Abstract
Although tissue engineering of the temporomandibular joint (TMJ) structures is in its infancy, tissue engineering provides the revolutionary possibility for treatment of temporomandibular disorders (TMDs). Recently, several reviews have provided a summary of knowledge of TMJ structure and function at the biochemical, cellular, or mechanical level for tissue engineering of mandibular cartilage, bone and the TMJ disc. As the TMJ enables large relative movements, joint lubrication can be considered of great importance for an understanding of the dynamics of the TMJ. The tribological characteristics of the TMJ are essential for reconstruction and tissue engineering of the joint. The purpose of this review is to provide a summary of advances relevant to the tribological characteristics of the TMJ and to serve as a reference for future research in this field. This review consists of four parts. Part 1 is a brief review of the anatomy and function of the TMJ articular components. In Part 2, the biomechanical and biochemical factors associated with joint lubrication are described: the articular surface topology with microscopic surface roughness and the biomechanical loading during jaw movements. Part 3 includes lubrication theories and possible mechanisms for breakdown of joint lubrication. Finally, in Part 4, the requirement and possibility of tissue engineering for treatment of TMDs with degenerative changes as a future treatment regimen will be discussed in a tribological context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Joints are formed between bones during the growth of the skeleton.122 These so-called diarthrodial joints or synovial joints allow various degrees of relative motion of the bones produced by surrounding muscle forces.175 The bone ends come together within a fibrous joint capsule. The inner lining of this joint capsule is a metabolically active tissue, known as the synovium. The ends of the bones are covered by a thin and highly deformable layer of dense connective tissue known as articular cartilage.175 There are two types of articular cartilage; hyaline cartilage and fibrocartilage. The joint cavity, formed by the cartilaginous surfaces and the synovium, is filled with a small amount of synovial fluid. Ligaments, tendons, and other soft tissues inside and outside the joint cavity give stability to the joint and maintain the proper alignment of the articulating bone ends during motion.175 Daily activity accompanies joint motion, resulting in joint loads. These loads must be sustained by these biological bearings, the diarthrodial joints, with tribological characteristics such as friction, lubrication, and wear.114
The temporomandibular joint (TMJ) is one of the diarthrodial synovial joints in human body. Like other synovial joints, the TMJ enables large relative movements between separate bones.61,128 A dense fibrocartilaginous articular disc is located between the bones in each TMJ. The TMJ disc divides the joint cavity into two compartments (superior and inferior) and is a structure with an important functional role. The disc provides a largely passive movable articular surface accommodating the translatory movement made by the condyle. In fact, the condyle undertakes translatory as well as rotary movement and therefore the human TMJ is also described as a synovial sliding-ginglymus joint.
Since the fibrocartilage covering both the TMJ condyle and articular eminence is avascular, intra-articular synovial fluid provides nourishment to these fibrocartilage cells, which also have limited ability for self-repair.15,56,158 The fibrocartilaginous nature of the TMJ disc and articular cartilage, along with the lubrication function of the intra-articular synovial fluid, allow the cartilaginous structures of the TMJ to conform under function and ensure that loads are absorbed and spread over larger contact areas.43,120,133,165,166
Like other synovial joints, the articular surfaces of the TMJ are highly incongruent. Due to this incongruency, the contact areas of the opposing articular surfaces in the absence of the TMJ disc would be very small, and upon joint loading this would lead to large peak loads and friction. The presence of the TMJ disc, articular cartilage, and synovial fluid in this joint is believed to prevent these peak loads,43,120,133,165,166 as the TMJ disc is capable of deforming and adapting its shape to that of the articular surfaces. During jaw movement, the disc moves with respect to both the mandibular condyle and the articular eminence. When the disc slides along the articular surfaces, shear loading of the disc has been considered to be negligible, due to very low friction.119 Unfortunately, the pristine structures of the articular surfaces often deteriorate with aging, internal derangement, and arthritis, becoming increasingly roughened and eroded, with development of pain and dysfunction, and progressing to osteoarthritis (OA). Internal derangement of the TMJ is defined as an abnormal positional relationship of the disc relative to the mandibular condyle and the articular eminence, and is classified in terms of a series of five stages of increasing severity.179 It should be noted that internal derangement frequently precedes the onset of TMJ–OA. The process of TMJ–OA is characterized by degenerative joint changes such as deterioration and abrasion of articular cartilage and disc surfaces, and occurrence of thickening and remodeling of the underlying bone. These could lead to a reduction in boundary lubrication between the articular surfaces, resulting in an increase of the frictional coefficient.120 As a consequence, joint lubrication can be considered of great importance for an understanding of the dynamics of the TMJ. More information about the tribological characteristics of the TMJ are essential for reconstruction and tissue engineering of the joint.
To date, our understanding of diarthrodial joint lubrication is based on knowledge of the structural and deformational characteristics of articular cartilage, the biochemical and biorheological properties of synovial fluid, the topological design and microscopic roughness of the articulating surfaces of the joint, the kinematics of the joints, and the subsequent biomechanical loading on the articular surfaces. This review is divided into four parts. Part 1 will review the anatomy and function of the TMJ articular components, articular cartilage, disc, and synovial fluid. Part 2 will discuss the biomechanical and biochemical factors associated with regulation of joint lubrication: the articular surface topology with microscopic surface roughness and the biomechanical loading during jaw movements. Part 3 includes lubrication theories (boundary lubrication and fluid film lubrication) and possible mechanisms for breakdown of joint lubrication. Finally, in Part 4, the requirement and possibility of artificial replacements and tissue engineering for treatment of TMJ–OA with degenerative changes will be discussed.
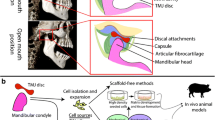
Part 1: Anatomy and Function of Articular Components in the TMJ (Fig. 1)
TMJ Disc
The TMJ disc is composed of variable amounts of cells and extracellular matrix. It is noteworthy that the characterization data in the literature are derived from a number of animal species (especially rats, rabbits, dogs, cows, and pigs) in addition to humans,42 and thus inherent interspecies differences are reflected in differences in data sets among related studies. The extracellular matrix is composed of macromolecules and fluid. The macromolecules comprise about 15–35% of the wet weight of the disc, while the tissue fluid comprises about 65–80%.48,117,118,148 The dry weight of the TMJ disc consists mainly of collagen (68–83%) and proteoglycans (0.6–10%).13,19,48,117,118,148 The cells of the TMJ disc are a heterogeneous combination of fibrochondrocytes and fibroblast-like cells, which are distinctly different from chondrocytes of hyaline cartilage 26,47,90,105,106,108,128,133 (Fig. 1).
(a) Side view of the human skull by means of CT images. (b) Sagittal views of the TMJ. The TMJ consists of the bone components (mandibular condyle and articular eminence) and soft tissues (condylar cartilage, fossa cartilage, joint capsule, articular disc, and retrodiscal tissue). In the healthy joint, the articular disc moves forward and downward when the mandibular condyle moves along the posterior slope of the articular eminence (mouth opening). Both at closing and opening position, the disc is located between the two bone components. The articular surfaces are covered with thin fibrous layers. Synovial fluid inside the joints acts as a lubricant during movement
Collagen fibers maintain the shape of the disc, while elastin is associated with restoration of shape during unloading.133 Collagen fibers commonly exhibit waviness (“crimping”). When tension is applied to the disc, the first effect is to straighten the crimp; accounting for the initial toe region of the curve.25,133,155 Beyond this initial phase, the collagen fibers begin to extend and become tensile load-bearing. When further loaded, the collagen network deforms and water is squeezed out of the disc while the orientation of the collagen fibers is rearranged.25 The rearrangement of the collagen fibers is reversible when the disc is not deformed beyond the physiologic strain range. This enables the disc to continuously adapt its shape to fit in the space between the opposing articular surfaces and to suitably distribute loads in the TMJ. Collagen gives the disc much of its tensile stiffness and strength. The thin surface layers of the disc have a different architecture from the thick inner layer.134 In the superior and inferior surface layers, the collagen fibers are more or less perpendicularly arranged in an anteroposterior and mediolateral direction.107 In the inner layer, the orientation of collagen fibers varies markedly in different regions of the disc. The fibers run primarily anteroposteriorly in the intermediate zone and mediolaterally in the anterior and posterior bands. The anteroposterior fibers from the intermediate zone are interlaced with the mediolateral fibers in both bands.168 In the central region of the bands, the fibers from the intermediate zone flare superiorly and inferiorly and turn medially and laterally, merging structurally with those of the bands.106,133 In the medial and lateral regions of the disc, near the condylar poles, the anteroposterior fibers of the intermediate zone are attached tightly to the poles of the condyle.168 These differences in collagen fiber orientation are associated with the regional differences and anisotropy in the mechanical properties of the disc as described afterwards.
Proteoglycans are enmeshed in the network of collagen fibers and are virtually immobile. Several proteoglycans are detected in the disc. Biglycan and decorin belong to the group of small proteoglycans, consisting of a core protein of approximately 38 kDa to which either one (decorin) or two (biglycan) chondroitin/dermatan sulfate side-chains are attached.35,52 Aggrecan is a large proteoglycan containing both chondroitin sulfate and keratan sulfate.118 Aggrecan molecules possess high viscosity and large molecular size that reduce their capacity to diffuse through the collagen network, resulting in the retention of large amounts of water.116 The result is a stiff viscoelastic material surrounding the collagen fibers. Because of its molecular structure, aggrecan is ideally suited to resist compressive loading.
Mandibular Condylar Cartilage
The mandibular condyle is covered by a zonal cartilage layer from the articular surface to the underlying bone, which is composed of several zones: the fibrous, proliferative, mature, and hypertrophic zones.96,109 Essentially, the proliferative zone serves as a separating barrier between the fibrocartilaginous fibrous zone and the hyaline-like mature and hypertrophic zones. The fibrous zone is composed of fibroblast-like cells, which have a flat shape and endoplasmic reticulum surrounded by a dense intercellular matrix of collagen fibrils and ground substance.87 The proliferative zone plays an important role as a cell reservoir, which has mesenchymal cells distributed heterogeneously as chondrocyte precursors for the underlying zones.28 Differentiated chondrocytes are found in the mature and hypertrophic zones, where the degeneration of chondrocytes has been noted closer to the subchondral bone.87 The collagen fibers of fibrocartilage are arranged in several distinct zones,37 and provide mainly tensile and shear strength to the cartilage, whereas resistance to compressive forces is due to the presence of proteoglycans.101,155 Regarding the collagen types, collagen type I is found throughout all of the mandibular condylar cartilage zones.40 Collagen type II and X, commonly found in hyaline cartilage, are abundant in the mature and hypertrophic zones.170 When cartilage is loaded by compression, the small permeability of the collagen network impedes interstitial fluid flow through the collagen network.112 These features contribute to the viscoelastic properties of cartilage. In the articular cartilage, collagen forms a three-dimensional network and thus impacts the cartilage form, stability and tensile strength and resistance to shear forces. From MR assessment, the collagen matrix is organized in an arched structure.63 The fibers curve from a radial orientation at the subchondral bone into a tangential orientation at the articular surface.63 Then, on the articular surfaces collagen fibers run in parallel. In the mandibular condylar cartilage, collagen fibers mainly run in the anteroposterior direction,149 which may be an optimized structure concerning resistance to anteroposterior shear forces.
The major proteoglycan in the mandibular condylar cartilage is aggrecan. Aggrecan is mainly located in the hypertrophic and mature zones.101,130 Aggrecan provides osmotic swelling pressure to the cartilage and enables it to resist compressive loads.101,130 Versican and decorin have also been reported in the mandibular condylar cartilage.39,101,130
Synovial Fluid
Synovial fluid is a viscous gel and contains mostly water. This fluid acts as a lubricant in the upper and lower compartments of the TMJ as well as acting as a vehicle for nutrients as it passes through the surface layers of the disc and articular cartilage layers.84,165 The collagen and proteoglycans are dispersed in the fluid, making the cartilage a microporous material with a certain permeability. The mechanical response of the disc to compression depends on the permeability.85 A low permeability means that any significant exchange of fluid between the inside and outside of the disc must take place over a period of time (e.g., minutes) compared to the physiological loading cycle (1 s). As a consequence, the disc maintains its stiffness under compression. In the case of high permeability, a rapid exchange of fluid is possible that results in a substantial decrease in disc stiffness. It should be noted that stress relaxation under compression occurs rapidly in the TMJ disc, with viscoelastic time constants on the order of 5–50 s.8 By comparison, stress relaxation under tension is much slower.44,149
The synovial membrane lines the inner surface of the joint capsule. It contains specialized cell types with phagocytic and immunologic capacity, and produces the synovial fluid that provides the nutritional and metabolic requirements to the avascular tissues of the mandibular condylar and articular eminence fibrocartilage as well as to the disc. It also serves as a joint lubricant.
Hyaluronic acid (HA), 0.14–0.36% of synovial fluid in normal subjects,156 is one of the principal components determining the rheological properties of synovial fluid, especially the viscosity.182 Synovial viscosity depends on both the concentration of HA and its molecular weight.38,88,182 In synovial fluid, HA with high molecular weight released by type B synovial cells is generally believed to be essential for lubrication of joints by reducing friction.22,129 Meanwhile, in joints afflicted with OA, the synovial fluid has reduced viscosity due to the decrease in both concentration and molecular weight of HA84,110 (see Part 2).
Part 2: Biomechanical and Biochemical Factors Associated with Regulation of Joint Lubrication
Microscopic Roughness of the Articular Surfaces
The durability of joints depends primarily on their ability to articulate with low friction and wear, which are reduced by pathologies such as arthritis.51 Fluid films from 0.5–2 μm are required to separate the articular cartilage surfaces with roughnesses on the order of 1 μm.51,81 These films are only achieved with high-viscosity synovial fluid (see “Biochemical Compositions of Lubricant” section). However, mechanisms other than fluid film lubrication are required to protect cartilage over a lifetime of use. For example, high molecular weight proteins, phospholipids and glycoprotein complexes at the cartilage surface may provide a certain level of lubrication and temporary protection. In addition, models have shown that the highly porous nature of cartilage at its surface is capable of maintaining effective lubrication even in the absence of weeping flow, as only about 1% of the total contact area of cartilage layer interactions consists of solid–solid contacts, where friction occurs.151 Therefore, the initial friction coefficient following an applied load is decreased by trapped lubricant at the surface, independent of squeeze-film lubrication effects.151 However, it has been suggested that although compressive stresses prevent the initiation of fissures in a healthy joint, that normal movements in a pathological joint with a thin synovial fluid layer can easily cause fissures.82
A classic overview of surface characteristics of articular cartilage was presented in the mid 1970s.113 To put cartilage roughness dimensions in perspective, typical center line average (CLA) values of surface finishes are 0.05–0.2 μm for superfinishing, 0.1–0.5 μm for polishing, 0.1–2 μm for grinding, and 1–6 μm for milling.127 The CLA is the arithmetical average deviation from the center line, essentially the average roughness height. In comparison, early studies found the CLA of fetal cartilage to be 1 μm, compared to 2.75 μm for 67-year-old cartilage and 5.25 μm for osteoarthritic cartilage.181 Average surface roughnesses of bovine femoral cartilages have been measured on the order of 1 μm by interferometry and laser profilometry.54,60 This roughness was seen to increase from 800 ± 200 to 2100 ± 200 nm when the cartilage was loaded under contact with metal.54 Roughnesses of loaded articular surfaces of rabbit knees were measured at 10–30 nm, which was less than that observed in unloaded joints.36 Interestingly, irregularities of one surface had little or no effect on the contour of the opposite surface due to the fluid-containing space in between.36
The surface roughness of TMJ cartilages has scarcely been studied. Optical profilometry of cadaveric mandibular condyles revealed roughnesses of 30 ± 5 μm for healthy smooth surfaces and 140 ± 9 μm for remodeled condyles.49 Using an atomic force microscope (AFM), the superficial zone of mandibular condyles of 7-day-old rabbit exhibited roughnesses varying from 95 to 130 nm.125
Essentially, there is a cause and effect cycle between surface roughness and joint function, as the roughness will influence friction and wear, and degenerative joint pathologies will adversely affect roughness. In macrosystems, roughness is the main controlling factor of friction.137 Macroscale friction is chiefly the result of mechanical interlock caused by the roughness of the contacting surfaces, whereas nanoscale stick/slips are caused by atomic roughness.137 Adhesion and friction forces have an inverse relationship with roughness, both increasing as roughness decreases.137 In the presence of synovial fluid, the rougher the surface, the smaller the contact area, and decreasing contact area decreases friction due to a lower probability of liquid bridging. However, if synovial fluid is absent, macroscale friction force increases with roughness as a result of mechanical interlock.137
The first study to relate microscale AFM measurements of the friction coefficient of articular cartilage with measurements at the macroscale level found that the microscale AFM friction coefficient correlated well with the macroscale equilibrium friction coefficient, representing the friction response in the absence of cartilage interstitial fluid pressurization.124 However, the articular surface roughness of bovine humeral heads (462 ± 216 nm) was not found to correlate significantly with friction coefficients, as measured with AFM.
Recently, a model was developed for understanding the effects of both surface roughness and couple stresses on synovial joint lubrication.31 It was found that roughness increased load carrying capacity relative to a smooth surface, which was attributed to a rougher surface reducing the leakage of lubricant and increasing the pressure in the film region.
In summary, we are aware that micro-scale roughness and porosity of the articular cartilage surface provide for better lubrication than if the cartilage were a completely solid and smooth surface, and we are also aware that degenerative diseases appear to increase the apparent roughness. This apparent paradox can be explained by the number of other factors that lead to the breakdown of the lubrication system in these pathological cases. For example, a biphasic poroviscoelastic model has shown that a significant increase in permeability results from the absence of the superficial zone, as when damaged or fibrillated from arthritis or impact trauma.147 Moreover, pathological cases are accompanied by a reduction in synovial fluid viscosity181 and the presence of inflammatory cytokines. The micro-scale roughness is an integral part of the functioning tribology of healthy mandibular condylar cartilage, although its contribution in pathological cases is overshadowed by predominant factors that lead to the breakdown of the lubrication mechanisms.
Biomechanical Loading in the TMJ During Jaw Movements
Mandibular motions are divided into continuous and intermittent motion. These motions, sometimes combined together, result in static and dynamic loading in the TMJ, respectively. Static loading occurs, for example, during clenching, grinding, and bruxism; dynamic loading occurs during, for example, talking and chewing. Mechanical loading in the TMJ is necessary for the growth, development and maintenance of the joint tissue. Generally, dynamic loading is likely to lead to an anabolic effect for the joint tissues, while static loading, if prolonged or excessive, induces a catabolic effect. As both sliding and rotating with slightly lateral excursion occur simultaneously between articulating surfaces, the TMJ is subjected to a multitude of different loading regions during mandibular movements. Basically, three types of loading can be distinguished: compression, tension, and shear. Obviously, during natural loading of the joint, combinations of these basic types of loading do occur on the articulating surfaces. During every type of loading, the joint tissues such as articular cartilage and fibrocartilaginous disc undergo a deformation (strain) commensurate with their material properties, while internal forces are produced within the tissue.
Numerous works have focused primarily on calculating the absolute magnitude of TMJ loading with finite element models. Previously reported magnitudes of TMJ loading, however, differ significantly from one another because of different simulated conditions such as jaw geometry and musculature. For this reason, and due to large discrepancies in direct measurements as well, there is currently no universally agreed-upon value of TMJ loading. Our understanding is that the loading distribution produced by masticatory muscle forces during various mandibular movements is largely dependent on the biomechanical properties of the joint tissues, and that these properties are in turn dependent on the loading environment.
As described in our previous reviews,43,165,174 the TMJ disc and condylar cartilage are viscoelastic, being frequency-, region-, direction-, and time-dependent in nature. For evaluation of the basic biomechanical characteristic of a tissue, the elastic modulus or relaxed modulus is commonly used. This elastic modulus is defined as the slope of the elastic region of the stress–strain curve. With respect to the disc, tensile studies have shown that the disc is stiffer and stronger in the anterior and posterior bands than in the intermediate zone in the mediolateral direction.43,169 Furthermore, through its center, the disc is stiffer under tension and shear in the anteroposterior direction than in the mediolateral direction. For example, the tensile modulus of the porcine disc was 76.4 MPa in the anteroposterior direction, whereas it was 3.2 MPa in the mediolateral direction (strain rate, 500 mm/min24). This is due to the orientation of collagen fibers of the disc. Also in shear, the elastic modulus of the disc was about one-third smaller in the mediolateral direction than in the anteroposterior direction.160 Under compression, regional studies of the disc are contradictory, but more evidence suggests that the disc is stiffer in the center than in the periphery.7,43 The resistance to compression is mainly dependent on the density of proteoglycans, especially of the large chondroitin-sulfate molecules. Since the distribution and amount of the proteoglycans are different in various regions of the disc and articular cartilage, regional differences in its compressive modulus can be explained. Although the results of the various studies cannot be easily compared due to the large interspecies variation and different experimental protocols, the compressive modulus of the disc is considered to be smaller than its tensile modulus.
Of the three types of loading, shear loading is the most important from a tribological perspective. Shear can result in fatigue, damage, and irreversible deformation of cartilage.152,183,184 Furthermore, shear stress is associated with a breakdown of joint lubrication through a reduction of HA molecular weight (see Part 3). Previously our works have demonstrated that the shear behavior of the discs was dependent on the frequency and direction of shear load.160,162 In other studies it was reported that the shear stress in cartilage was very sensitive not only to the frequency and direction of the loading, but also to the amount of shear and compressive strain.115,152,183 This implies that the shear stress induced in the disc may be dependent on the compressive strain when the frequency and direction of the shear loading are kept constant. The dynamic shear properties of the disc are anisotropic. That is, the dynamic shear modulus of the disc is significantly larger in anteroposteriorly than in mediolaterally applied shear strain.160 The anisotropic behavior of the disc is mainly dependent on the orientation of collagen fibers as well as the tensile modulus. This implies that upon mediolateral shear deformation the collagen network bears a larger part of the loads and therefore, could be more vulnerable to damage.
The mandibular condylar cartilage is a nonlinear viscoelastic material, as is the TMJ disc. Anisotropy of the mechanical properties in mandibular condylar cartilage is confirmed by greater average tensile strength, tensile stiffness, and energy absorption in the anteroposterior direction than in the mediolateral direction. The reported Young’s moduli in the anteroposterior and mediolateral directions were, respectively, 9.0 and 6.6 MPa.83 Under dynamic compression, the dynamic elastic and viscous moduli were 1.36 and 0.34 MPa at a frequency of 1.0 Hz, respectively.166 Significant regional differences in the dynamic properties were detected, and the anterior area revealed significantly higher moduli than the posterior area.166 These findings were in agreement with the nanoindentation findings of Hu et al.76 The resistance to compression is mainly dependent on the density of proteoglycans, especially of the large chondroitin-sulfate molecules. As the distribution and amount of the proteoglycans are different in various regions of the mandibular condylar cartilage, regional differences in its compressive modulus can be explained. With respect to the dynamic shear modulus in the anteroposterior direction, the dynamic elastic and viscous moduli were, respectively, 1.56 and 0.34 MPa at a frequency of 2.0 Hz164 and these values were almost the same as those in dynamic compression.166 In contrast, the dynamic shear moduli in the mediolateral direction were about 30% of those in the anteroposterior direction (data not published), which implies that the dynamic shear behavior of mandibular condylar cartilage is also anisotropic. As described above, shear loading can induce a breakdown of cartilage. Therefore, the shear characteristics suggest that mandibular condylar cartilage has a weak resistance to mediolateral shear stress, which might lead to degradation of articular cartilage and synovial fluid.
Biochemical Compositions of Lubricant
HA in synovial fluid has been believed to be a crucial factor in articular joint lubrication.30, 150 The rheological property of HA in solution is characterized by remarkably high viscoelasticity.21, 57 High molecular weight HA plays an important role in maintaining the viscoelasticity of synovial fluid, whereas the increase of low molecular weight HA results in a reduction in the viscoelasticity, leading to the deterioration of joint lubrication88 (see Part 3).
HA imparts viscoelastic character to the solution due to its specific structure, which is generally explained as random coil-entanglement. HA is a glycosaminoglycan consisting of repeated disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine and with high molecular weight (800–1900 kDa) in its native state.91 The secondary and tertiary structures of HA in solution have been examined by means of rotary shadowing-electron microscopy143 and NMR.66,145,146 The HA forms reversible and ordered aggregates, extensively branched networks at physiological temperature in solution.143 This dynamic network formation is affected both by its concentration and molecular weight, resulting in changes in its viscoelastic behavior.30,88,111,143,144 Increasing the molecular weight more effectively enhances the HA network formation than the concentration.88 High molecular weight HA strands in solution have no ends, whereas low molecular weight HA forms islands of meshworks under similar conditions.143 The enzymatic digestion of HA resulted in lower stability,145 suggesting that stable intermolecular interactions can be achieved by high molecular weight HA. In contrast, low molecular weight HA disrupts the intermolecular network formation by high molecular weight HA.177
The accumulation of lower molecular weight HA in synovial fluid has been suggested to be due to various mechanisms such as depolymerization with reactive oxygen species (ROS),93,102 enzymatic cleavage123 and newly synthesized low molecular weight HA.167 IL-1β, TNF-α, and IFN-γ are highly distributed in the synovial fluid of joints with degenerative disease such as OA and rheumatoid arthritis (RA).75,138,176 A number of previous in vitro studies supported cytokine-induced HA synthesis, and the accumulation of low molecular weight HA in cultured synoviocytes.32,64,77,89,104 Low molecular weight HA modulates immune or inflammatory processes71,89 and decreases the viscoelasticity of synovial fluid.
Several molecules locally present in synovial fluid have been reported to contribute to joint lubrication, especially boundary lubrication.53,78,132,140 Among them, surface active phospholipids (SAPLs) are considered to be mostly responsible for the boundary lubrication of the articular cartilage surface by reducing the kinetic friction.53,69,132 Surface active phospholipids have been suggested to be coupled with HA under healthy conditions.53,121 Dipalmitoyl phosphatidylcholine (DPPC), the predominant surface active component, synergistically enhanced lubricating ability when mixed with HA.53 In addition, SAPLs are protected by adhesion to the high-molecular weight HA from phospholipase A2 (PLA2), with the SAPL-lysing enzyme secreted in synovial fluid121 (see Part 3).
A mucinous glycoprotein called lubricin, also known as PRG478 or articular cartilage superficial zone protein (SZP),140 is found in the synovial fluid. Lubricin provides boundary lubrication of articular surfaces under high contact pressure and quite low sliding speed.80 Since the boundary lubricant needs to be adsorbed to the surface before it exerts its ability, lubricin may contribute to boundary lubrication as a water-soluble carrier of SAPLs,70,142 although the detailed lubrication mechanism associated with lubricin is still unclear.
From these findings, it is suggested that the various contents of synovial fluid contribute to articular joint lubrication, and maintain the lubrication ability by synergistic interactions. Overloading and subsequent deterioration of these lubricants may cause high friction in joints, resulting in degenerative diseases.
Part 3: Development Theory and Breakdown Mechanism for TMJ Lubrication
Lubrication Theory
A number of physicochemical modes of lubrication occur in synovial joints and have been classified as fluid film and boundary. The former mainly depends on a synovial fluid, and the latter on articular components such as articular cartilage and the fibrocartilaginous disc. One type of fluid-mediated lubrication mode is hydrostatic. At the onset of loading and after a prolonged loading, the interstitial fluid within cartilage becomes pressurized, due to the biphasic nature of the tissue; fluid may also be forced into asperities between articular surfaces through a weeping mechanism.139 Pressurized interstitial fluid and trapped lubricant pools may therefore contribute significantly to the bearing of normal load with little resistance to shear force, facilitating a very low friction coefficient.17 In addition, at the onset of loading and/or motion, squeeze film, hydrodynamic, and elastohydrodynamic types of fluid film lubrication occur, with pressurization, motion, and deformation acting to drive viscous lubricant from and/or through the gap between the two surfaces in relative motion.139 The normal frictional coefficient between the cartilage surfaces of synovial joints is reported to be within a range of 0.001–0.1 (Fig. 2).54,94,97,98 This coefficient may increase due to deterioration in the lubrication mechanism.16,18,94 This mechanism is primarily dependent on the synovial fluid, where HA is considered to be the primary effective constituent.98,141 However, the composition of the lubricant may change upon joint loading, because then it mixes with water, which is exuded out of the cartilaginous tissue when it is compressed.55
(a) Frictional coefficients of the TMJ measured by a pendulum type friction tester. Means and standard deviations of frictional coefficient are provided for the TMJ, as measured in the intact joint and after washing with PBS and scouring with gauze and sandpaper. Double asterisks indicate a significant difference between the groups at 1% of confidence.84,159,161 (b) Frictional coefficients of the TMJ after scouring with gauze, and the effect of the application of HA with different molecular weights and concentration in the TMJ with experimentally reduced lubricating ability. The error bars indicate standard deviations. Double asterisks indicate a significant difference between the groups at 1% of confidence.84,159,161 (C) Microscopic observations of the cartilage surfaces by scanning electron microscopy after PBS washing and scouring with gauze and sandpaper. After PBS washing, the amorphous layer (arrows) still existed. After scouring with gauze, the amorphous layer collapsed in part although the congruent articular surface still existed. After scouring with sandpaper, the amorphous layer was completely disrupted and the inner layer exposed as an irregular surface.84,159,161
A number of studies are available concerning joint lubrication, which strongly suggest that SAPLs provide highly efficient boundary lubrication and act as protectors of the articular surfaces (Fig. 3).68,142 SAPLs are associated with lubricin. According to Nitzan,120 SAPLs are polar lipids that bind to the articular surface by their polar ends, thus orientating their non-polar moieties outward. The latter impart a hydrophobic surface, which has a relatively low surface energy that is much less conductive to friction than the articular surface without SAPLs. The hydrogen bonds between the phospholipid molecules provide excellent cohesion, a factor on which load bearing is dependent.68 In contrast, HA is a multipotential, high molecular weight, viscous component of the synovial fluid that has a negligible load-bearing capacity.79,92 To date, an important indirect role in the lubrication process has been assigned to HA especially with high-molecular-weight. PLA2, which is secreted by the synoviocytes, chondrocytes, and osteoblasts into the synovial fluid, poses a constant threat to the continuity of SAPL layers.173 An in vitro study showed that dose-dependent inhibition of PLA2 activity occurred in the presence of increasing concentration and molecular weight of HA.121 It has also been shown that the high-molecular-weight HA actually adheres to SAPLs, protecting their continuity from lysis by PLA2.121
Concepts of a breakdown of TMJ lubrication. (a) According to Nitzan,120 SAPLs are polar lipids that bind to the articular surface by their polar ends. In the healthy joint, the hydrogen bonds between the phospholipid molecules provide excellent cohesion. PLA2 poses a constant threat to the continuity of SAPL layers, and HA adheres to the SAPLs, protecting their continuity from lysis by PLA2. (b) Overloading may impair the lubrication by biochemical reaction. The excessive loading decreases the blood flow and causes hypoxia in the joint. On reperfusion, xanthine oxidase generates reactive oxygen species (ROS) in the presence of re-supplied oxygen with hypoxanthine as a substance. ROS in joints inhibits the biosynthesis of HA and degrades it. Reduction of HA causes not only a decrease in viscosity of synovial fluid, but also an inability to protect phospholipids from PLA2. (c) On lysis of the SAPLs, the articular surfaces are stripped of their lubricants, and as a result, friction is generated between the exposed surfaces of the disc and articular eminence
Breakdown Mechanism
The major cause of breakdown of the joint lubrication is overloading.55,120,163 The lubrication of synovial joints generally accepted is the multimodal, including fluid film and boundary mechanisms. Fluid film lubrication is the dominant mechanism and joints can withstand dynamic and static loading by this lubricating mechanism as long as the loading is not excessive. However, fluid film lubrication exists only during short periods of overloading. After prolonged overloading, only solid contact may exist between the articular surfaces, and then there is probably no longer any fluid film lubrication but only boundary lubrication.97,163 That is to say, the fluid film lubrication of synovial joints achieves low friction only when the articular surfaces are kept apart; then the lubricating mode is changed to boundary lubrication, because the squeeze film mechanism is disrupted due to the thinning of the fluid film and the solid contact of articular surfaces.
Overloading also impairs lubrication by biochemical reaction. The loading pressure that exceeds the capillary perfusion pressure decreases blood flow and causes hypoxia in the joint.29 Under hypoxia, adenosine triphosphate (ATP) is degraded to hypoxanthine. When the joint is released from the loading pressure, blood flow recovers and oxygen is supplied to intracapsular tissues. On reperfusion, xanthine oxidase generates superoxide in the presence of re-supplied oxygen with hypoxanthine as a substance. This mechanism is hypoxic-reperfusion injury and it explains the production of ROS in the joint on overloading (Fig. 3).29 ROS in joints inhibits the biosynthesis of HA and degrades it.62 Articular surfaces are covered with a phospholipid that is attached to HA as a fluid film, and HA in the joint space protects phospholipids from PLA2. Reduction of HA causes not only a decrease in viscosity of synovial fluid, but also an inability to protect phospholipids from PLA2.121 As a result, the lubrication of the joint breaks down.
Friction in synovial joints is associated with its lubrication mechanism, and breakdown of the joint lubrication increases friction in joints.16,94 It is generally accepted that increased friction in the joint is a major contributing factor in disc displacement.120 Tanaka et al.159,161,163 and Kawai et al.84 investigated the association between friction in the porcine TMJ and the condition of the joint lubrication by using a pendulum-type friction tester. To experimentally mimic pathology, the joint space and disc surface were washed with phosphate-buffered saline (PBS) to replace the synovial fluid with PBS, and the cartilage and disc surface were scoured with PBS gauze or sandpaper to remove the fluid film. The frictional coefficient of the TMJ increased with the duration of loading because the fluid film got thinner and fluid film lubrication was damaged. The more the TMJ was damaged, the more friction in the TMJ was increased. Therefore, it is likely that overloading the TMJ increases the friction by damaging fluid film lubrication, and in the impaired TMJ, friction increases markedly because of reduction in viscosity of the synovial fluid and damage to the fluid film. The articular disc is important to the lubrication. The frictional coefficient of the TMJ was increased significantly by removal of the TMJ disc.159 This means that the friction in the diseased TMJ, especially in which the disc is displaced, is large and the damage of the TMJ may progress to OA under large shear stresses. As described above, HA has an important role in lubrication, but HA in the synovial fluid of the damaged TMJ can be degraded.157 The addition of HA to the damaged TMJ was proven effective in reducing the friction.84,159,161 However, the frictional coefficient did not recover to the level of the intact TMJ.
In a clinical setting, HA injection into the TMJ has been attempted for use as a treatment remedy of TMDs with severe pain and movement disability, although HA injection has not yet been FDA approved for use in the TMJ. In double blind studies in other joints after 2–12 months, HA has been shown to provide significantly better results than saline. However, no significant differences were noted in radiographic progression of the disease.95 In the TMJ, Bertolami et al.27 reported that when using HA in TMJ–OA cases, there were no differences in outcomes among the placebo and saline control group measured variables. Meanwhile, Alpaslan and Alpaslan14 reported that arthrocentesis with the addition of HA revealed superior results compared to that without HA addition, although arthrocentesis both with and without HA provided beneficial results. Consequently, the effectiveness of HA injection into the TMJ is still controversial.
Part 4: Looking to the Future
Requirement and Possibility of Artificial Replacement and Tissue Engineering for TMJ
Joint Lubrication in Tissue Engineering Strategy
It is clear that as a result of a breakdown in the lubrication system of the TMJ the tissues may be irreparably destroyed. While more conservative treatments are preferred when possible, in severe cases or after multiple operations, the current end stage treatment is total joint replacement. There are now long-term studies available in the literature that support the safety and efficacy of total joint replacement under appropriate circumstances.103 The next generation of joint replacements will incorporate live tissues in an effort to reconstruct the joint to its normal state. However, with a few exceptions, orthopedic and craniofacial tissue engineering technology have not yet reached the point where joint lubrication has become an integral part of design strategy. One notable exception is a recent study that proposed a strategy to promote joint lubrication by layering PRG4-secreting superficial zone chondrocytes at the articular surface of an engineered construct.86 In addition, a recent review of tissue engineering and biomechanics in the knee joint by Ateshian and Hung17 highlighted the need to match the material properties of the native articular cartilage in tissue engineering to maintain not only the function, but also the lubrication of the joint as well. In another example of considering joint lubrication in tissue engineering, Sander and Nauman131 have summarized mathematical relationships between microstructure and permeability, with the emphasis that controlling the permeability of a scaffold or engineered construct may assist in providing the desired hydrodynamic lubrication of the joint. Strategies such as these may be essential for successful implementation of engineered constructs. It should be clear from the preceding sections that even with a “good as new” engineered joint, that without a functioning lubrication mechanism the engineered tissues may befall the same fate as the ravaged tissues they replaced.
TMJ Disc Tissue Engineering
The TMJ disc and the mandibular condyle have been the focus of tissue engineering efforts for the TMJ. In the long term, TMJ tissue engineering strategies may need to combine both of these structures into a single implant, perhaps along with other TMJ tissues such as the retrodiscal tissue.46 Thorough reviews of TMJ disc tissue engineering are available in the literature,7,9,42,43,59 which describe the structure and function of the TMJ disc in comparison to other cartilages and summarize previous TMJ disc tissue engineering studies. TMJ disc tissue engineering efforts date back to 1991,171 although the majority of related studies were not published until 2004 or later. Early studies focused on cell source, biomaterials, and shape-specific scaffolds.58,126,153,171 More recent studies have supported the use of polyglycolic acid (PGA) over agarose,12 promoted the spinner flask as the preferred seeding method with PGA scaffolds,12 demonstrated the importance of using growth factors such as platelet-derived growth factor (PDGF)-BB65 and insulin-like growth factor (IGF)-I,6,11,41 revealed the detrimental effects of passaging and pellet culture,5,6 recommended 25 μg/mL as a preferred ascorbic acid concentration,23 and investigated the effects of hydrostatic pressure10 and rotating wall bioreactors.45
Overall, the TMJ disc tissue engineering studies to date have utilized various cell sources and biomaterials, evaluating the effects of different bioactive signals and bioreactors. The next major investigations into TMJ disc tissue engineering will be the incorporation of stem cell sources and the evaluation of in vivo performance of engineered TMJ discs.
Mandibular Condyle/Ramus Tissue Engineering
Although mandibular condyle/ramus tissue engineering studies were not published until this decade, several publications have appeared in the past 5 years, with comprehensive reviews available in the literature.99,100 The largest contributions, thus far, have come from the groups of Hollister50,72–74,135,136,180 and Mao.2–4 Hollister and colleagues have developed a solid free-form fabrication (SFF) method for producing patient-specific condyle-shaped scaffolds based on CT and/or MRI, allowing for precise control over overall shape, internal architecture, pore size, porosity, permeability, and mechanical integrity. Their in vivo studies have collectively demonstrated substantial bone ingrowth and glycosaminoglycan (GAG) formation.73,135,136,180 Mao’s group has taken another approach, encapsulating marrow-derived mesenchymal stem cells (MSCs) in a polyethylene glycol diacrylate (PEG-DA) hydrogel to create stratified bone and cartilage layers in the shape of a human condyle. After 12 weeks in vivo, it was shown that collagen type II and GAGs were localized in the chondrogenic layer, and osteopontin, osteonectin, and collagen type I were localized in the osteogenic layer.4
Beyond these two primary groups, various different approaches have been employed, most of which were in vivo studies using only histology and/or imaging to validate engineered constructs. One approach was to mold coral into the shape of a human condyle, seed them with MSCs, and implant with bone morphogenetic protein (BMP)-2 to demonstrate osteogenesis in rats and angiogenesis in rabbits.33,34 Another approach was to implant acellular poly (lactic-co-glycolic acid) (PLGA) based constructs with growth factors in rat mandibular defects, either demonstrating the efficacy of transforming growth factor (TGF)-β1 and IGF-I154 or the lack of efficacy of BMP-2172 under the respective study conditions. In another study, osteoblasts were seeded into condyle-shaped PGA/polylactic acid (PLA) scaffolds and chondrocytes were painted on the surface prior to implantation in mice, after which positive histological results were observed.178 In a related study, porcine MSCs seeded in condyle-shaped PLGA scaffolds were cultured under osteogenic conditions in a custom-built rotating bioreactor, which also yielded positive histological results.1 Another approach examined a new cell source, comparing human umbilical cord matrix MSCs with porcine condylar cartilage cells in vitro, showing that the umbilical cord matrix stem cells outperformed the cartilage cells with regard to biosynthesis and proliferation.20
The next major step for mandibular condyle/ramus tissue engineering will be demonstrating long-term in vivo efficacy with osteochondral condyle/ramus replacements in larger animals (e.g., pig), which will require an understanding of the growth and mechanics of the native tissue.67
Conclusions
The lubrication system of the TMJ is an essential function for mandibular dynamics. Understanding the development and breakdown mechanisms of TMJ lubrication may enable us to develop a “good as new” treatment remedy for TMDs. Future studies attempting to artificially replace and tissue engineer the TMJ should be aware of the wealth of joint lubrication data, as it will be necessary to incorporate tribological considerations in design criteria as tissue engineered constructs reach clinical application.
References
Abukawa H., H. Terai, D. Hannouche, J. P. Vacanti, L. B. Kaban, M. J. Troulis. Formation of a mandibular condyle in vitro by tissue engineering. J. Oral. Maxillofac. Surg. 61:94–100, 2003
Alhadlaq A., J. H. Elisseeff, L. Hong, C. G. Williams, A. I. Caplan, B. Sharma, R. A. Kopher, S. Tomkoria, D. P. Lennon, A. Lopez, J. J. Mao. Adult stem cell driven genesis of human-shaped articular condyle. Ann. Biomed. Eng. 32:911–923, 2004
Alhadlaq A., J. J. Mao. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J. Dent. Res. 82:951–956, 2003
Alhadlaq A., J. J. Mao. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J. Bone Joint. Surg. Am. 87:936–944, 2005
Allen K. D., K. A. Athanasiou. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue Eng. 13:101–110, 2007
Allen K. D., K. A. Athanasiou. Growth factor effects on passaged TMJ disk cells in monolayer and pellet cultures. Orthod. Craniofac. Res. 9:143–152, 2006
Allen K. D., K. A. Athanasiou. Tissue engineering of the TMJ disc: a review. Tissue Eng. 12:1183–1196, 2006
Allen K. D., K. A. Athanasiou. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J. Biomech. 39:312–322, 2006
Almarza A. J., K. A. Athanasiou. Design characteristics for the tissue engineering of cartilaginous tissues. Ann. Biomed. Eng. 32:2–17, 2004
Almarza A. J., K. A. Athanasiou. Effects of hydrostatic pressure on TMJ disc cells. Tissue Eng. 12:1285–1294, 2006
Almarza A. J., K. A. Athanasiou. Evaluation of three growth factors in combinations of two for temporomandibular joint disc tissue engineering. Arch. Oral. Biol. 51:215–221, 2006
Almarza A. J., K. A. Athanasiou. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 10:1787–1795, 2004
Almarza A. J., A. C. Bean, L. S. Baggett, K. A. Athanasiou. Biochemical analysis of the porcine temporomandibular joint disc. Br. J. Oral Maxillofac. Surg. 44:124–128, 2006
Alpaslan G. H., C. Alpaslan. Efficacy of temporomandibular joint arthrocentesis with and without injection of sodium hyaluronate in treatment of internal derangements. J. Oral. Maxillofac. Surg. 59:613–618; discussion 618–619, 2001
Aoyama J., E. Tanaka, M. Miyauchi, T. Takata, K. Hanaoka, Y. Hattori, A. Sasaki, M. Watanabe, K. Tanne. Immunolocalization of vascular endothelial growth factor in rat condylar cartilage during postnatal development. Histochem. Cell Biol. 122:35–40, 2004
Ateshian G. A. A theoretical formulation for boundary friction in articular cartilage. J. Biomech. Eng. 119:81–86, 1997
Ateshian G. A., C. T. Hung. Patellofemoral joint biomechanics and tissue engineering. Clin. Orthop. Relat. Res. 436:81–90, 2005
Ateshian G. A., W. M. Lai, W. B. Zhu, V. C. Mow. An asymptotic solution for the contact of two biphasic cartilage layers. J. Biomech. 27:1347–1360, 1994
Axelsson S., A. Holmlund, A. Hjerpe. Glycosaminoglycans in normal and osteoarthrotic human temporomandibular joint disks. Acta Odontol. Scand. 50:113–119, 1992
Bailey M. M., L. Wang, C. J. Bode, K. E. Mitchell, M. S. Detamore. A comparison of human umbilical cord matrix stem cells and TMJ condylar chondrocytes for tissue engineering TMJ condylar cartilage. Tissue Eng. 13:1–11 (Epub ahead of print), 2007
Balazs, E. A. Viscoelastic properties of hyaluronic acid and biological lubrication. Univ. Mich. Med. Cent. J. 255–259, 1968
Balazs E. A., D. Watson, I. F. Duff, S. Roseman. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 10:357–376, 1967
Bean A. C., A. J. Almarza, K. A. Athanasiou. Effects of ascorbic acid concentration on the tissue engineering of the temporomandibular joint disc. Proc. Inst. Mech. Eng. [H]. 220:439–447, 2006
Beatty M. W., M. J. Bruno, L. R. Iwasaki, J. C. Nickel. Strain rate dependent orthotropic properties of pristine and impulsively loaded porcine temporomandibular joint disk. J. Biomed. Mater. Res. 57:25–34, 2001
Berkovitz B. K. Collagen crimping in the intra-articular disc and articular surfaces of the human temporomandibular joint. Arch. Oral. Biol. 45:749–756, 2000
Berkovitz B. K., J. Pacy. Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Arch. Oral. Biol. 45:987–995, 2000
Bertolami C. N., T. Gay, G. T. Clark, J. Rendell, V. Shetty, C. Liu, D. A. Swann. Use of sodium hyaluronate in treating temporomandibular joint disorders: a randomized, double-blind, placebo-controlled clinical trial. J. Oral. Maxillofac. Surg. 51:232–242, 1993
Bibb C. A., A. G. Pullinger, F. Baldioceda. The relationship of undifferentiated mesenchymal cells to TMJ articular tissue thickness. J. Dent. Res. 71:1816–1821, 1992
Blake D. R., P. Merry, J. Unsworth, B. L. Kidd, J. M. Outhwaite, R. Ballard, C. J. Morris, L. Gray, J. Lunec. Hypoxic-reperfusion injury in the inflamed human joint. Lancet 1:289–293, 1989
Bothner H., O. Wik. Rheology of hyaluronate. Acta Otolaryngol. Suppl. 442:25–30, 1987
Bujurke N. M., R. B. Kudenatti, and V. B. Awati. Effect of surface roughness on squeeze film poroelastic bearings with special reference to synovial joints. Math. Biosci. 209:76–89, 2007
Butler D. M., G. F. Vitti, T. Leizer, J. A. Hamilton. Stimulation of the hyaluronic acid levels of human synovial fibroblasts by recombinant human tumor necrosis factor alpha, tumor necrosis factor beta (lymphotoxin), interleukin-1 alpha, and interleukin-1 beta. Arthritis Rheum. 31:1281–1289, 1988
Chen F., S. Chen, K. Tao, X. Feng, Y. Liu, D. Lei, T. Mao. Marrow-derived osteoblasts seeded into porous natural coral to prefabricate a vascularised bone graft in the shape of a human mandibular ramus: experimental study in rabbits. Br. J .Oral. Maxillofac. Surg. 42:532–537, 2004
Chen F., T. Mao, K. Tao, S. Chen, G. Ding, X. Gu. Bone graft in the shape of human mandibular condyle reconstruction via seeding marrow-derived osteoblasts into porous coral in a nude mice model. J .Oral. Maxillofac. Surg. 60:1155–1159, 2002
Chopra R. K., C. H. Pearson, G. A. Pringle, D. S. Fackre, P. G. Scott. Dermatan sulphate is located on serine-4 of bovine skin proteodermatan sulphate. Demonstration that most molecules possess only one glycosaminoglycan chain and comparison of amino acid sequences around glycosylation sites in different proteoglycans. Biochem. J. 232:277–279, 1985
Clark J. M., A. G. Norman, M. J. Kaab, H. P. Notzli. The surface contour of articular cartilage in an intact, loaded joint. J. Anat. 195(Pt 1):45–56, 1999
de Bont L. G., G. Boering, P. Havinga, R. S. Liem. Spatial arrangement of collagen fibrils in the articular cartilage of the mandibular condyle: a light microscopic and scanning electron microscopic study. J. Oral. Maxillofac. Surg. 42:306–313, 1984
De Smedt S. C., P. Dekeyser, V. Ribitsch, A. Lauwers, J. Demeester. Viscoelastic and transient network properties of hyaluronic acid as a function of the concentration. Biorheology 30:31–41, 1993
Del Santo M. Jr., F. Marches, M. Ng, R. J. Hinton. Age-associated changes in decorin in rat mandibular condylar cartilage. Arch. Oral. Biol. 45:485–493, 2000
Delatte M., J. W. Von den Hoff, R. E. van Rheden, A. M. Kuijpers-Jagtman. Primary and secondary cartilages of the neonatal rat: the femoral head and the mandibular condyle. Eur. J. Oral. Sci. 112:156–162, 2004
Detamore M. S., K. A. Athanasiou. Evaluation of three growth factors for TMJ disc tissue engineering. Ann. Biomed. Eng. 33:383–390, 2005
Detamore M. S., K. A. Athanasiou. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 9:1065–1087, 2003
Detamore M. S., K. A. Athanasiou. Structure and function of the temporomandibular joint disc: implications for tissue engineering. J. Oral. Maxillofac. Surg. 61:494–506, 2003
Detamore M. S., K. A. Athanasiou. Tensile properties of the porcine temporomandibular joint disc. J. Biomech. Eng. 125:558–565, 2003
Detamore M. S., K. A. Athanasiou. Use of a rotating bioreactor toward tissue engineering the temporomandibular joint disc. Tissue Eng. 11:1188–1197, 2005
Detamore M. S., K. A. Athanasiou, J. Mao. A call to action for bioengineers and dental professionals: Directives for the future of TMJ bioengineering. Ann. Biomed. Eng. 35:1301–1311, 2007
Detamore M. S., J. N. Hegde, R. R. Wagle, A. J. Almarza, D. Montufar-Solis, P. J. Duke, K. A. Athanasiou. Cell type and distribution in the porcine temporomandibular joint disc. J. Oral. Maxillofac. Surg. 64:243–248, 2006
Detamore M. S., J. G. Orfanos, A. J. Almarza, M. M. French, M. E. Wong, K. A. Athanasiou. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 24:45–57, 2005
Dirksen D., U. Stratmann, J. Kleinheinz, G. von Bally, F. Bollmann. Three-dimensional visualization and quantification of the mandibular articular surface by optical profilometry. Cells Tissues Organs. 164:212–220, 1999
Feinberg S. E., S. J. Hollister, J. W. Halloran, T. M. Chu, P. H. Krebsbach. Image-based biomimetic approach to reconstruction of the temporomandibular joint. Cells Tissues Organs. 169:309–321, 2001
Fisher J. Biomedical applications, in Modern Tribology Handbook, Bhushan B., Ed.. 2001, CRC Press: New York, NY. 1593–1609
Fisher L. W., J. D. Termine, M. F. Young. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J. Biol. Chem. 264:4571–4576, 1989
Forsey R. W., J. Fisher, J. Thompson, M. H. Stone, C. Bell, E. Ingham. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials 27:4581–4590, 2006
Forster H., J. Fisher. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc. Inst. Mech. Eng. [H]. 213:329–345, 1999
Forster H., J. Fisher. The influence of loading time and lubricant on the friction of articular cartilage. Proc. Inst. Mech. Eng. [H]. 210:109–119, 1996
Fujisawa T., T. Kuboki, T. Kasai, W. Sonoyama, S. Kojima, J. Uehara, C. Komori, H. Yatani, T. Hattori, M. Takigawa. A repetitive, steady mouth opening induced an osteoarthritis-like lesion in the rabbit temporomandibular joint. J. Dent. Res. 82:731–735, 2003
Gibbs D. A., E. W. Merrill, K. A. Smith, E. A. Balazs. Rheology of hyaluronic acid. Biopolymers 6:777–791, 1968
Girdler N. M. In vitro synthesis and characterization of a cartilaginous meniscus grown from isolated temporomandibular chondroprogenitor cells. Scand. J. Rheumatol. 27:446–453, 1998
Glowacki J. Engineered cartilage, bone, joints, and menisci. Potential for temporomandibular joint reconstruction. Cells Tissues Organs. 169:302–308, 2001
Graindorge S., W. Ferrandez, E. Ingham, Z. Jin, P. Twigg, J. Fisher. The role of the surface amorphous layer of articular cartilage in joint lubrication. Proc. Inst. Mech. Eng. [H]. 220:597–607, 2006
Gray R. J. M., S. J. Davies, A. A. Quayle. Temporomandibular Disorders: A Clinical Approach. London: British Dental Association. 1995
Grootveld M., E. B. Henderson, A. Farrell, D. R. Blake, H. G. Parkes, P. Haycock. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal low-molecular-mass metabolites by proton-n.m.r. spectroscopy. Biochem. J. 273(Pt 2):459–467, 1991
Grunder W. MRI assessment of cartilage ultrastructure. NMR Biomed. 19:855–876, 2006
Hamerman D., D. D. Wood. Interleukin 1 enhances synovial cell hyaluronate synthesis. Proc. Soc. Exp. Biol. Med. 177:205–210, 1984
Hanaoka K., E. Tanaka, T. Takata, M. Miyauchi, J. Aoyama, N. Kawai, D. A. Dalla-Bona, E. Yamano, K. Tanne. Platelet-derived growth factor enhances proliferation and matrix synthesis of temporomandibular joint disc-derived cells. Angle Orthod. 76:486–492, 2006
Heatley F., J. E. Scott. A water molecule participates in the secondary structure of hyaluronan. Biochem. J. 254:489–493, 1988
Herring S. W., P. Ochareon. Bone-special problems of the craniofacial region. Orthod. Craniofac. Res. 8:174–182, 2005
Hills B. A. Synovial surfactant and the hydrophobic articular surface. J. Rheumatol. 23:1323–1325, 1996
Hills B. A., B. D. Butler. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann. Rheum. Dis. 43:641–648, 1984
Hills B. A., M. K. Monds. Enzymatic identification of the load-bearing boundary lubricant in the joint. Br. J. Rheumatol. 37:137–142, 1998
Hodge-Dufour J., P. W. Noble, M. R. Horton, C. Bao, M. Wysoka, M. D. Burdick, R. M. Strieter, G. Trinchieri, E. Pure. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J. Immunol. 159:2492–2500, 1997
Hollister S. J., R. A. Levy, T. M. Chu, J. W. Halloran, S. E. Feinberg. An image-based approach for designing and manufacturing craniofacial scaffolds. Int. J. Oral. Maxillofac. Surg. 29:67–71, 2000
Hollister S. J., C. Y. Lin, E. Saito, C. Y. Lin, R. D. Schek, J. M. Taboas, J. M. Williams, B. Partee, C. L. Flanagan, A. Diggs, E. N. Wilke, G. H. Van Lenthe, R. Muller, T. Wirtz, S. Das, S. E. Feinberg, P. H. Krebsbach. Engineering craniofacial scaffolds. Orthod. Craniofac. Res. 8:162–173, 2005
Hollister S. J., R. D. Maddox, J. M. Taboas. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 23:4095–4103, 2002
Horiuchi T., T. Yoshida, Y. Koshihara, H. Sakamoto, H. Kanai, S. Yamamoto, H. Ito. The increase of parathyroid hormone-related peptide and cytokine levels in synovial fluid of elderly rheumatoid arthritis and osteoarthritis. Endocr. J. 46:643–649, 1999
Hu K., P. Radhakrishnan, R. V. Patel, J. J. Mao. Regional structural and viscoelastic properties of fibrocartilage upon dynamic nanoindentation of the articular condyle. J. Struct. Biol. 136:46–52, 2001
Huey G., A. Moiin, R. Stern. Levels of [3H]glucosamine incorporation into hyaluronic acid by fibroblasts is modulated by culture conditions. Matrix 10:75–83, 1990
Ikegawa S., M. Sano, Y. Koshizuka, Y. Nakamura. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet. Cell Genet. 90:291–297, 2000
Jay G. D., K. Haberstroh, C. J. Cha. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. J. Biomed. Mater. Res. 40:414–418, 1998
Jay G. D., U. Tantravahi, D. E. Britt, H. J. Barrach, C. J. Cha. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J. Orthop. Res. 19:677–687, 2001
Jin Z. M., D. Dowson, J. Fisher. The effect of porosity of articular cartilage on the lubrication of a normal human hip joint. Proc. Inst. Mech. Eng. [H]. 206:117–124, 1992
Kafka V. Surface fissures in articular cartilage: effect of pathological changes in synovial fluid. Clin. Biomech. (Bristol, Avon). 17:713–715, 2002
Kang H., G. Bao, Y. Dong, X. Yi, Y. Chao, M. Chen. [Tensile mechanics of mandibular condylar cartilage]. West Chin. J. Stomatol. 18:85–87, 2000
Kawai N., E. Tanaka, T. Takata, M. Miyauchi, M. Tanaka, M. Todoh, T. van Eijden, K. Tanne. Influence of additive hyaluronic acid on the lubricating ability in the temporomandibular joint. J. Biomed. Mater. Res. A 70:149–153, 2004
Kim K. W., M. E. Wong, J. F. Helfrick, J. B. Thomas, K. A. Athanasiou. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann. Biomed. Eng. 31:924–930, 2003
Klein T. J., B. L. Schumacher, M. E. Blewis, T. A. Schmidt, M. S. Voegtline, E. J. Thonar, K. Masuda, R. L. Sah. Tailoring secretion of proteoglycan 4 (PRG4) in tissue-engineered cartilage. Tissue Eng. 12:1429–1439, 2006
Klinge R. F. The structure of the mandibular condyle in the monkey (Macaca mulatta). Micron 27:381–387, 1996
Kobayashi Y., A. Okamoto, K. Nishinari. Viscoelasticity of hyaluronic acid with different molecular weights. Biorheology 31:235–244, 1994
Konttinen Y. T., H. Saari, D. C. Nordstrom. Effect of interleukin-1 on hyaluronate synthesis by synovial fibroblastic cells. Clin. Rheumatol. 10:151–154, 1991
Landesberg R., E. Takeuchi, J. E. Puzas. Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Arch. Oral. Biol. 41:761–767, 1996
Laurent T. C., J. R. Fraser. Hyaluronan. Faseb. J. 6:2397–2404, 1992
Laurent T. C., U. B. Laurent, J. R. Fraser. The structure and function of hyaluronan: an overview. Immunol. Cell. Biol. 74:A1–A7, 1996
Li M., L. Rosenfeld, R. E. Vilar, M. K. Cowman. Degradation of hyaluronan by peroxynitrite. Arch. Biochem. Biophys. 341:245–250, 1997
Linn F. C. Lubrication of animal joints. I. The arthrotripsometer. J. Bone Joint. Surg. Am. 49:1079–1098, 1967
Lohmander L. S., N. Dalen, G. Englund, M. Hamalainen, E. M. Jensen, K. Karlsson, M. Odensten, L. Ryd, I. Sernbo, O. Suomalainen, A. Tegnander. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: A randomised, double blind, placebo controlled multicentre trial. Ann. Rheum. Diseases 55:424–431, 1996
Luder H. U., C. P. Leblond, K. von der Mark. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am. J. Anat. 182:197–214, 1988
Mabuchi K., T. Obara, K. Ikegami, T. Yamaguchi, T. Kanayama. Molecular weight independence of the effect of additive hyaluronic acid on the lubricating characteristics in synovial joints with experimental deterioration. Clin. Biomech. (Bristol, Avon). 14:352–356, 1999
Mabuchi K., Y. Tsukamoto, T. Obara, T. Yamaguchi. The effect of additive hyaluronic acid on animal joints with experimentally reduced lubricating ability. J. Biomed. Mater. Res. 28:865–870, 1994
Mao J. J. Stem-cell-driven regeneration of synovial joints. Biol. Cell. 97:289–301, 2005
Mao J. J., W. V. Giannobile, J. A. Helms, S. J. Hollister, P. H. Krebsbach, M. T. Longaker, S. Shi. Craniofacial tissue engineering by stem cells. J. Dent. Res. 85:966–979, 2006
Mao J. J., F. Rahemtulla, P. G. Scott. Proteoglycan expression in the rat temporomandibular joint in response to unilateral bite raise. J. Dent. Res. 77:1520–1528, 1998
McNeil J. D., O. W. Wiebkin, W. H. Betts, L. G. Cleland. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann. Rheum. Dis. 44:780–789, 1985
Mercuri L. G., N. R. Edibam, A. Giobbie-Hurder. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J. Oral. Maxillofac. Surg. 65:1140–1148, 2007
Meyer F. A., I. Yaron, M. Yaron. Synergistic, additive, and antagonistic effects of interleukin-1 beta, tumor necrosis factor alpha, and gamma-interferon on prostaglandin E, hyaluronic acid, and collagenase production by cultured synovial fibroblasts. Arthritis Rheum. 33:1518–1525, 1990
Milam S. B., R. J. Klebe, R. G. Triplett, D. Herbert. Characterization of the extracellular matrix of the primate temporomandibular joint. J. Oral. Maxillofac. Surg. 49:381–391, 1991
Mills D. K., D. J. Fiandaca, R. P. Scapino. Morphologic, microscopic, and immunohistochemical investigations into the function of the primate TMJ disc. J. Orofac. Pain. 8:136–154, 1994
Minarelli A. M., M. Del Santo Jr, E. A. Liberti. The structure of the human temporomandibular joint disc: a scanning electron microscopy study. J. Orofac. Pain. 11:95–100, 1997
Minarelli A. M., E. A. Liberti. A microscopic survey of the human temporomandibular joint disc. J. Oral. Rehabil. 24:835–840, 1997
Mizoguchi I., I. Takahashi, M. Nakamura, Y. Sasano, S. Sato, M. Kagayama, H. Mitani. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch. Oral. Biol. 41:863–869, 1996
Mori S., M. Naito, S. Moriyama. Highly viscous sodium hyaluronate and joint lubrication. Int. Orthop. 26:116–121, 2002
Morris E. R., D. A. Rees, E. J. Welsh. Conformation and dynamic interactions in hyaluronate solutions. J. Mol. Biol. 138:383–400, 1980
Mow V. C., G. A. Ateshian, R. L. Spilker. Biomechanics of diarthrodial joints: a review of twenty years of progress. J. Biomech. Eng. 115:460–467, 1993
Mow V. C., W. M. Lai, I. Redler. Some surface characteristics of articular cartilage. I. A scanning electron microscopy study and a theoretical model for the dynamic interaction of synovial fluid and articular cartilage. J. Biomech. 7:449–456, 1974
Mow V. C., and A. F. Mak. Lubrication of diarthrodial joints. In: Handbook of Bioengineering, Chapter 5, edited by R. Skalak and S. Chen. New York, NY: McGraw-Hill Book Co., 1988, pp. 1–34
Mow V. C., A. Ratcliffe, K. Y. Chern, M. A. Kelly. “Structure and function relationships of the menisci of the knee,” in Knee Meniscus: Basic and Clinical Foundations, Mow V. C., Arnoczky S. P., Jackson D. W., Editors 1992, Raven Press: New York, NY. 37–57
Muir H. 1973 “The chemistry of the ground substance of joint cartilage,” in Sokoloff L., Editor, Joints and Synovial Fluid, vol. 18, Academic Press: New York, NY. 1011–1020
Nakano T., P. G. Scott. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch. Oral. Biol. 41:845–853, 1996
Nakano T., P. G. Scott. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch. Oral. Biol. 34:749–57, 1989
Nickel J. C., K. R. McLachlan. In vitro measurement of the frictional properties of the temporomandibular joint disc. Arch. Oral. Biol. 39:323–331, 1994
Nitzan D. W. The process of lubrication impairment and its involvement in temporomandibular joint disc displacement: a theoretical concept. J. Oral. Maxillofac. Surg. 59:36–45, 2001
Nitzan D. W., U. Nitzan, P. Dan, S. Yedgar. The role of hyaluronic acid in protecting surface-active phospholipids from lysis by exogenous phospholipase A(2). Rheumatology (Oxford) 40:336–340, 2001
O’Rahilly R., E. Gardner. “The embryology of movable joints,” in Joints and Synovial Fluids, Sokoloff L., Editor. 1978, Academic Press Inc.: New York, NY. 49–103
Orkin R. W., B. P. Toole. Isolation and characterization of hyaluronidase from cultures of chick embryo skin- and muscle-derived fibroblasts. J. Biol. Chem. 255:1036–1042, 1980
Park S., K. D. Costa G. A. Ateshian. Microscale frictional response of bovine articular cartilage from atomic force microscopy. J. Biomech. 37:1679–1687, 2004
Patel R. V., J. J. Mao. Microstructural and elastic properties of the extracellular matrices of the superficial zone of neonatal articular cartilage by atomic force microscopy. Front Biosci. 8:a18–a25, 2003
Puelacher W. C., J. Wisser, C. A. Vacanti, N. F. Ferraro, D. Jaramillo, J. P. Vacanti. Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. J. Oral. Maxillofac. Surg. 52:1172–1177, 1994
Quinn T. F. J. Physical Analysis for Tribology. New York, NY: Cambridge University Press. 1991
Rees L. A. The structure and function of the mandibular joint. Br. Dent. J. 96:125–133, 1954
Roberts B. J., A. Unsworth, N. Mian. Modes of lubrication in human hip joints. Ann. Rheum. Dis. 41:217–224, 1982
Roth S., K. Muller, D. C. Fischer, K. H. Dannhauer. Specific properties of the extracellular chondroitin sulphate proteoglycans in the mandibular condylar growth centre in pigs. Arch. Oral. Biol. 42:63–76, 1997
Sander E. A., E. A. Nauman. Permeability of musculoskeletal tissues and scaffolding materials: experimental results and theoretical predictions. Crit. Rev. Biomed. Eng. 31:1–26, 2003
Sarma A. V., G. L. Powell, M. LaBerge. Phospholipid composition of articular cartilage boundary lubricant. J. Orthop. Res. 19:671–676, 2001
Scapino R. P., P. B. Canham, H. M. Finlay, D. K. Mills. The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch. Oral. Biol. 41:1039–1052, 1996
Scapino R. P., A. Obrez, D. Greising. Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs. 182:201–225, 2006
Schek R. M., J. M. Taboas, S. J. Hollister, P. H. Krebsbach. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod. Craniofac. Res. 8:313–319, 2005
Schek R. M., J. M. Taboas, S. J. Segvich, S. J. Hollister, P. H. Krebsbach. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 10:1376–1385, 2004
Scherge M., S. Gorb. Biological Micro- and Nanotribology: Nature’s Solutions. New York, NY: Springer. 2001
Schlaak J. F., I. Pfers, K. H. Meyer Zum Buschenfelde, E. Marker-Hermann. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 14:155–162, 1996
Schmidt T. A., R. L. Sah. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 15:35–47, 2007
Schumacher B. L., J. A. Block, T. M. Schmid, M. B. Aydelotte, K. E. Kuettner. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch. Biochem. Biophys. 311:144–152, 1994
Schurz J., V. Ribitsch. Rheology of synovial fluid. Biorheology. 24:385–399, 1987
Schwarz I. M., B. A. Hills. Surface-active phospholipid as the lubricating component of lubricin. Br. J. Rheumatol. 37:21–26, 1998
Scott J. E., C. Cummings, A. Brass, Y. Chen. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem. J. 274(Pt 3):699–705, 1991
Scott J. E., F. Heatley. Biological properties of hyaluronan in aqueous solution are controlled and sequestered by reversible tertiary structures, defined by NMR spectroscopy. Biomacromolecules 3:547–553, 2002
Scott J. E., F. Heatley. Hyaluronan forms specific stable tertiary structures in aqueous solution: a 13C NMR study. Proc. Natl. Acad. Sci. USA 96:4850–4855, 1999
Scott J. E., F. Heatley, W. E. Hull. Secondary structure of hyaluronate in solution. A 1H-n.m.r. investigation at 300 and 500 MHz in [2H6]dimethyl sulphoxide solution. Biochem. J. 220:197–205, 1984
Setton L. A., W. Zhu, V. C. Mow. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. J. Biomech. 26:581–592, 1993
Sindelar B. J., S. P. Evanko, T. Alonzo, S. W. Herring, T. Wight. Effects of intraoral splint wear on proteoglycans in the temporomandibular joint disc. Arch. Biochem. Biophys. 379:64–70, 2000
Singh, M., and M. S. Detamore. Tensile properties of the mandibular condylar cartilage. J. Biomech. Eng., Accepted, 2007
Smith M. M., P. Ghosh. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol. Int. 7:113–122, 1987
Soltz M. A., I. M. Basalo, G. A. Ateshian. Hydrostatic pressurization and depletion of trapped lubricant pool during creep contact of a rippled indenter against a biphasic articular cartilage layer. J. Biomech. Eng. 125:585–593, 2003
Spirt A. A., A. F. Mak, R. P. Wassell. Nonlinear viscoelastic properties of articular cartilage in shear. J. Orthop. Res. 7:43–49, 1989
Springer I. N., B. Fleiner, S. Jepsen, Y. Acil. Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials 22:2569–2577, 2001
Srouji S., A. Rachmiel, I. Blumenfeld, E. Livne. Mandibular defect repair by TGF-beta and IGF-1 released from a biodegradable osteoconductive hydrogel. J. Craniomaxillofac. Surg. 33:79–84, 2005
Stegenga B., L. G. de Bont, G. Boering, J. D. van Willigen. Tissue responses to degenerative changes in the temporomandibular joint: a review. J. Oral Maxillofac. Surg. 49:1079–1088, 1991
Sundblad L. Glycosaminoglycans and glycoproteins in synovial fluid, in The Amino Sugars. The Chemistry and Biology of Compounds Containing Amino Sugars, Balazs E. A., Jeanloz R. W., Editors. 1965, Academic Press: New York, NY. 229–250
Takahashi T., K. Tominaga, H. Takano, W. Ariyoshi, M. Habu, J. Fukuda, H. Maeda. A decrease in the molecular weight of hyaluronic acid in synovial fluid from patients with temporomandibular disorders. J. Oral. Pathol. Med. 33:224–229, 2004
Tanaka E., J. Aoyama, M. Miyauchi, T. Takata, K. Hanaoka, T. Iwabe, K. Tanne. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochem. Cell Biol. 123:275–281, 2005
Tanaka E., D. A. Dalla-Bona, T. Iwabe, N. Kawai, E. Yamano, T. van Eijden, M. Tanaka, M. Miyauchi, T. Takata, K. Tanne. The effect of removal of the disc on the friction in the temporomandibular joint. J. Oral Maxillofac. Surg. 64:1221–1224, 2006
Tanaka E., K. Hanaoka, T. van Eijden, M. Tanaka, M. Watanabe, M. Nishi, N. Kawai, H. Murata, T. Hamada, K. Tanne. Dynamic shear properties of the temporomandibular joint disc. J. Dent Res. 82:228–231, 2003
Tanaka E., T. Iwabe, D. A. Dalla-Bona, N. Kawai, T. van Eijden, M. Tanaka, S. Kitagawa, T. Takata, K. Tanne. The effect of experimental cartilage damage and impairment and restoration of synovial lubrication on friction in the temporomandibular joint. J. Orofac. Pain. 19:331–336, 2005
Tanaka E., N. Kawai, K. Hanaoka, T. Van Eijden, A. Sasaki, J. Aoyama, M. Tanaka, K. Tanne. Shear properties of the temporomandibular joint disc in relation to compressive and shear strain. J. Dent. Res. 83:476–479, 2004
Tanaka E., N. Kawai, M. Tanaka, M. Todoh, T. van Eijden, K. Hanaoka, D. A. Dalla-Bona, T. Takata, K. Tanne. The frictional coefficient of the temporomandibular joint and its dependency on the magnitude and duration of joint loading. J. Dent. Res. 83:404–407, 2004
Tanaka, E., E. B. Rego, Y. Iwabuchi, T. Inubushi, J. H. Koolstra, T. M. G. J. van Eijden, N. Kawai, Y. Kudo, T. Takata, and K. Tanne. Biomechanical response of condylar cartilage-on-bone to dynamic shear. J. Biomed. Mater. Res. A., in press, 2007
Tanaka E., T. van Eijden. Biomechanical behavior of the temporomandibular joint disc. Crit. Rev. Oral. Biol. Med. 14:138–150, 2003
Tanaka E., E. Yamano, D. A. Dalla-Bona, M. Watanabe, T. Inubushi, M. Shirakura, R. Sano, K. Takahashi, T. van Eijden, K. Tanne. Dynamic compressive properties of the mandibular condylar cartilage. J. Dent. Res. 85:571–575, 2006
Tanimoto K., S. Ohno, K. Fujimoto, K. Honda, C. Ijuin, N. Tanaka, T. Doi, M. Nakahara, K. Tanne. Proinflammatory cytokines regulate the gene expression of hyaluronic acid synthetase in cultured rabbit synovial membrane cells. Connect. Tissue Res. 42:187–195, 2001
Teng S., Y. Xu. Biomechanical properties and collagen fiber orientation of TMJ discs in dogs: Part 1. Gross anatomy and collagen fiber orientation of the discs. J. Craniomandib. Disord. 5:28–34, 1991
Teng S., Y. Xu, M. Cheng, Y. Li. Biomechanical properties and collagen fiber orientation of TMJ discs in dogs: Part 2. Tensile mechanical properties of the discs. J. Craniomandib. Disord. 5:107–114, 1991
Teramoto M., S. Kaneko, S. Shibata, M. Yanagishita, K. Soma. Effect of compressive forces on extracellular matrix in rat mandibular condylar cartilage. J. Bone Miner. Metab. 21:276–286, 2003
Thomas M., D. Grande, R. H. Haug. Development of an in vitro temporomandibular joint cartilage analog. J. Oral. Maxillofac. Surg. 49:854–856; discussion 857, 1991
Ueki K., D. Takazakura, K. Marukawa, M. Shimada, K. Nakagawa, S. Takatsuka, E. Yamamoto. The use of polylactic acid/polyglycolic acid copolymer and gelatin sponge complex containing human recombinant bone morphogenetic protein-2 following condylectomy in rabbits. J. Craniomaxillofac. Surg. 31:107–114, 2003
Vadas P., J. Browning, J. Edelson, W. Pruzanski. Extracellular phospholipase A2 expression and inflammation: the relationship with associated disease states. J. Lipid. Mediat. 8:1–30, 1993
Wang L., M. S. Detamore. Tissue engineering the TMJ condyle. Tissue Eng. 13:1–17 (Epub ahead of print), 2007
Warwick R., P. L. Williams. Gray’s Anatomy. Philadelphia, PA: Saunders Co. 1973
Webb G. R., C. I. Westacott, C. J. Elson. Osteoarthritic synovial fluid and synovium supernatants up-regulate tumor necrosis factor receptors on human articular chondrocytes. Osteoarthritis Cartilage. 6:167–176, 1998
Welsh E. J., D. A. Rees, E. R. Morris, J. K. Madden. Competitive inhibition evidence for specific intermolecular interactions in hyaluronate solutions. J. Mol. Biol. 138:375–382, 1980
Weng Y., Y. Cao, C. A. Silva, M. P. Vacanti, C. A. Vacanti. Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J. Oral Maxillofac. Surg. 59:185–190, 2001
Wilkes C. H. Internal derangements of the temporomandibular joint. Pathological variations. Arch. Otolaryngol. Head Neck Surg. 115:469–477, 1989
Williams J. M., A. Adewunmi, R. M. Schek, C. L. Flanagan, P. H. Krebsbach, S. E. Feinberg, S. J. Hollister, S. Das. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 26:4817–4827, 2005
Wright V., D. Dowson. Lubrication and cartilage. J. Anat. 121:107–118, 1976
Yanaki T., T. Yamaguchi. Temporary network formation of hyaluronate under a physiological condition. 1. Molecular-weight dependence. Biopolymers 30:415–425, 1990
Zhu W., K. Y. Chern, V. C. Mow. Anisotropic viscoelastic shear properties of bovine meniscus. Clin. Orthop. Relat. Res. 306:34–45, 1994
Zhu W., V. C. Mow, T. J. Koob, D. R. Eyre. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J. Orthop. Res. 11:771–781, 1993
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, E., Detamore, M.S., Tanimoto, K. et al. Lubrication of the Temporomandibular Joint. Ann Biomed Eng 36, 14–29 (2008). https://doi.org/10.1007/s10439-007-9401-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-007-9401-z