hierarchical features. IEEE Trans. Med. Imaging 27:331–341, 2008.
Bijari, P. B., L. Antiga, and D. A. Steinman. Reliability of vascular geometry factors derived from clinical MRA. In: Proc. SPIE Medical Imaging, San Diego, CA, 2009.
Blanco, P. J., R. A. Feijoo, and S. A. Urquiza. A unified variational approach for coupling 3d–1d models and its blood flow applications. Comput. Meth. Appl. Mech. Eng. 196:4391–4410, 2007.
Botti, L., M. Piccinelli, B. Ene-Iordache, A. Remuzzi, and L. Antiga. An adaptive mesh refinement solver for large-scale simulation of biological flows. Int. J. Numer. Meth. Biomed. Eng. 26:86–100, 2010.
Boussel, L., V. Rayz, A. Martin, G. Acevedo-Bolton, M. T. Lawton, R. Higashida, W. S. Smith, W. L. Young, and D. Saloner. Phase-contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: Comparison with computational fluid dynamics. Magn. Reson. Med. 61:409–417, 2009.
Boussel, L., V. Rayz, C. McCulloch, A. Martin, G. Acevedo-Bolton, M. Lawton, R. Higashida, W. S. Smith, W. L. Young, and D. Saloner. Aneurysm growth occurs at region of low wall shear stress: Patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 39:2997–3002, 2008.
Bove, E. L., M. R. de Leval, F. Migliavacca, R. Balossino, and G. Dubini. Toward optimal hemodynamics: computer modeling of the Fontan circuit. Pediatr. Cardiol. 28:477–481, 2007.
Castro, M. A., C. M. Putman, and J. R. Cebral. Patient-specific computational fluid dynamics modeling of anterior communicating artery aneurysms: a study of the sensitivity of intra-aneurysmal flow patterns to flow conditions in the carotid arteries. AJNR Am. J. Neuroradiol. 27:2061–2068, 2006.
Causin, P., J. F. Gerbeau, and F. Nobile. Added-mass effect in the design of partitioned algorithms for fluid–structure problems. Comput. Meth. Appl. Mech. Eng. 194:4506–4527, 2005.
Cebral, J. R., M. A. Castro, S. Appanaboyina, C. M. Putman, D. Millan, and A. F. Frangi. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans. Med. Imaging 24:457–467, 2005.
Cebral, J. R., M. A. Castro, J. E. Burgess, R. S. Pergolizzi, M. J. Sheridan, and C. M. Putman. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. Am. J. Neuroradiol. 26:2550–2559, 2005.
Cebral, J. R., and R. Lohner. Efficient simulation of blood flow past complex endovascular devices using an adaptive embedding technique. IEEE Trans. Med. Imaging 24:468–476, 2005.
Cebral, J. R., R. S. Pergolizzi, and C. M. Putman. Computational fluid dynamics modeling of intracranial aneurysms: qualitative comparison with cerebral angiography. Acad. Radiol. 14:804–813, 2007.
Chatzizisis, Y. S., M. Jonas, A. U. Coskun, R. Beigel, B. V. Stone, C. Maynard, R. G. Gerrity, W. Daley, C. Rogers, E. R. Edelman, C. L. Feldman, and P. H. Stone. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation 117:993–1002, 2008.
Cheng, C., F. Helderman, D. Tempel, D. Segers, B. Hierck, R. Poelmann, A. van Tol, D. J. Duncker, D. Robbers-Visser, N. T. Ursem, R. van Haperen, J. J. Wentzel, F. Gijsen, A. F. van der Steen, R. de Crom, and R. Krams. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195:225–235, 2007.
Cheng, C., D. Tempel, R. van Haperen, A. van der Baan, F. Grosveld, M. J. Daemen, R. Krams, and R. de Crom. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753, 2006.
Dalman, R. L., M. M. Tedesco, J. Myers, and C. A. Taylor. AAA disease: mechanism, stratification, and treatment. Ann. NY Acad. Sci. 1085:92–109, 2006.
de Leval, M. R., P. Kilner, M. Gewillig, and C. Bull. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J. Thorac. Cardiovasc. Surg. 96:682–695, 1988.
de Zelicourt, D. A., K. Pekkan, J. Parks, K. Kanter, M. Fogel, and A. P. Yoganathan. Flow study of an extracardiac connection with persistent left superior vena cava. J. Thorac. Cardiovasc. Surg. 131:785–791, 2006.
DeGroff, C., B. Birnbaum, R. Shandas, W. Orlando, and J. Hertzberg. Computational simulations of the total cavo-pulmonary connection: insights in optimizing numerical solutions. Med. Eng. Phys. 27:135–146, 2005.
Dyverfeldt, P., J. P. Kvitting, A. Sigfridsson, J. Engvall, A. F. Bolger, and T. Ebbers. Assessment of fluctuating velocities in disturbed cardiovascular blood flow: in vivo feasibility of generalized phase-contrast MRI. J. Magn. Reson. Imaging 28:655–663, 2008.
Ethier, C. R. Computational modeling of mass transfer and links to atherosclerosis. Ann. Biomed. Eng. 30:461–471, 2002.
Feintuch, A., P. Ruengsakulrach, A. Lin, J. Zhang, Y. Q. Zhou, J. Bishop, L. Davidson, D. Courtman, F. S. Foster, D. A. Steinman, R. M. Henkelman, and C. R. Ethier. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 292:H884–H892, 2007.
Fernandez, M. A., J.-F. Gerbeau, and C. Grandmont. A projection semi-implicit scheme for the coupling of an elastic structure with an incompressible fluid. Int. J. Numer. Meth. Eng. 69:794–821, 2007.
Figueroa, C. A., S. Baek, C. A. Taylor, and J. D. Humphrey. A computational framework for coupled fluid–solid growth modeling in cardiovascular simulations. Comput. Meth. Appl. Mech. Eng. 198:3583–3602, 2009.
Figueroa, C. A., C. A. Taylor, A. J. Chiou, V. Yeh, and C. K. Zarins. Effect of curvature on displacement forces acting on aortic endografts: a three dimensional computational analysis. J. Endovas. Ther. 16:284–294, 2009.
Figueroa, C. A., C. A. Taylor, A. J. Chiou, V. Yeh, and C. K. Zarins. Magnitude and direction of pulsatile displacement forces acting on thoracic aortic endografts. J. Endovasc. Ther. 16:350–358, 2009.
Figueroa, C. A., I. E. Vignon-Clementel, K. C. Jansen, T. J. R. Hughes, and C. A. Taylor. A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput. Meth. Appl. Mech. Eng. 195:5685–5706, 2006.
Ford, M. D., S. W. Lee, S. P. Lownie, D. W. Holdsworth, and D. A. Steinman. On the effect of parent-aneurysm angle on flow patterns in basilar tip aneurysms: towards a surrogate geometric marker of intra-aneurismal hemodynamics. J. Biomech. 41:241–248, 2008.
Ford, M. D., H. N. Nikolov, J. S. Milner, S. P. Lownie, E. M. Demont, W. Kalata, F. Loth, D. W. Holdsworth, and D. A. Steinman. PIV-measured versus CFD-predicted flow dynamics in anatomically realistic cerebral aneurysm models. J. Biomech. Eng. 130:021015, 2008.
Ford, M. D., G. R. Stuhne, H. N. Nikolov, D. F. Habets, S. P. Lownie, D. W. Holdsworth, and D. A. Steinman. Virtual angiography for visualization and validation of computational models of aneurysm hemodynamics. IEEE Trans. Med. Imaging 24:1586–1592, 2005.
Ford, M. D., Y. J. Xie, B. A. Wasserman, and D. A. Steinman. Is flow in the common carotid artery fully developed? Physiol. Meas. 29:1335–1349, 2008.
Förster, C., W. A. Wall, and E. Ramm. Artificial added mass instabilities in sequential staggered coupling of nonlinear structures and incompressible viscous flows. Comput. Meth. Appl. Mech. Eng. 196:1278–1293, 2007.
Friedman, M. H., H. A. Himburg, and J. A. LaMack. Statistical hemodynamics: a tool for evaluating the effect of fluid dynamic forces on vascular biology in vivo. J. Biomech. Eng. 128:965–968, 2006.
Frydrychowicz, A., A. Berger, M. F. Russe, A. F. Stalder, A. Harloff, S. Dittrich, J. Hennig, M. Langer, and M. Markl. Time-resolved magnetic resonance angiography and flow-sensitive 4-dimensional magnetic resonance imaging at 3 tesla for blood flow and wall shear stress analysis. J. Thorac. Cardiovasc. Surg. 136:400–407, 2008.
Gerbeau, J.-F., M. Vidrascu, and P. Frey. Fluid–structure interaction in blood flows on geometries based on medical imaging. Comput. Struct. 83:155–165, 2005.
Gijsen, F. J., J. J. Wentzel, A. Thury, B. Lamers, J. C. Schuurbiers, P. W. Serruys, and A. F. van der Steen. A new imaging technique to study 3-d plaque and shear stress distribution in human coronary artery bifurcations in vivo. J. Biomech. 40:2349–2357, 2007.
Gijsen, F. J., J. J. Wentzel, A. Thury, F. Mastik, J. A. Schaar, J. C. Schuurbiers, C. J. Slager, W. J. van der Giessen, P. J. de Feyter, A. F. van der Steen, and P. W. Serruys. Strain distribution over plaques in human coronary arteries relates to shear stress. Am. J. Physiol. Heart Circ. Physiol. 295:H1608–H1614, 2008.
Glor, F. P., B. Ariff, A. D. Hughes, L. A. Crowe, P. R. Verdonck, D. C. Barratt, G. T. S. A. Mc, D. N. Firmin, and X. Y. Xu. Image-based carotid flow reconstruction: a comparison between MRI and ultrasound. Physiol. Meas. 25:1495–1509, 2004.
Glor, F. P., B. Ariff, A. D. Hughes, P. R. Verdonck, D. C. Barratt, A. D. Augst, S. A. Thom, and X. Y. Xu. Influence of head position on carotid hemodynamics in young adults. Am. J. Physiol. Heart Circ. Physiol. 287:H1670–H1681, 2004.
Glor, F. P., B. Ariff, A. D. Hughes, P. R. Verdonck, S. A. Thom, D. C. Barratt, and X. Y. Xu. Operator dependence of 3-d ultrasound-based computational fluid dynamics for the carotid bifurcation. IEEE Trans. Med. Imaging 24:451–456, 2005.
Glor, F. P., Q. Long, A. D. Hughes, A. D. Augst, B. Ariff, S. A. Thom, P. R. Verdonck, and X. Y. Xu. Reproducibility study of magnetic resonance image-based computational fluid dynamics prediction of carotid bifurcation flow. Ann. Biomed. Eng. 31:142–151, 2003.
Greve, J. M., A. S. Les, B. T. Tang, M. T. Draney Blomme, N. M. Wilson, R. L. Dalman, N. J. Pelc, and C. A. Taylor. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am. J. Physiol. Heart Circ. Physiol. 291:H1700–H1708, 2006.
Groen, H. C., F. J. Gijsen, A. van der Lugt, M. S. Ferguson, T. S. Hatsukami, A. F. van der Steen, C. Yuan, and J. J. Wentzel. Plaque rupture in the carotid artery is localized at the high shear stress region: a case report. Stroke 38:2379–2381, 2007.
Harloff, A., F. Albrecht, J. Spreer, A. F. Stalder, J. Bock, A. Frydrychowicz, J. Schollhorn, A. Hetzel, M. Schumacher, J. Hennig, and M. Markl. 3d blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4d MRI at 3T. Magn. Reson. Med. 61:65–74, 2009.
Hassan, T., M. Ezura, E. V. Timofeev, T. Tominaga, T. Saito, A. Takahashi, K. Takayama, and T. Yoshimoto. Computational simulation of therapeutic parent artery occlusion to treat giant vertebrobasilar aneurysm. AJNR Am. J. Neuroradiol. 25:63–68, 2004.
Hassan, T., E. V. Timofeev, M. Ezura, T. Saito, A. Takahashi, K. Takayama, and T. Yoshimoto. Hemodynamic analysis of an adult vein of Galen aneurysm malformation by use of 3d image-based computational fluid dynamics. AJNR Am. J. Neuroradiol. 24:1075–1082, 2003.
Himburg, H. A., S. E. Dowd, and M. H. Friedman. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am. J. Physiol. Heart Circ. Physiol. 293:H645–H653, 2007.
Himburg, H. A., and M. H. Friedman. Correspondence of low mean shear and high harmonic content in the porcine iliac arteries. J. Biomech. Eng. 128:852–856, 2006.
Himburg, H. A., D. M. Grzybowski, A. L. Hazel, J. A. LaMack, X. M. Li, and M. H. Friedman. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am. J. Physiol. Heart Circ. Physiol. 286:H1916–H1922, 2004.
Hofer, M., G. Rappitsch, K. Perktold, W. Trubel, and H. Schima. Numerical study of wall mechanics and fluid dynamics in end-to-side anastomoses and correlation to intimal hyperplasia. J. Biomech. 29:1297–1308, 1996.
Hoi, Y., S. H. Woodward, M. Kim, D. B. Taulbee, and H. Meng. Validation of CFD simulations of cerebral aneurysms with implication of geometric variations. J. Biomech. Eng. 128:844–851, 2006.
Hope, M. D., D. D. Purcell, T. A. Hope, C. von Morze, D. B. Vigneron, M. T. Alley, and W. P. Dillon. Complete intracranial arterial and venous blood flow evaluation with 4d flow MR imaging. AJNR Am. J. Neuroradiol. 30:362–366, 2009.
Hope, T. A., M. Markl, L. Wigstrom, M. T. Alley, D. C. Miller, and R. J. Herfkens. Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J. Magn. Reson. Imaging 26:1471–1479, 2007.
Howell, B. A., T. Kim, A. Cheer, H. Dwyer, D. Saloner, and T. A. Chuter. Computational fluid dynamics within bifurcated abdominal aortic stent-grafts. J. Endovasc. Ther. 14:138–143, 2007.
http://www.itk.org. Accessed on March 22, 2009.
http://www.simtk.org. Accessed on March 22, 2009.
http://www.vmtk.org. Accessed on March 22, 2009.
http://www.vtk.org. Accessed on March 22, 2009.
Humphrey, J. D. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50:53–78, 2008.
Humphrey, J. D., and C. A. Taylor. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu. Rev. Biomed. Eng. 10:221–246, 2008.
Ideker, T., T. Galitski, and L. Hood. A new approach to decoding life: systems biology. Annu. Rev. Genomics Hum. Genet. 2:343–372, 2001.
Jou, L. D., C. M. Quick, W. L. Young, M. T. Lawton, R. Higashida, A. Martin, and D. Saloner. Computational approach to quantifying hemodynamic forces in giant cerebral aneurysms. AJNR Am. J. Neuroradiol. 24:1804–1810, 2003.
Jou, L. D., G. Wong, B. Dispensa, M. T. Lawton, R. T. Higashida, W. L. Young, and D. Saloner. Correlation between lumenal geometry changes and hemodynamics in fusiform intracranial aneurysms. AJNR Am. J. Neuroradiol. 26:2357–2363, 2005.
Kadirvel, R., Y. H. Ding, D. Dai, H. Zakaria, A. M. Robertson, M. A. Danielson, D. A. Lewis, H. J. Cloft, and D. F. Kallmes. The influence of hemodynamic forces on biomarkers in the walls of elastase-induced aneurysms in rabbits. Neuroradiology 49:1041–1053, 2007.
Kakalis, N. M., A. P. Mitsos, J. V. Byrne, and Y. Ventikos. The haemodynamics of endovascular aneurysm treatment: a computational modelling approach for estimating the influence of multiple coil deployment. IEEE Trans. Med. Imaging 27:814–824, 2008.
Kassab, G. S. Scaling laws of vascular trees: of form and function. Am. J. Physiol. Heart Circ. Physiol. 290:H894–H903, 2006.
Kim, T., A. Y. Cheer, and H. A. Dwyer. A simulated dye method for flow visualization with a computational model for blood flow. J. Biomech. 37:1125–1136, 2004.
Kleinstreuer, C., S. Hyun, J. R. Buchanan, Jr., P. W. Longest, J. P. Archie, Jr., and G. A. Truskey. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit. Rev. Biomed. Eng. 29:1–64, 2001.
Kleinstreuer, C., Z. Li, and M. A. Farber. Fluid–structure interaction analyses of stented abdominal aortic aneurysms. Annu. Rev. Biomed. Eng. 9:169–204, 2007.
Krams, R., J. J. Wentzel, J. A. Oomen, R. Vinke, J. C. Schuurbiers, P. J. de Feyter, P. W. Serruys, and C. J. Slager. Evaluation of endothelial shear stress and 3d geometry as factors determining the development of atherosclerosis and remodeling in human coronary arteries in vivo. Combining 3d reconstruction from angiography and IVUS (ANGUS) with computational fluid dynamics. Arterioscler. Thromb. Vasc. Biol. 17:2061–2065, 1997.
Ku, J. P., C. J. Elkins, and C. A. Taylor. Comparison of CFD and MRI flow and velocities in an in vitro large artery bypass graft model. Ann. Biomed. Eng. 33:257–269, 2005.
LaDisa, Jr., J. F., I. Guler, L. E. Olson, D. A. Hettrick, J. R. Kersten, D. C. Warltier, and P. S. Pagel. Three-dimensional computational fluid dynamics modeling of alterations in coronary wall shear stress produced by stent implantation. Ann. Biomed. Eng. 31:972–980, 2003.
Lagana, K., R. Balossino, F. Migliavacca, G. Pennati, E. L. Bove, M. R. de Leval, and G. Dubini. Multiscale modeling of the cardiovascular system: application to the study of pulmonary and coronary perfusions in the univentricular circulation. J. Biomech. 38:1129–1141, 2005.
LaMack, J. A., H. A. Himburg, X. M. Li, and M. H. Friedman. Interaction of wall shear stress magnitude and gradient in the prediction of arterial macromolecular permeability. Ann. Biomed. Eng. 33:457–464, 2005.
Lee, S. E., S. W. Lee, P. F. Fischer, H. S. Bassiouny, and F. Loth. Direct numerical simulation of transitional flow in a stenosed carotid bifurcation. J. Biomech. 41:2551–2561, 2008.
Lee, S. W., L. Antiga, J. D. Spence, and D. A. Steinman. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 39:2341–2347, 2008.
Lee, S. W., L. Antiga, and D. A. Steinman. Correlations among indicators of disturbed flow at the normal carotid bifurcation. J. Biomech. Eng. 131:061013, 2009.
Lee, S.-W., D. S. Smith, F. Loth, P. F. Fischer, and H. S. Bassiouny. Numerical and experimental simulation of transitional flow in a blood vessel junction. Numer. Heat Transf. A-Appl. 51:1–22, 2007.
Lee, S. W., and D. A. Steinman. On the relative importance of rheology for image-based CFD models of the carotid bifurcation. J. Biomech. Eng. 129:273–278, 2007.
Les, A. S., S. C. Shadden, C. A. Figueroa, J. M. Park, M. M. Tedesco, R. J. Herfkens, R. L. Dalman, and C. A. Taylor. Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng., In press.
Leuprecht, A., K. Perktold, M. Prosi, T. Berk, W. Trubel, and H. Schima. Numerical study of hemodynamics and wall mechanics in distal end-to-side anastomoses of bypass grafts. J. Biomech. 35:225–236, 2002.
Li, Z. H., and C. Kleinstreuer. Blood flow and structure interactions in a stented abdominal aortic aneurysm model. Med. Eng. Phys. 27:369–382, 2005.
Löhner, R., J. R. Cebral, F. E. Camelli, S. Appanaboyina, J. D. Baum, E. L. Mestreau, and O. A. Soto. Adaptive embedded and immersed unstructured grid techniques. Comput. Meth. Appl. Mech. Eng. 197:2173–2197, 2008.
Lundstrom, C., P. Ljung, A. Persson, and A. Ynnerman. Uncertainty visualization in medical volume rendering using probabilistic animation. IEEE Trans. Vis. Comput. Graph. 13:1648–1655, 2007.
Markl, M., F. P. Chan, M. T. Alley, K. L. Wedding, M. T. Draney, C. J. Elkins, D. W. Parker, R. Wicker, C. A. Taylor, R. J. Herfkens, and N. J. Pelc. Time-resolved three-dimensional phase-contrast MRI. J. Magn. Reson. Imaging 17:499–506, 2003.
Marsden, A. L., A. J. Bernstein, V. M. Reddy, S. C. Shadden, R. L. Spilker, F. P. Chan, C. A. Taylor, and J. A. Feinstein. Evaluation of a novel y-shaped extracardiac Fontan baffle using computational fluid dynamics. J. Thorac. Cardiovasc. Surg. 137:394–403, 2009.
Marsden, A. L., J. A. Feinstein, and C. A. Taylor. A computational framework for derivative-free optimization of cardiovascular geometries. Comput. Meth. Appl. Mech. Eng. 197:1890–1905, 2008.
Marsden, A. L., I. E. Vignon-Clementel, F. P. Chan, J. A. Feinstein, and C. A. Taylor. Effects of exercise and respiration on hemodynamic efficiency in CFD simulations of the total cavopulmonary connection. Ann. Biomed. Eng. 35:250–263, 2007.
Meckel, S., A. F. Stalder, F. Santini, E. W. Radu, D. A. Rufenacht, M. Markl, and S. G. Wetzel. In vivo visualization and analysis of 3-d hemodynamics in cerebral aneurysms with flow-sensitized 4-d MR imaging at 3T. Neuroradiology. 50:473–484, 2008.
Meng, H., D. D. Swartz, Z. Wang, Y. Hoi, J. Kolega, E. M. Metaxa, M. P. Szymanski, J. Yamamoto, E. Sauvageau, and E. I. Levy. A model system for mapping vascular responses to complex hemodynamics at arterial bifurcations in vivo. Neurosurgery 59:1094–1100, 2006 (discussion 1100-1).
Migliavacca, F., R. Balossino, G. Pennati, G. Dubini, T. Y. Hsia, M. R. de Leval, and E. L. Bove. Multiscale modelling in biofluidynamics: application to reconstructive paediatric cardiac surgery. J. Biomech. 39:1010–1020, 2006.
Migliavacca, F., M. R. de Leval, G. Dubini, and R. Pietrabissa. A computational pulsatile model of the bidirectional cavopulmonary anastomosis: the influence of pulmonary forward flow. J. Biomech. Eng. 118:520–528, 1996.
Migliavacca, F., M. R. de Leval, G. Dubini, R. Pietrabissa, and R. Fumero. Computational fluid dynamic simulations of cavopulmonary connections with an extracardiac lateral conduit. Med. Eng. Phys. 21:187–193, 1999.
Migliavacca, F., G. Dubini, G. Pennati, R. Pietrabissa, R. Fumero, T.-Y. Hsia, and M. R. de Leval. Computational model of the fluid dynamics in systemic-to-pulmonary shunts. J. Biomech. 33:549–557, 2000.
Milner, J. S., J. A. Moore, B. K. Rutt, and D. A. Steinman. Hemodynamics of human carotid artery bifurcations: computational studies with models reconstructed from magnetic resonance imaging of normal subjects. J. Vasc. Surg. 28:143–156, 1998.
Mistretta, C. A. Undersampled radial mr acquisition and highly constrained back projection (hypr) reconstruction: potential medical imaging applications in the post-nyquist era. J. Magn. Reson. Imaging 29:501–516, 2009.
Morbiducci, U., R. Ponzini, M. Grigioni, and A. Redaelli. Helical flow as fluid dynamic signature for atherogenesis risk in aortocoronary bypass. A numeric study. J. Biomech. 40:519–534, 2007.
Moyle, K. R., L. Antiga, and D. A. Steinman. Inlet conditions for image-based CFD models of the carotid bifurcation: is it reasonable to assume fully developed flow? J. Biomech. Eng. 128:371–379, 2006.
Müller, J., O. Sahni, X. Li, K. E. Jansen, M. S. Shephard, and C. A. Taylor. Anisotropic adaptive finite element method for modelling blood flow. Comput. Meth. Appl. Mech. Eng. 8:295–305, 2005.
Olufsen, M. S. Structured tree outflow condition for blood flow in larger systemic arteries. Am. J. Physiol. 276:H257–H268, 1999.
Pekkan, K., B. Whited, K. Kanter, S. Sharma, D. de Zelicourt, K. Sundareswaran, D. Frakes, J. Rossignac, and A. Yoganathan. Patient-specific surgical planning and hemodynamic computational fluid dynamics optimization through free-form haptic anatomy editing tool (SURGEM). Med. Biol. Eng. Comput. 46:1139–1152, 2008.
Perktold, K., and G. Rappitsch. Computer simulation of local blood flow and vessel mechanics in a compliant carotid artery bifurcation model. J. Biomech. 28:845–856, 1995.
Prakash, S., and C. R. Ethier. Requirements for mesh resolution in 3d computational hemodynamics. J. Biomech. Eng. 123:134–144, 2001.
Prosi, M., K. Perktold, Z. H. Ding, and M. H. Friedman. Influence of curvature dynamics on pulsatile coronary artery flow in a realistic bifurcation model. J. Biomech. 37:1767–1775, 2004.
Radaelli, A. G., L. Augsburger, J. R. Cebral, M. Ohta, D. A. Rufenacht, R. Balossino, G. Benndorf, D. R. Hose, A. Marzo, R. Metcalfe, P. Mortier, F. Mut, P. Reymond, L. Socci, B. Verhegghe, and A. F. Frangi. Reproducibility of haemodynamical simulations in a subject-specific stented aneurysm model—a report on the virtual intracranial stenting challenge 2007. J. Biomech. 41:2069–2081, 2008.
Rafii, B. Y., O. J. Abilez, P. Benharash, and C. K. Zarins. Lateral movement of endografts within the aneurysm sac is an indicator of stent-graft instability. J. Endovasc. Ther. 15:335–343, 2008.
Rayz, V. L., L. Boussel, G. Acevedo-Bolton, A. J. Martin, W. L. Young, M. T. Lawton, R. Higashida, and D. Saloner. Numerical simulations of flow in cerebral aneurysms: comparison of CFD results and in vivo MRI measurements. J. Biomech. Eng. 130:051011, 2008.
Rayz, V. L., L. Boussel, M. T. Lawton, G. Acevedo-Bolton, L. Ge, W. L. Young, R. T. Higashida, and D. Saloner. Numerical modeling of the flow in intracranial aneurysms: prediction of regions prone to thrombus formation. Ann. Biomed. Eng. 36:1793–1804, 2008.
Roache, P. J. Verification and Validation in Computational Science and Engineering. Albuquerque: Hermosa Publishers, p. 446, 1998.
Ryval, J., A. G. Straatman, and D. A. Steinman. Two-equation turbulence modeling of pulsatile flow in a stenosed tube. J. Biomech. Eng. 126:625–635, 2004.
Sahni, O., K. E. Jansen, M. S. Shephard, C. A. Taylor, and M. W. Beall. Adaptive boundary layer meshing for viscous flow simulations. Eng. Comput. 24:267–285, 2008.
Sahni, O., K. E. Jansen, C. A. Taylor, and M. S. Shephard. Automated adaptive cardiovascular flow simulations. Eng. Comput. 25:25–36, 2009.
Sahni, O., J. Müller, K. E. Jansen, M. S. Shephard, and C. A. Taylor. Efficient anisotropic adaptive discretization of the cardiovascular system. Comput. Meth. Appl. Mech. Eng. 195:5634–5655, 2006.
Schmidt, J. P., S. L. Delp, M. A. Sherman, C. A. Taylor, V. S. Pande, and R. B. Altman. The simbios national center: systems biology in motion. Proc. IEEE 96:1266–1280, 2008.
Sforza, D. M., C. M. Putman, and J. R. Cebral. Hemodynamics of cerebral aneurysms. Annu. Rev. Fluid Mech. 41:91–107, 2009.
Shadden, S. C., and C. A. Taylor. Characterization of coherent structures in the cardiovascular system. Ann. Biomed. Eng. 36:1152–1162, 2008.
Sharma, S., S. Goudy, P. Walker, S. Panchal, A. Ensley, K. Kanter, V. Tam, D. Fyfe, and A. Yoganathan. In vitro flow experiments for determination of optimal geometry of total cavopulmonary connection for surgical repair of children with functional single ventricle. J. Am. Coll. Cardiol. 27:1264–1269, 1996.
Shimogonya, Y., T. Ishikawa, Y. Imai, N. Matsuki, and T. Yamaguchi. Can temporal fluctuation in spatial wall shear stress gradient initiate a cerebral aneurysm? A proposed novel hemodynamic index, the gradient oscillatory number (GON). J. Biomech. 42:550–554, 2009.
Shojima, M., M. Oshima, K. Takagi, R. Torii, M. Hayakawa, K. Katada, A. Morita, and T. Kirino. Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke 35:2500–2505, 2004.
Slager, C. J., J. J. Wentzel, F. J. Gijsen, A. Thury, A. C. van der Wal, J. A. Schaar, and P. W. Serruys. The role of shear stress in the destabilization of vulnerable plaques and related therapeutic implications. Nat. Clin. Pract. Cardiovasc. Med. 2:456–464, 2005.
Slager, C. J., J. J. Wentzel, J. C. Schuurbiers, J. A. Oomen, J. Kloet, R. Krams, C. von Birgelen, W. J. van der Giessen, P. W. Serruys, and P. J. de Feyter. True 3-dimensional reconstruction of coronary arteries in patients by fusion of angiography and IVUS (ANGUS) and its quantitative validation. Circulation 102:511–516, 2000.
Spilker, R. L., J. A. Feinstein, D. W. Parker, V. M. Reddy, and C. A. Taylor. Morphometry-based impedance boundary conditions for patient-specific modeling of blood flow in pulmonary arteries. Ann. Biomed. Eng. 35:546–559, 2007.
Spilker, R. L., and C. A. Taylor. Incorporating physiological measurements in image-based hemodynamic simulations. In: Fifth International Biofluids Symposium and Workshop, Pasadena, CA, 2008.
Steele, B. N., M. S. Olufsen, and C. A. Taylor. Fractal network model for simulating abdominal and lower extremity blood flow during resting and exercise conditions. Comput. Meth. Biomech. Biomed. Eng. 10:39–51, 2007.
Steinman, D. A. Image-based computational fluid dynamics modeling in realistic arterial geometries. Ann. Biomed. Eng. 30:483–497, 2002.
Steinman, D. A., J. S. Milner, C. J. Norley, S. P. Lownie, and D. W. Holdsworth. Image-based computational simulation of flow dynamics in a giant intracranial aneurysm. AJNR Am. J. Neuroradiol. 24:559–566, 2003.
Steinman, D. A., and C. A. Taylor. Flow imaging and computing: large artery hemodynamics. Ann. Biomed. Eng. 33:1704–1709, 2005.
Steinman, D. A., J. B. Thomas, H. M. Ladak, J. S. Milner, B. K. Rutt, and J. D. Spence. Reconstruction of carotid bifurcation hemodynamics and wall thickness using computational fluid dynamics and MRI. Magn. Reson. Med. 47:149–159, 2002.
Stone, P. H., A. U. Coskun, S. Kinlay, M. E. Clark, M. Sonka, A. Wahle, O. J. Ilegbusi, Y. Yeghiazarians, J. J. Popma, J. Orav, R. E. Kuntz, and C. L. Feldman. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 108:438–444, 2003.
Stonebridge, P. A., and C. M. Brophy. Spiral laminar flow in arteries? Lancet 338:1360–1361, 1991.
Stuhne, G. R., and D. A. Steinman. Finite-element modeling of the hemodynamics of stented aneurysms. J. Biomech. Eng. 126:382–387, 2004.
Suo, J., D. E. Ferrara, D. Sorescu, R. E. Guldberg, W. R. Taylor, and D. P. Giddens. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler. Thromb. Vasc. Biol. 27:346–351, 2007.
Tambasco, M., and D. A. Steinman. On assessing the quality of particle tracking through computational fluid dynamic models. J. Biomech. Eng. 124:166–175, 2002.
Tang, B. T., C. P. Cheng, M. T. Draney, N. M. Wilson, P. S. Tsao, R. J. Herfkens, and C. A. Taylor. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am. J. Physiol. Heart Circ. Physiol. 291:H668–H676, 2006.
Tang, D., C. Yang, S. Mondal, F. Liu, G. Canton, T. S. Hatsukami, and C. Yuan. A negative correlation between human carotid atherosclerotic plaque progression and plaque wall stress: in vivo MRI-based 2d/3d FSI models. J. Biomech. 41:727–736, 2008.
Taylor, C. A., and M. T. Draney. Experimental and computational methods in cardiovascular fluid mechanics. Annu. Rev. Fluid Mech. 36:197–231, 2004.
Taylor, C. A., M. T. Draney, J. P. Ku, D. Parker, B. N. Steele, K. Wang, and C. K. Zarins. Predictive medicine: computational techniques in therapeutic decision-making. Comput. Aided Surg. 4:231–247, 1999.
Taylor, C. A., T. J. R. Hughes, and C. K. Zarins. Computational investigations in vascular disease. Comput. Phys. 10:224–232, 1996.
Taylor, C. A., T. J. R. Hughes, and C. K. Zarins. Finite element modeling of blood flow in arteries. Comput. Meth. Appl. Mech. Eng. 158:155–196, 1998.
Tezduyar, T. E., S. Sathe, M. Schwaab, and B. S. Conklin. Arterial fluid mechanics modeling with the stabilized space-time fluid–structure interaction technique. Int. J. Numer. Meth. Fluids 57:601–629, 2008.
Thomas, J. B., J. S. Milner, B. K. Rutt, and D. A. Steinman. Reproducibility of image-based computational fluid dynamics models of the human carotid bifurcation. Ann. Biomed. Eng. 31:132–141, 2003.
Torii, R., M. Oshima, T. Kobayashi, K. Takagi, and T. E. Tezduyar. Fluid–structure interaction modeling of a patient-specific cerebral aneurysm: Influence of structural modeling. Comput. Mech. 43:151–159, 2008.
Torii, R., N. B. Wood, N. Hadjiloizou, A. W. Dowsey, A. R. Wright, A. D. Hughes, J. Davies, D. P. Francis, J. Mayet, G. Z. Yang, S. A. Thom, and X. Y. Xu. Stress phase angle depicts differences in coronary artery hemodynamics due to changes in flow and geometry after percutaneous coronary intervention. Am. J. Physiol. Heart Circ. Physiol. 296:H765–H776, 2009.
Vermeersch, S. J., E. R. Rietzschel, M. L. De Buyzere, D. De Bacquer, G. De Backer, L. M. Van Bortel, T. C. Gillebert, P. R. Verdonck, and P. Segers. Determining carotid artery pressure from scaled diameter waveforms: comparison and validation of calibration techniques in 2026 subjects. Physiol. Meas. 29:1267–1280, 2008.
Vignon-Clementel, I. E., C. A. Figueroa, K. C. Jansen, and C. A. Taylor. Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput. Meth. Appl. Mech. Eng. 195:3776–3796, 2006.
Wake, A. K., J. N. Oshinski, A. R. Tannenbaum, and D. P. Giddens. Choice of in vivo versus idealized velocity boundary conditions influences physiologically relevant flow patterns in a subject-specific simulation of flow in the human carotid bifurcation. J. Biomech. Eng. 131:021013, 2009.
Wang, K. C., R. W. Dutton, and C. A. Taylor. Improving geometric model construction for blood flow modeling. Eng. Med. Biol. 18:33–39, 1999.
Weinberg, P. D., and C. Ross Ethier. Twenty-fold difference in hemodynamic wall shear stress between murine and human aortas. J. Biomech. 40:1594–1598, 2007.
Wellnhofer, E., L. Goubergrits, U. Kertzscher, K. Affeld, and E. Fleck. Novel non-dimensional approach to comparison of wall shear stress distributions in coronary arteries of different groups of patients. Atherosclerosis 202:483–490, 2009.
Wentzel, J. J., F. J. Gijsen, J. C. Schuurbiers, R. Krams, P. W. Serruys, P. J. De Feyter, and C. J. Slager. Geometry guided data averaging enables the interpretation of shear stress related plaque development in human coronary arteries. J. Biomech. 38:1551–1555, 2005.
Whitehead, K. K., K. Pekkan, H. D. Kitajima, S. M. Paridon, A. P. Yoganathan, and M. A. Fogel. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation 116:I165–I171, 2007.
Wilson, N. M., F. R. Arko, and C. A. Taylor. Predicting changes in blood flow in patient-specific operative plans for treating aortoiliac occlusive disease. Comput. Aided Surg. 10:257–277, 2005.
Wolters, B., M. C. M. Rutten, G. W. H. Schurink, U. Kose, J. de Hart, and F. N. van de Vosse. A patient-specific computational model of fluid–structure interaction in abdominal aortic aneurysms. Med. Eng. Phys. 27:871–883, 2005.
Womersley, J. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J. Physiol. 127:553–563, 1955.
Womersley, J. R. Oscillatory motion of a viscous liquid in a thin-walled elastic tube. I. The linear approximation for long waves. Philos. Mag. 7:199–221, 1955.
Wood, N. B., S. Z. Zhao, A. Zambanini, M. Jackson, W. Gedroyc, S. A. Thom, A. D. Hughes, and X. Y. Xu. Curvature and tortuosity of the superficial femoral artery: a possible risk factor for peripheral arterial disease. J. Appl. Physiol. 101:1412–1418, 2006.
Yamashita, S., H. Isoda, M. Hirano, H. Takeda, S. Inagawa, Y. Takehara, M. T. Alley, M. Markl, N. J. Pelc, and H. Sakahara. Visualization of hemodynamics in intracranial arteries using time-resolved three-dimensional phase-contrast MRI. J. Magn. Reson. Imaging 25:473–478, 2007.
Yeung, J. J., H. J. Kim, T. A. Abbruzzese, I. E. Vignon-Clementel, M. T. Draney-Blomme, K. K. Yeung, I. Perkash, R. J. Herfkens, C. A. Taylor, and R. L. Dalman. Aortoiliac hemodynamic and morphologic adaptation to chronic spinal cord injury. J. Vasc. Surg. 44:1254–1265, 2006.
Young, H. K., P. G. Walker, A. A. Fontaine, S. Panchal, A. E. Ensley, J. Oshinski, S. Sharma, B. Ha, C. L. Lucas, and A. P. Yoganathan. Hemodynamics of the Fontan connection: an in-vitro study. J. Biomech. Eng. 117:423–428, 1995.
Younis, H. F., M. R. Kaazempur-Mofrad, R. C. Chan, A. G. Isasi, D. P. Hinton, A. H. Chau, L. A. Kim, and R. D. Kamm. Hemodynamics and wall mechanics in human carotid bifurcation and its consequences for atherogenesis: investigation of inter-individual variation. Biomech. Model. Mechanobiol. 3:17–32, 2004.
Zarins, C. K., and C. A. Taylor. Endovascular device design in the future: transformation from trial and error to computational design. J. Endovasc. Ther. 16:I-12–21, 2009.
Acknowledgments
This review is based on material presented at the International Biofluids Symposium and Workshop, Pasadena, CA, March 28–30, 2008. Taylor’s research on which this work is based has been supported, in part, by grants from the National Science Foundation (ITR-0205741) and the National Institutes of Health through the NIH Roadmap for Medical Research Grant U54 GM072970. Information on the National Centers for Biomedical Computing can be obtained from http://nihroadmap.nih.gov/bioinformatics. Steinman’s work on image-based modeling has been supported by a Career Investigator Award from the Heart & Stroke Foundation, as well as by grants from that agency and from the Canadian Institutes of Health Research. The invaluable contributions from our many collaborators, staff, trainees and study volunteers are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Larry V. McIntire oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Taylor, C.A., Steinman, D.A. Image-Based Modeling of Blood Flow and Vessel Wall Dynamics: Applications, Methods and Future Directions. Ann Biomed Eng 38, 1188–1203 (2010). https://doi.org/10.1007/s10439-010-9901-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9901-0