Abstract
Mesenchymal stem cells (MSCs) are an attractive cell source for regenerative medicine and the study of skeletal development. Despite considerable interest in MSC chondrogenesis, the signal transduction and molecular mechanisms underlying this process remain largely undefined. To explore the signaling topology regulating chondrogenic differentiation, as well as to discover novel modulators, we developed and validated a high-throughput screening (HTS) assay for MSC chondrogenesis. Adapting standard assay procedures to enable HTS, we successfully minimized cell number, handling, and culture duration. Using our optimized methodology with automation, we evaluated a comprehensive screen using four growth factors, TGF-β3, BMP-2, IGF-1, and FGF-2, to demonstrate the feasibility of large combinatorial screens. We examined the chondrogenic effects of these growth factors in different combinations and doses (81 combinations total with 16 replicates per group) and found variable effects on GAG content with different combinations. In general, TGF-β3 had a pro-chondrogenic effect while FGF-2 had a proliferative effect. BMP-2 was both proliferative and pro-chondrogenic while the effect of IGF-1 in our system was variable. We also carried out an HTS campaign of the National Institute of Neurological Disorders and Stroke (NINDS) chemical library of small molecules (1040 compounds) and identified 5 potential inducers and 24 potential inhibitors of chondrogenesis. Of these compounds, several were identified from the hypnotic, anti-neoplastic, or anti-protein synthesis classes of molecules. These studies demonstrate our ability to carry out high-throughput screening assays for modulators of chondrogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult bone marrow derived mesenchymal stem cells (MSCs) are a multi-potential and self-renewing cell type that can be induced to differentiate along a variety of tissue-specific pathways, including cartilage (chondrogenesis) and bone (osteogenesis). In the original description of MSC chondrogenesis, cells were collected into high-density pellets to induce a rounded morphology (mimicking mesenchymal condensation in the limb bud) and treated with specific biofactors, including transforming growth factor-β (TGF-β) superfamily members and dexamethasone, in a chemically defined medium.8,28,29 Under these conditions, MSCs synthesize a cartilage-specific extracellular matrix (ECM) rich in glycosaminoglycan (GAG) and type II collagen and express cartilage markers, including the transcription factor Sox 9. Given their chondrogenic potential, MSCs are a promising cell source for investigating skeletal developmental paradigms and cartilage tissue engineering applications. Indeed, recent studies have shown that MSCs undergo chondrogenesis in 3D environments, such as fibrous networks, porous foams, and hydrogels,2,12,18,22 and deposit a cartilage-like extracellular matrix (ECM). However, the quantity and functional capacity of ECM formed by chondrogenic MSCs is reduced relative to the ECM formed by fully differentiated chondrocytes cultured under identical conditions.13,22 Thus, further work is necessary to optimize MSC chondrogenesis for engineering replacement tissues.
A better understanding of the signaling pathways underlying MSC differentiation is critical to understanding this process. To date, growth factors have been widely used to induce chondrogenesis, however, the molecular mechanisms involved in this phenotypic conversion are only partially defined. Several studies have used synthetic molecules to investigate signaling pathways known to be involved in cartilage development or chondrocyte biosynthesis to determine their functional roles in MSC differentiation. For example, MAPK and Wnt signaling pathways have been implicated in TGF-β1-mediated chondrogenesis,36,37 and synthetic MAPK inhibitors block pellet formation. It has also been shown that Insulin-Like Growth Factor-1 (IGF-1) activates the PI3K pathway during chondrognesis, while induction by TGF-β1 does not.23 In micromass cultures of limb-bud cells (a related, but distinct, cell type), synthetic compounds that inhibit Rac and ROCK activity alter chondrogenic progression.42,43 Disruption of cytoskeletal dynamics in these same cells with cytochalasin D and colchicine can also influence chondrogenesis.41 Analogous to results from chondrocyte de- and re-differentiation studies,7 actin cytoskeleton disruption improves the chondrogenic differentiation of embryonic stem cells.47 More recently, investigators have taken an informed approach by identifying factors involved in MSC chondrogenesis. For example, expression analysis suggests a decrease in retinoic acid receptor β is associated with chondrogenesis; subsequent treatment with a synthetic inhibitor (LE135) of this pathway induced chondrogenesis via a Sox 9-independent pathway.17 These studies demonstrate that a clearer understanding of the molecular mechanisms governing MSC chondrogenesis may provide insight into methods for optimizing functional differentiation, and that small molecule modulators will be critical in these efforts.
While induction of MSC chondrogenesis is well-defined using existing protocols and media formulations, the capacity and potency of alternative factors to regulate chondrogenesis remains largely unexplored. Although several studies have examined the chondrogenic effects of various combinations of growth factors and media supplements, they have been limited in scope since execution, maintenance, and analysis remains a laborious and time-consuming process.5,10,15,16,19,35 Most chondrogenesis assays require a ‘macro’ pellet (>225,000 cells in 200 μL of media) cultured in individual tubes to ensure sufficient ECM for subsequent quantification steps. Given this pellet size, the screening of large chemical libraries or numerous conditions is impractical; for example, a single screen of the >200,000 compounds in the NIH Small Molecule Repository (SMR) would require ∼46 billion cells from a single donor. Recent efforts in minimizing handling have used 96-well conical plates27,40; however, cell number in each pellet and time required to analyze differentiation end-points remains a limiting factor.
These limitations may be overcome by high-throughput screening (HTS), wherein the simultaneous layout and query of a large number of conditions may be realized within a single plate. HTS depends on the use of robotic liquid handling systems and on the development of sensitive and readily quantifiable assays.39 In a typical screen, a drug target or model system is reacted against a large range of chemicals contained in a compound library. Identified agents that modulate pathways of biologic interest are then verified via secondary confirmatory assays and characterization of dosage response. Numerous chemical libraries, such as the National Institute of Neurological Disorders and Stroke (NINDS) library and the Library of Pharmacologically Active Compounds (LOPAC), contain small molecules of known pharmacologic activity. Additionally, the recently developed SMR is populated by a vast number of compounds with unknown activity. Few studies have assayed these libraries to study MSC differentiation towards skeletal phenotypes with HTS methods. One recent study used zebrafish as a model to screen >5000 compounds from commercial libraries and identified dorsomorphin, an inhibitor of bone morphogenic protein (BMP) type I receptor signaling.45 HTS was also used to identify osteogenic suppressors in MSC monolayers using an siRNA library48 and the osteogenic inducer purmorphamine from a custom chemical library.44 These studies illustrate the power of HTS for identifying new compounds. However, chondrogenic differentiation protocols are more complicated than the assays used to date and require specific adaptation of the standard protocol to be useful in HTS. This is the first report of a high-throughput assay for screening MSC chondrogenesis.
An HTS assay for chondrogenesis would enable the rapid optimization of effective media formulations for tissue engineering and would provide a platform for pharmaceutical screening to identify new chemical agents for the treatment of musculoskeletal pathologies. To optimize cell culture and assay procedures to enable high throughput screening of MSC chondrogensis, we have focused on minimizing the cell number required, manual handling, and culture durations. A novel in-well digestion protocol was developed to enable rapid post-processing and to further minimize handling. In addition, a precise and robotic approach for layout, culture, and analysis of ECM deposition using ‘micro’ MSC pellets (10,000 cells in 50 μL of media) in a 384-well format was validated. Following validation in this 384-well format, a combinatorial study analyzing the chondrogenic effects of TGF-β3, BMP-2, IGF-1, Fibroblast Growth Factor-2 (FGF-2), and their combinations (81 combinations) using three different doses per growth factor (none, low, high) was executed. Finally, we carried out an initial screen of the NINDS small molecule library containing 1040 known compounds and demonstrated the feasibility of this technology for use in HTS for potential effectors of chondrogenesis. These methods and results provide a new approach to the rapid identification of compounds that influence chondrogenic fate decisions by MSCs.
Methods
Mesenchymal Stem Cell Isolation and Culture
Bone marrow derived MSCs were harvested from the carpal bones of 3–6 month old calves (Research 87, Boylston, MA) as in Huang et al. 13 Briefly, trabecular bone regions were removed and agitated in high glucose Dulbecco’s Modified Eagle’s Medium (hgDMEM) supplemented with 2% penicillin/streptomycin/Fungizone (PSF) and 300 U/mL of heparin. The marrow mixture was separated from fat by centrifugation (5 min at 300× g) and plated onto 15 cm tissue culture plates. After 48 h to allow for cell attachment, media was changed and cultures were maintained in hgDMEM supplemented with 1% PSF and 10% fetal bovine serum. Medium was changed twice weekly until confluence and sub-culturing was carried out at a 1:3 expansion ratio up to passage three.
HTS Assay Development
Minimization of Pellet Size, Media Exchange, and In-well Analysis
Mesenchymal stem cell pellets were formed via centrifugation (300× g) for 5 min in conical 96-well polypropylene plates (Nalge Nunc International, Rochester, NY). Pellets contained 225,000, 150,000, 75,000, 30,000, and 15,000 MSCs with all assays performed in triplicate. Each pellet was maintained in 150 μL of chemically defined medium (CM) consisting of hgDMEM without phenol red supplemented with 1X PSF, 0.1 μM dexamethasone, 50 mg/mL ascorbate 2-phosphate, 40 mg/mL l-proline, 100 mg/mL sodium pyruvate, 1X ITS+ (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenous acid, 1.25 mg/mL BSA, and 5.35 μg/mL linoleic acid). Pellets were cultured with (CM+) or without (CM−) 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN) for 7 days, with media changed twice, once, or not at all. Three replicate experiments were carried out with consistent results (one replicate shown). On day 7, pellets were digested with direct addition of 30 μL concentrated papain solution (8.96 units/mL in 0.1 M sodium acetate, 10 M cysteine HCL, 0.05 M EDTA, pH 6.0) into each well. Plates were sealed with optical adhesive covers (Applied Biosystems, Foster City, CA) and incubated in a 60 °C waterbath overnight. To assess GAG content, 40 μL of digestate was manually combined with 250 μL of dimethylmethylene blue (DMMB) reagent and absorbance read at 540/595 nm.11 In a separate study, cartilage gene expression was evaluated in pellets of different sizes (30,000 and 225,000 cells/pellet) with culture as above with no media changes. After 7 days, pellets were combined and gene expression analyzed via real-time PCR as in Mauck et al. 22 For gene expression analysis, 18 pellets (30 K pellet size) or 3 pellets (225 K pellet size) were pooled for each sample with n = 3 samples per group assessed. PCR amplification was carried out with primers specific for aggrecan and type II collagen and expression was normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Inhibition of Chondrogenesis with IL-1β
To confirm that chemical screens may be performed, Interleukin-1β (IL-1β, R&D Systems, Minneapolis, MN), a known inhibitor of chondrogenesis, was used to block the chondrogenic effect of TGF-β3.20 Large (225,000) and small (30,000) pellets were formed as described above in 96-well plates in 150 μL of CM− or CM+ media supplemented with IL-1β at 0, 0.1, 1.0, 10.0 ng/mL. Pellets were cultured with no media changes and GAG content assessed on day 7.
Assessing DMSO Sensitivity
To assess the sensitivity of MSCs to dimethyl sulfoxide (DMSO), a common solvent in chemical libraries, small pellets (30,000) were formed in 96-well plates and exposed to CM− or CM+ media in the presence of graded concentrations of DMSO (0, 0.10, 0.25, 0.50, 0.75, 1%) for 7 days. GAG and DNA content was assessed via the DMMB and PicoGreen assays, respectively. The PicoGreen dsDNA assay (Molecular Probes, Eugene, OR) was carried out by reacting 10 μL of digested sample with 100 μL of PicoGreen working reagent (50 μL PicoGreen reagent, 500 μL 20× TE buffer, 9.45 mL deionized water).12 Following a 5-min incubation in the dark, plates were read at 480 nm excitation/520 nm emission (Bio-Tek Synergy HT Multi-Mode Microplate Reader).
Automation of Layout and Analysis in 384-Well Format
For optimization of culture and analysis techniques in a 384-well format for HTS, a stream-lined protocol for chondrogenesis was applied (Fig. 1). Total media volume and cell number was further reduced from 96-well procedures by a factor of three. Cells were dispensed automatically using a Matrix Technologies WellMate system with 10,000 cells pelleted in 50 μL of CM in 384-well conical plates (Greiner Bio-One, San Diego, CA). After centrifugation (5 min at 300× g), pellets were cultured for 7 days in CM− or CM+, with a Breathe-Easy membrane (Research Products International Corp., Mt. Prospect, IL) sealing all wells to minimize evaporation and allow for gas exchange. After culture for 7 days, the Breathe-Easy membrane was removed and in-well digestion was performed by automated addition of 10 μL of concentrated papain solution (3.36 units/mL) using the WellMate system. Plates were then sealed with ArctiSeal sealing mats (ArcticWhite LLC, Bethlehem, PA) and incubated in a 60 °C waterbath as described above. Subsequently, a robotic liquid handling system (PerkinElmer Evolution P3 Pipetting Platform) mixed and transferred 10 μL of the digestate to a new flat-bottomed 384-well assay plate (Corning Inc., Corning, NY), and the GAG assay was performed by reaction with 60 μL DMMB dye solution as described above (PerkinElmer Envision 2102 Multilabel Reader). DNA content was similarly assessed by reaction of 1 μL of digestate with 10 μL of Picgoreen working reagent.
Growth Factor Combinatorial Screen
To demonstrate the feasibility of assaying combinations of multiple growth factors at varying dosages, a screen was carried out using TGF-β3, BMP-2 (R&D Systems, Minneapolis, MN), IGF-1 (Peprotech, Rocky Hill, NJ), and FGF-2 (Peprotech, Rocky Hill, NJ). The effects of three different concentrations (none, low, high) were assessed for each growth factor. For TGF-β3 and FGF-2, the low and high concentrations were chosen as 1 and 10 ng/mL, respectively. Low and high concentrations of BMP-2 and IGF-1 were chosen as 10 and 100 ng/mL. Growth factor concentrations were selected based on previous studies of MSC chondrogenesis.6,10,32,35 The chondrogenic and proliferative effects of each growth factor was assessed alone and in combinations of two, three, and four growth factors at all dosages for a total number of 81 groups (n = 16 pellets per group). To carry out this study, MSCs were pelleted in 384-well plates as described above with 10,000 cells in 30 μL CM−. Growth factors were dispensed via automation at 5 μL volumes to achieve desired concentrations in 50 μL. CM− medium (5–20 μL) was dispensed into wells containing three or fewer growth factors to bring the final volume per well to 50 μL. Papain digestion was executed as described above. An initial analysis of GAG deposition indicated that for certain combinations of growth factors, the amount of GAG accumulated exceeded the measurable range when assayed using our standard volume ratios. Therefore, the DMMB assay was performed by combining 5 μL of digestate with 80 μL of DMMB dye. The DNA assay was performed via automation as described above.
NINDS Library Screen
After confirming assay sensitivity in the 384-well culture format and showing robustness of chondrogenesis in the presence of DMSO, HTS was performed using the NINDS library (1040 compounds, listed at http://www.msdiscovery.com/ninds.html) of small molecules. To execute this study, micro-pellets were formed as above in conical 384-well plates with 10,000 cells in 45 μL of CM− or CM+ media. Compounds from the NINDS library were prepared by diluting 10 mM stock solutions in CM−. Diluted compound solutions (5 μL) were added to achieve a final concentration of 10 μM in 50 μL (0.1% final DMSO concentration). Media only (CM−) and cell only (in CM− or CM+) controls were maintained in each plate. Papain digestion and GAG assays were performed via automation as described above. Possible inducers of chondrogenesis (CM− “hits”) were identified by selecting values above a threshhold (150% of average CM− control values) in CM−. Possible inhibitors (CM+ “hits”) were identified by selecting values below a threshhold (50% of average CM+ control values) in CM+. DNA content of the identified inhibitors was assessed to identify compounds that were cytotoxic rather than anti-chondrogenic. Compounds that reduced DNA content by more than 40% compared to CM+ control values were considered cytotoxic.
Statistics
Statistical analysis was performed using SYSTAT software (v10.2, SYSTAT Software Inc., San Jose, CA). For the following studies, data was analyzed via two-way ANOVA with significance set at p < 0.05. For cell pellet minimization studies, pellet size and media condition were the independent variables. For IL-1β inhibition studies, IL-1β concentration and media condition were the independent variables. For DMSO sensitivity studies, DMSO concentration and media condition were set as the independent variables. Where significance was indicated by ANOVA analysis, Tukey’s posthoc testing was carried out to enable comparisons between groups. For the growth factor combinations study, one-way ANOVA was performed. Due to the large number of conditions, where significance was indicated by ANOVA analysis, posthoc testing was performed with Bonferroni corrections and significance set at p < 0.05. Assay sensitivities in 96-well and 384-well formats were assessed via Z-factor analysis.46
Results
Minimization of Cell Number and Media Exchange
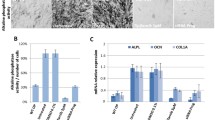
In order for HTS to be conducted practically and efficiently, screening assays must use a minimum number of cells and limited handling steps. We therefore optimized a standard MSC chondrogenesis assay for HTS, using GAG content as the measure for chondrogenesis. In these optimization studies, GAG content was dependent on both cell pellet size (p < 0.001) and the presence of TGF-β3 in the media (p < 0.001). Increasing cell number/pellet increased GAG content of pellets maintained in either media with (CM+) or without (CM−) TGF-β3 (Fig. 2a). Above 15,000 cells/pellet, differences in GAG became appreciable, with higher levels in CM+ pellets compared to CM− (p < 0.05). Normalizing GAG content to cell number showed that deposition was most efficient in CM+ at 30,000 cells/pellet or lower, regardless of media changes (Fig. 2b). At 30,000 cells/pellet, the assay Z-factor was 0.6 (excellent). Expression of aggrecan and collagen II mRNAs by quantitative rt-PCR showed at least 4- and 10-fold increases, respectively, in CM+ compared to CM− at both sizes, confirming MSC chondrogenesis at the molecular level (Fig. 2c, d).
GAG deposition and cartilage gene expression on day 7 with variation in pellet size, media exchange, and media condition. (a) GAG content with increasing pellet size (no media change). (b) GAG content normalized to cell number. CM− or CM+ media (white or black markers) were changed twice, once, or not at all (circles, diamonds, or squares); increasing stars indicate greater than previous cell number within medium type (p < 0.05), # lower than CM+ of corresponding pellet size (p < 0.001), n = 3. (c) Aggrecan and (d) type II collagen gene expression of large (225,000 cells/pellet) and small (30,000 cells/pellet) pellets, n = 3
Optimization of Culture and Analysis for 384-Well Format
To further reduce handling for HTS and enable robotic liquid dispensing, automated layout and analysis procedures were validated in micro-pellets (10,000 cells/pellet in 50 μL of media) in 384-well plates, with GAG content results similar to those found with large (225,000 cells/pellet) and small (30,000 cells/pellet) pellets in 96-well plates. In this 384-well format, GAG content was 62 ± 6 μg/pellet in CM+ compared to 8 ± 3 μg/pellet in CM− (p = 0.00025). Z-factor analysis showed that the assay sensitivity remained excellent (Z = 0.6).
Effect of Combinations of TGF-β3, BMP-2, IGF-1, and FGF-2 on MSC Chondrogenesis
To assess the effects of different growth factors, their combinations and their doses, we carried out an aggressive combinatorial study comprised of four growth factors (TGF-β3, BMP-2, IGF-1, and FGF-2) at three doses (Fig. 3a). When MSC pellets were cultured with a single growth factor, FGF-2, TGF-β3, and BMP-2 at both high and low doses enhanced GAG deposition compared to CM− controls (p < 0.025, Fig. 3b). IGF-1 had no discernible effect on GAG production at either dose (p > 0.13, Fig. 3b). When pellets were cultured with combinations of two growth factors, the effect on matrix accumulation became less clear, although all combinations yielded higher GAG values compared to CM− (p < 0.001). While the high dose of BMP-2 showed a strong combinatorial effect with TGF-β3 and FGF-2 at both doses (p < 0.001), the same effect was not found with the low dose of BMP-2 (p > 0.05, Fig. 3c). Combinations of IGF-1 or FGF-2 with TGF-β3 or BMP-2 yielded mixed results. A low dose of IGF-1 with a high dose of TGF-β3 enhanced GAG deposition (p < 0.001), but IGF-1 had no effect on TGF-β3 activity when TGF-β3 was provided at the low dose (p > 0.4, Fig. 3d). Similarly, a low dose of IGF-1 enhanced the chondrogenic effect of low dose BMP-2 (p < 0.025), but did not enhance high dose BMP-2 activity (p > 0.75). Combinations of low dose TGF-β3 with both doses of FGF-2 improved GAG production compared to the low dose of TGF-β3 alone (p < 0.02); however these values did not reach high dose TGF-β3 levels (p < 0.001, Fig. 3d). Interestingly, combinations of FGF-2 and IGF-1 at any dose significantly increased GAG accumulation compared to FGF-2 alone, IGF-1 alone, or low dose TGF-β3 (p < 0.005, Fig. 3e). With combinations of three or more growth factors, the effects of individual growth factors and their doses on chondrogenesis became more difficult to assess. While combinations involving high dose TGF-β3 with high dose BMP-2 consistently outperformed all other combinations (GAG values greater than 130 μg/pellet), improvements in GAG accrual were observed for other combinations as well (Supplementary Fig. 1). Analysis of DNA content suggests that while FGF-2 increased cell proliferation (p < 0.05) and TGF-β3 enhanced matrix deposition (without increasing cell number, p > 0.25), a high dose of BMP-2 improved both matrix deposition and cell number (p < 0.001, Supplementary Fig. 2).
Growth factor combinatorial screen. (a) Growth factor combinations of TGF-β3 (T), BMP-2 (B), IGF-1 (I), and FGF-2 (F) were assayed in 81 combinations. CM− represents control condition. Each growth factor was given at one of three doses (none, low, high) where indicated by X. (b) GAG content of pellets cultured in the presence of a single growth factor: TGF-β3 (T), BMP-2 (B), IGF-1 (I), or FGF-2 (F) at a low (L) or high (H) dose. GAG content of pellets cultured with combinations of two growth factors: (c) BMP-2 with another growth factor, (d) TGF-β3 with another growth factor, and (e) combinations of FGF-2 and IGF-1. *Greater than control (p < 0.05). +Greater than all other groups within the same dose (p < 0.05)
Sensitivity of MSC pellets to IL-1β and DMSO
A pilot screen using IL-1β, a known inhibitor of chondrogenesis, showed that GAG deposition was dependent on both IL-1β concentration (p < 0.001) and media condition (p < 0.001). Increasing concentrations of IL-1β inhibited GAG production in CM+ (Fig. 4a, b). For smaller pellets (30,000), inhibition was apparent at 0.1 ng/mL IL-1β (p < 0.01), although complete inhibition was not seen until 1.0 ng/mL (compared to CM−, p > 0.29, Fig. 4a). At a concentration of 1.0 ng/mL, GAG deposition was ∼50% of control CM+ values. Based on these results, 50% of control CM+ GAG was chosen as the inhibition threshhold for future screens. Interestingly, large pellets (225,000) were less sensitive to inhibition by IL-1β; GAG deposition decreased from control values at 1.0 ng/mL (p < 0.01) and complete inhibition was only noted at the highest dose (compared to CM−, p > 0.1, Fig. 4b). As expected, GAG content for CM− pellets did not change with addition of IL-1β (p > 0.5, Fig. 4a, b).
GAG accumulation with variation in IL-1β concentration, pellet size and media condition and the effect of DMSO on MSC chondrogenesis. (a) GAG content with increasing concentrations of IL-1β at the 30,000 pellet size. (b) GAG content with increasing concentrations of IL-1β at the 225,000 pellet size. *Lower than 0 ng/mL IL-1β within medium type (p < 0.01), #lower than CM+ within IL-1β concentration (p < 0.001), n = 4. (c) DNA and (d) GAG content of micro-pellets with exposure to graded levels of DMSO. **Indicates lower than 0, 0.1, 0.25% DMSO within the same medium type (p < 0.05). DNA: n = 3; GAG: n = 8
Since chemical compounds are often solubilized in DMSO, the effect of DMSO on chondrogenesis was examined. When exposed to graded concentrations of DMSO, pellets were insensitive to DMSO at levels up to 0.5%, regardless of media condition as assessed by DNA (CM−: p > 0.45, CM+: p > 0.96) and GAG (CM−: p > 0.85, CM+: p > 0.7) contents (Fig. 4c, d). We therefore used 0.5% DMSO as the maximum allowable thresholds for library screens when compounds are dissolved in DMSO.
Identification of Potential Inducers and Inhibitors of Chondrogenesis with NINDS Library Screen
After optimization and validation of our miniaturized culture and assay procedures, a screen of the NINDS library was undertaken to demonstrate the feasibility of HTS for MSC chondrogenesis. From this screen, 5 potential inducers and 39 potential inhibitors of chondrogenesis were identified based on GAG content assays (Fig. 5, full listing provided in Supplementary Fig. 3). Of these potential inhibitors, 15 were cytotoxic at the doses investigated, lowering DNA contents by 40–80% compared to control CM+ values (Fig. 6). The remaining 24 compounds were selected as viable “hits” (Table 1). With a Z-factor of 0.5, assay sensitivity remained excellent for this screen.
Identification of cytotoxic compounds from NINDS library screen. GAG (μg/pellet) and DNA (% deviation from control) content of identified inducers and inhibitors of chondrogenesis. Compounds with DNA content greater than 40% below CM+ control values were considered cytotoxic and excluded from further analysis
Discussion
Mesenchymal stem cells from bone marrow are an attractive cell source for regenerative medicine and the study of skeletal development. MSCs can differentiate into a number of relevant phenotypes, as well as transition from one phenotype to another.24,33 Despite considerable interest in MSC chondrogenesis, the signal transduction and molecular mechanisms that underlie this process remain largely undefined. Furthermore, while defined media formulations have been developed to induce this phenotype, these media have yet to be optimized with respect to the functional capacity of the formed tissue. For example, using standard media formulations (containing TGF-β3 and dexamethasone), MSCs in 3D culture systems produce cartilaginous tissues with lower properties than donor matched chondrocytes maintained similarly.22 To explore the signaling topology that underlies chondrogenesis, as well as to discover novel modulators of this process, we developed and optimized a high-throughput screening methodology compatible with MSC chondrogenesis. In traditional pellet studies, chondrogenesis is evaluated after 21 days of culture using sizes of 200–250,000 cells/pellet. Here, we successfully reduced the number of cells required to 10,000 per pellet and assessed chondrogenesis after 7 days. Further, we developed an in-well digestion protocol to enable high-throughput analysis and minimize handling (Fig. 1). We also demonstrated our ability to fully automate the layout, culture, and analysis of cartilage matrix production by MSCs in a 384-well format with Z-factor analysis confirming the excellent sensitivity of these miniaturized growth and assay procedures.
After validation of our assay system, we executed a comprehensive combinatorial screen of four growth factors: TGF-β3, BMP-2, IGF-1, and FGF-2. While previous studies have analyzed the effects of these factors on chondrogenesis, there has been no study assessing all combinations of these growth factors using multiple doses in a single experiment. Over a 7-day culture period, treatment with TGF-β3, BMP-2, and FGF-2 improved matrix production compared to control, while treatment with IGF-1 failed to induce chondrogenesis. Our results also showed an enhanced effect when high doses of TGF-β3 and BMP-2 were given in concert. These findings are consistent with several previous studies of growth factor effects on MSC chondrogenesis.16,20,26,31,35 Although the chondrogenic effects of TGF-β3 and BMP-2 are well characterized, the effects of FGF-2 and IGF-1 are less established. In our study, short-term pellet culture with FGF-2 increased cell proliferation when given alone or with other growth factors. In monolayer studies, FGF-2 increased cell proliferation and enhanced the chondrogenic potential of MSCs.15,34 While IGF-1 has been shown to improve chondrogenesis when given with TGF-β3,16 our results were less decisive. Interestingly, combinations of IGF-1 and FGF-2 appeared to increase GAG deposition, independent of dosage.
In addition to our growth factor screen, we conducted an HTS campaign on the NINDS library (1040 compounds) and identified several potential inducers and inhibitors of chondrogenesis (Table 1). Inducers were from several different classes of molecules, including hypnotic agents and vasodilators. Inhibitors were largely anti-neoplastic or anti-protein synthesis agents. To eliminate cytotoxic inhibitors, the threshold for DNA content was set at 40% below control values. Dosage response studies will be necessary to determine whether inhibitory effects will be retained with minimal cytotoxicity at lower doses. Interestingly, of the identified inhibitors, several compounds, including those that altered sodium/potassium levels (ouabain and valinomycin), inhibited protein synthesis (puromycin and cyclohexamide), or inhibited microtubule formation (colchicine and vinblastine), have previously been shown to block sulfate incorporation into GAGs in embryonic chick cartilage.1,4 These results reaffirm our ability to identify specific inhibitors and inducers of chondrogenesis. Unexpectedly, glucosamine hydrochloride, an amino monosaccharide with possible anti-arthritic properties,30 was also identified as a potential inhibitor of MSC chondrogenesis. While the benefits of glucosamine as a therapeutic agent for osteoarthritis remains controversial,38 in one study of human MSC pellets, treatment with 100 μM of glucosamine enhanced chondrogenesis while treatment with 10 mM significantly inhibited chondrogenesis.9 However, the pellet size used in these experiments (1.5 M cells per pellet) was 150 times greater than the size used in our screen (10,000 cells per pellet). Our validation studies with IL-1β suggest that smaller pellet sizes may be more sensitive to inhibition, perhaps owing to decreased diffusional distances. Therefore, while 10 μM of glucosamine was inhibitory in our miniaturized pellets, a lower dose may prove to be beneficial.
The ability to effectively screen large numbers of compounds and uncover both inducers and inhibitors of chondrogenesis is a key strength of HTS. From this initial screen of a small chemical library, we were able to identify several known effectors of chondrogenesis and GAG production. Moreover, we identified several modulators whose actions on MSC chondrogenesis had been previously unknown. While inducers of chondrogenesis are of clear utility for tissue engineering applications, they may also possess chondroprotective properties useful for direct systemic treatment of osteoarthritis. Similarly, inhibitors of chondrogenesis may be of clinical interest as therapeutic agents for skeletal pathologies, such as fibrodysplasia ossificans progressive (FOP). In FOP, progenitor cells in the soft tissues undergo chondogenesis and ultimately osteogenesis, forming ectopic bone in an endochondral fashion. In addition to discovering novel modulators of chondrogenesis, we were also able to identify compounds widely used in orthopaedic practice that displayed no effect on chondrogenesis. These compounds include common antibiotics (vancomycin, ciprofloxacin, and gentamicin), non-steroidal anti-inflammatory agents (rofecoxib and celecoxib) and corticosteroids (cortisone and methylprednisolone). Interestingly, while ciprofloxacin did not affect chondrogenesis, at high doses it has been shown to inhibit fracture healing in rats and inhibit proliferation and extracellular mineralization by osteoblastic cells.14 Vancomycin has also been shown to reduce cell proliferation in chondrocytes and osteoblasts; however, these effects were only evident at doses exceeding 2000 μg/ml.3 Although we were able to identify several inducers and inhibitors of chondrogenesis, secondary screening tools must be developed to confirm our findings and eliminate false positives.
This work describes a new method for assaying MSC chondrogenesis using an HTS approach and presents findings from a preliminary chemical screen and combinatorial growth factor study. While the results are exciting, several limitations remain. In terms of methodology, we focused on the DMMB assay of GAG production as a primary screening tool. GAG is a sensitive and cost-effective (0.01 ¢ per well) measure of chondrogenesis. However, in this setup, we capture all GAG produced by MSCs over the time course of the study, rather than that which is fixed within the ECM and most important for tissue formation. Future work may discriminate between soluble GAG and fixed GAG by assaying the media prior to papain digest. Additionally, more sensitive assays will be required to confirm early-stage chondrogenesis at the molecular level in identified compounds of interest. For example, we have previously used both aggrecan and type II collagen promoter-reporter assays to monitor cartilaginous gene expression in differentiating MSCs,21 and these assays may be adapted for secondary screening in this HTS format. These assays are more expensive, but will serve as a specific indicator of the chondrogenic state. On a mechanistic level, it has recently been reported that centrifugation is not necessary for pellet formation40; removal of this step might further decrease processing time for our anticipated larger library screens. In our studies, we observed a natural heterogeneity in response between the different donor MSC populations investigated. While differences with chondrogenic induction in all screens remain robust (Z-factors > 0.5), as we transition from bovine to human sources, these differences will have to be carefully monitored. Additionally, we only assayed a single dose for the chemical factors in the library screen. As with all library screening, this allows for the existence of false-negatives—factors that are in fact inducers or inhibitors that fail to present based on improper dosing. Additional assays at higher doses may be performed to account for this limitation. Finally, these studies were executed over a relatively short duration of 7 days. By focusing our screen for factors that induce chondrogenesis, we may fail to recognize late-stage modulators of this process that may prove valuable for tissue engineering efforts. To implement longer culture durations, the protocol may be tailored to include partial media exchanges, with half the volume of media replaced once a week, to avoid aspiration of cell pellets during the exchange.
Conclusions
The results of this study demonstrate for the first time that HTS approaches can be used to screen large molecular libraries for modulators of MSC chondrogenesis. Furthermore, by fully automating the layout and analysis techniques, as well as reducing pellet size to 10,000 cells/pellet, we are now poised to screen even larger chemical libraries, such as the >200,000 compound NIH Small Molecule Repository, which contains many factors of chemical interest but unknown action. Newly identified modulators of chondrogenesis may find use in tissue engineering as well as in the treatment of musculoskeletal pathologies. For example, it has been reported that MSCs from osteoarthritic (OA) patients are themselves less robust than those from healthy patients of the same age.25 Factors that improve MSC activity in these diseased cells may prove beneficial to this large and growing OA population. For skeletal pathologies like FOP, factors may be identified that block the promiscuous chondrogenesis that occurs in these cells, while not adversely impacting the natural chondrogenic events that are necessary for skeletal growth in this young population. In the same way that microarray technologies allow for unbiased discovery of new genes involved in a specific cellular process, HTS identifies novel modulators of biochemical action. Using this HTS approach coupled with large chemical libraries, new modulators of chondrogenesis can be identified and the signaling topology of this important event in cartilage and bone formation will be better understood. These advances will improve the clinical application of MSCs for cartilage formation for regenerative medicine applications as well as for the treatment of skeletal pathologies.
References
Adamson L. F., C. S. Anast 1966 Amino acid, potassium, and sulfate transport and incorporation by embryonic chick cartilage: the mechanism of the stimulatory effects of serum. Biochim. Biophys. Acta 121(1):10–20
Angele P., R. Kujat, M. Nerlich, J. Yoo, V. Goldberg, B. Johnstone 1999 Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng. 5(6):545–554 doi:10.1089/ten.1999.5.545
Antoci V. Jr., C. S. Adams, N. J. Hickok, I. M. Shapiro, J. Parvizi 2007 Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin. Orthop. Relat. Res. 462, 200–206 doi:10.1097/BLO.0b013e31811ff866
Audhya T. K., K. D. Gibson 1976 Effects of medium composition and metabolic inhibitors on glycosaminoglycan synthesis in chick embryo cartilage and its stimulation by serum and triiodothyronine. Biochim. Biophys. Acta 437(2):364–376
Awad H. A., Y. D. Halvorsen, J. M. Gimble, F. Guilak 2003 Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 9(6):1301–1312 doi:10.1089/10763270360728215
Barry F., R. E. Boynton, B. Liu, J. M. Murphy 2001 Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 268(2):189–200. doi:10.1006/excr.2001.5278
Brown P. D., P. D. Benya 1988 Alterations in chondrocyte cytoskeletal architecture during phenotypic modulation by retinoic acid and dihydrocytochalasin b-induced reexpression. J. Cell Biol. 106(1):171–179. doi:10.1083/jcb.106.1.171
Caplan, A. I. 1991 Mesenchymal stem cells. J. Orthop. Res. 9(5):641–650 doi:10.1002/jor.1100090504
Derfoul A., A. D. Miyoshi, D. E. Freeman, R. S. Tuan 2007 Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits mmp-13 expression and matrix degradation. Osteoarthr. Cartil. 15(6):646–655 doi:10.1016/j.joca.2007.01.014
Estes B. T., A. W. Wu, F. Guilak 2006 Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 54(4):1222–1232 doi:10.1002/art.21779
Farndale R. W., D. J. Buttle, A. J. Barrett 1986 Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 883(2):173–177
Huang C. Y., K. L. Hagar, L. E. Frost, Y. Sun, H. S. Cheung 2004 Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22(3):313–323. doi:10.1634/stemcells.22–3-313
Huang A. H., M. Yeger-McKeever, A. Stein, R. L. Mauck 2008 Tensile properties of engineered cartilage formed from chondrocyte- and msc-laden hydrogels. Osteoarthr. Cartil. 16:1074–1082
Huddleston P. M., J. M. Steckelberg, A. D. Hanssen, M. S. Rouse, M. E. Bolander, R. Patel 2000 Ciprofloxacin inhibition of experimental fracture healing. J. Bone Joint Surg. Am. 82(2):161–173
Im G. I., N. H. Jung, S. K. Tae 2006 Chondrogenic differentiation of mesenchymal stem cells isolated from patients in late adulthood: the optimal conditions of growth factors. Tissue Eng. 12(3):527–536. doi:10.1089/ten.2006.12.527
Indrawattana N., G. Chen, M. Tadokoro, L. H. Shann, H. Ohgushi, T. Tateishi, J. Tanaka, A. Bunyaratvej 2004 Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem. Biophys. Res. Commun. 320(3):914–919. doi:10.1016/j.bbrc.2004.06.029
Kafienah W., S. Mistry, M. J. Perry, G. Politopoulou, A. P. Hollander 2007 Pharmacological regulation of adult stem cells: chondrogenesis can be induced using a synthetic inhibitor of the retinoic acid receptor. Stem Cells 25(10):2460–2468 doi:10.1634/stemcells.2007-0059
Li W. J., R. Tuli, X. Huang, P. Laquerriere, R. S. Tuan 2005 Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 26(25):5158–5166 doi:10.1016/j.biomaterials.2005.01.002
Longobardi L., L. O’Rear, S. Aakula, B. Johnstone, K. Shimer, A. Chytil, W. A. Horton, H. L. Moses, A. Spagnoli 2006 Effect of igf-i in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of tgf-beta signaling. J. Bone Miner. Res. 21(4):626–636. doi:10.1359/jbmr.051213
Majumdar M. K., E. Wang, E. A. Morris 2001 Bmp-2 and bmp-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of il-1. J. Cell Physiol. 189(3):275–284. doi:10.1002/jcp.10025
Mauck R. L., B. A. Byers, X. Yuan, R. S. Tuan 2007 Regulation of cartilaginous ecm gene transcription by chondrocytes and mscs in 3d culture in response to dynamic loading. Biomech. Model Mechanobiol. 6(1–2):113–125. doi:10.1007/s10237-006-0042-1
Mauck R. L., X. Yuan, R. S. Tuan 2006 Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 14(2):179–189. doi:10.1016/j.joca.2005.09.002
McMahon L. A., P. J. Prendergast, V. A. Campbell 2008 A comparison of the involvement of p38, erk1/2 and pi3k in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 368(4):990–995
Mueller M. B., R. S. Tuan 2008 Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 58(5):1377–1388. doi:10.1002/art.23370
Murphy J. M., K. Dixon, S. Beck, D. Fabian, A. Feldman, F. Barry 2002 Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 46(3):704–713. doi:10.1002/art.10118
Palmer G. D., A. Steinert, A. Pascher, E. Gouze, J. N. Gouze, O. Betz, B. Johnstone, C. H. Evans, S. C. Ghivizzani 2005 Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol. Ther. 12(2):219–228. doi:10.1016/j.ymthe.2005.03.024
Penick K. J., L. A. Solchaga, J. F. Welter 2005 High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques 39(5):687–691
Pittenger M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, D. R. Marshak 1999 Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147. doi:10.1126/science.284.5411.143
Prockop D. J. 1997 Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276(5309):71–74. doi:10.1126/science.276.5309.71
Reginster J. Y., R. Deroisy, L. C. Rovati, R. L. Lee, E. Lejeune, O. Bruyere, G. Giacovelli, Y. Henrotin, J. E. Dacre, C. Gossett 2001 Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 357(9252):251–256. doi:10.1016/S0140-6736(00)03610-2
Schmitt B., J. Ringe, T. Haupl, M. Notter, R. Manz, G. R. Burmester, M. Sittinger, C. Kaps 2003 Bmp2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation 71(9–10):567–577. doi:10.1111/j.1432-0436.2003.07109003.x
Solchaga L. A., K. Penick, J. D. Porter, V. M. Goldberg, A. I. Caplan, J. F. Welter 2005 Fgf-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell Physiol. 203(2):398–409. doi:10.1002/jcp.20238
Song L., R. S. Tuan 2004 Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 18(9):980–982
Stewart A. A., C. R. Byron, H. Pondenis, M. C. Stewart 2007 Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am. J. Vet. Res. 68(9):941–945. doi:10.2460/ajvr.68.9.941
Toh W. S., H. Liu, B. C. Heng, A. J. Rufaihah, C. P. Ye, T. Cao 2005 Combined effects of tgfbeta1 and bmp2 in serum-free chondrogenic differentiation of mesenchymal stem cells induced hyaline-like cartilage formation. Growth Factors 23(4):313–321. doi:10.1080/08977190500252763
Tuli R., M. R. Seghatoleslami, S. Tuli, M. S. Howard, K. G. Danielson, R. S. Tuan 2002 P38 map kinase regulation of ap-2 binding in tgf-beta1-stimulated chondrogenesis of human trabecular bone-derived cells. Ann. N. Y. Acad. Sci. 96, 172–177
Tuli R., S. Tuli, S. Nandi, X. Huang, P. A. Manner, W. J. Hozack, K. G. Danielson, D. J. Hall, R. S. Tuan 2003 Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves n-cadherin and mitogen-activated protein kinase and wnt signaling cross-talk. J. Biol. Chem. 278(42):41227–41236. doi:10.1074/jbc.M305312200
Verbruggen G. 2006 Chondroprotective drugs in degenerative joint diseases. Rheumatology (Oxford) 45(2):129–138. doi:10.1093/rheumatology/kei171
Walters W. P., M. Namchuk 2003 Designing screens: how to make your hits a hit. Nat. Rev. Drug Discov. 2(4):259–266. doi:10.1038/nrd1063
Welter, J. F., L. A. Solchaga, and K. J. Penick. Simplification of aggregate culture of human mesenchymal stem cells as a chondrogenic screening assay. Biotechniques 42(6):732, 734–737, 2007
Woods A., F. Beier 2006 Rhoa/rock signaling regulates chondrogenesis in a context-dependent manner. J. Biol. Chem. 281(19):13134–13140. doi:10.1074/jbc.M509433200
Woods A., G. Wang, F. Beier 2005 Rhoa/rock signaling regulates sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 280(12):11626–11634. doi:10.1074/jbc.M409158200
Woods A., G. Wang, H. Dupuis, Z. Shao, F. Beier 2007 Rac1 signaling stimulates n-cadherin expression, mesenchymal condensation, and chondrogenesis. J. Biol. Chem. 282(32):23500–23508. doi:10.1074/jbc.M700680200
Wu X., S. Ding, Q. Ding, N. S. Gray, P. G. Schultz 2002 A small molecule with osteogenesis-inducing activity in multipotent mesenchymal progenitor cells. J. Am. Chem. Soc. 124(49):14520–14521. doi:10.1021/ja0283908
Yu P. B., C. C. Hong, C. Sachidanandan, J. L. Babitt, D. Y. Deng, S. A. Hoyng, H. Y. Lin, K. D. Bloch, R. T. Peterson 2008 Dorsomorphin inhibits bmp signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4(1):33–41. doi:10.1038/nchembio.2007.54
Zhang J. H., T. D. Chung, K. R. Oldenburg 1999 A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4(2):67–73. doi:10.1177/108705719900400206
Zhang Z., J. Messana, N. S. Hwang, J. H. Elisseeff 2006 Reorganization of actin filaments enhances chondrogenic differentiation of cells derived from murine embryonic stem cells. Biochem. Biophys. Res. Commun. 348(2):421–427. doi:10.1016/j.bbrc.2006.07.073
Zhao Y., S. Ding 2007 A high-throughput sirna library screen identifies osteogenic suppressors in human mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 104(23):9673–9678. doi:10.1073/pnas.0703407104
Acknowledgments
This work was supported by the Center for FOP and Related Disorders, the National Institutes of Health (RO3 AR053668), and an NSF graduate research fellowship (AHH). Additional support was provided by the Penn Institute for Regenerative Medicine (IRM) and the Chemical Biology in Translation (CBIT) program. The authors also thank Dr. Gwo-Chin Lee for helpful discussions regarding pharmaceuticals commonly used in orthopaedic procedures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, A.H., Motlekar, N.A., Stein, A. et al. High-Throughput Screening for Modulators of Mesenchymal Stem Cell Chondrogenesis. Ann Biomed Eng 36, 1909–1921 (2008). https://doi.org/10.1007/s10439-008-9562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9562-4