Abstract
We analyzed intraventricular blood flow numerically to study the influence of the mode of mitral valve opening on the flow field in the left ventricle. Four different types of opening mode were examined: gradually axisymmetric, gradually anteroposterior (anatomical), gradually bilateral (anti-anatomical), or instantaneous opening and closing. In these models, the shape of the valve orifice was the same when the mitral valve was opened fully. The results demonstrated that the framework of the velocity profile of transmitral flow was built during the phase of mitral valve opening, which was characterized by the mode of valve opening. After the mitral valve opened completely, the transmitral velocity profile developed while maintaining its topological features. Consequently, each mode of mitral valve opening had its own pattern of intraventricular flow, although mitral valve opening accounted for less than 4% of a cardiac cycle. Particle tracking in the resulting flow field revealed that ventricular ejection was more efficient in the anteroposterior and axisymmetric opening modes. These results addressed the importance of the mode of mitral valve opening in intraventricular flow dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The mitral valve is a bicuspid valve located between the left ventricle and left atrium. It regulates blood flow between these two chambers. During systole, it closes tightly to prevent blood from flowing backward into the atrium, and once the left ventricle expands, it opens at the commissure of the leaflets, allowing blood to flow into the ventricular cavity. When one or both valve leaflets are damaged, the mitral valve is unable to seal tightly. In addition, if the mitral valve calcifies, mitral stenosis and regurgitation can occur. The gravest consequence of this is progressive, irreversible ventricular dysfunction, which ultimately leads to enlargement of the left ventricle (dilated cardiomyopathy).12
Severe mitral valve dysfunction requires surgical treatment.1 If the valve is too damaged to permit repair, it must be replaced with an artificial valve. A wide variety of the artificial valves have been devised.1 The performance of these valves is evaluated in engineering terms, i.e., the noise level,26 energy loss,6 , 33 and regurgitation,17 as well as biologically, i.e., hemolysis and the thrombogenicity of the valve itself.9 , 23 These factors are linked mainly to the fluid dynamics near the valve. In contrast, relatively few studies have examined the hemodynamics downstream from the valve, despite the fact that if stagnation occurs in the ventricular cavity, the risk of thrombosis increases. Furthermore, poor washout causes red blood cells to remain in the heart for a number of cardiac cycles without being ejected, leading to their deterioration.
According to Tsakiris et al.,30 the time required for the mitral valve to open fully is approximately 0.04 s with a standard deviation of 0.01 s. This is only 4% of the entire cardiac cycle. Therefore, it is generally believed that the opening of the mitral valve does not affect flow dynamics in the left ventricle. However, our previous study showed that even this length of opening time of the mitral valve has a pronounced influence on the intraventricular flow field throughout diastole.20 Therefore, we postulated that the intraventricular flow dynamics downstream from the valve could change with the mode of mitral valve opening. In support of this postulate, experimental studies conducted by Akutsu’s group2 – 4 showed that a small difference in the design of mechanical heart valves could generate noticeable flow differences within the ventricular cavity.
Therefore, we investigated the effect of the mode of mitral valve opening on the flow dynamics in the left ventricle. Four different modes were examined: the valve orifice opens axisymmetrically, anteroposteriorly (anatomically), or bilaterally (anti-anatomically) over 0.035 s, or it opens instantaneously. The intraventricular flow dynamics were analyzed numerically using a three-dimensional model of a left ventricle with dilated cardiomyopathy because this often requires mitral valve replacement surgery.12 , 24 The velocity profile of the transmitral flow, flow patterns, and efficiency of ventricular ejection were analyzed.
METHODS
Left Ventricle Model
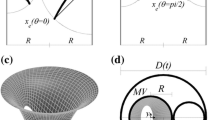
We modeled an enlarged left ventricle to represent dilated cardiomyopathy, neglecting major irregularities of the luminal surface, such as the papillary muscles and chordae tendinae. The method of modeling an enlarged left ventricle is similar to that used to model a normal left ventricle.21 The configuration of the left ventricle model at the end of diastole is shown in Fig. 1. For simplicity, the geometry of this model is symmetric with respect to the bisector plane of the mitral and aortic orifices, both of which are circular. A global Cartesian coordinate system, with coordinates (x, y, z), is defined at the origin, O, which is located at the point at which the two planes containing the mitral and aortic orifices intersected the bisector plane. The x-axis lies on the intersection of the plane containing the mitral orifice and the bisector plane, and the y-axis lies on the intersection of the two planes containing the mitral and aortic orifices. With this definition, the long-axis plane of the left ventricle is the x–z plane. At this stage, the ventricular volume was 180 cm3, the diameters of the mitral and aortic valves were 2.6 cm, and the angle between the inlet plane of the aorta and the mitral valve plane was 140°.
The left ventricle was regarded as a U-shaped tube with the mitral valve at one end and the aortic valve at the other end. We defined Ψ as the cross section of the left ventricle obtained by cutting it with a plane radiating from the line of intersection of the planes containing the two valve orifices, as shown in Fig. 2. The angle between Ψ and the plane of the mitral valve was α. For each Ψ, a point O′ was set at its centroid. Line f was defined as the intersection between Ψ and the plane of symmetry of the left ventricle, and line g was the straight line connecting O′ with an arbitrary point P on the circumference of Ψ. The angle between lines f and g was defined as θ. In designing the fully expanded model left ventricle, all Ψ cross sections were assumed to be elliptical.
In modeling the ventricular wall motion, we ignored the untwisting and twisting motion and assumed that the left ventricle deforms independently of the internal blood pressure. In addition, we assumed that each point on the ventricular surface moved in a radial direction from the centroid of cross section Ψ to which the point belongs.21 With these assumptions, the relationship between the moving velocity of the ventricular wall, v w (α, θ, t), and the rate of volume change of the left ventricle, dV/dt, can be expressed as
where e is a unit vector in the direction of line g, dS is the element of the area of the surface of the left ventricle model, and n denotes the components of a unit vector normal to the surface S. In this simulation, for simplicity, we decomposed the moving velocity of the ventricular wall v w (α, θ, t) as
where v a (t) is the velocity of the ventricular apex and W 1(θ) and W 2(α) are weighting functions for the moving velocity of the wall. In order to increase the wall movement from the base to the apex, while not moving the walls at the mitral and aortic valves to maintain their shape,21 we used W 1(θ) and W 2(α) given by
where D(α) is the length of the long axis of the cross section at angle α at the maximum expansion of the left ventricle, and D max is the maximum of D(α) at α=4π/9, which corresponds to the cross section containing the ventricular apex. The temporal variation in the volume during a cardiac cycle was given with some modifications of clinical data for a normal heart,25 as illustrated in Fig. 3. Note that the isovolumetric phases, which exist between diastole and systole in a real heart, are neglected here. The stroke volume was 80 cm3 with an ejection fraction of 0.44. The lengths of diastole and systole were 0.5 and 0.3 s, respectively. Using W 1(θ), W 2(α) and dV/dt, we can calculate v α (t) from Eqs. (1) and (2) and determine the moving velocity of the entire surface of the left ventricle.
Valve Orifice Model
We modeled the valve as a planar unit by neglecting the valve leaflets. Four different modes of opening and closing of the valve orifice were examined, as illustrated in Fig. 4(a) the valve opens axisymmetrically from its center, while keeping the orifice as a circular core; Fig. 4(b) the valve opens anteroposteriorly and symmetrically with respect to the y-axis (parallel to the anatomical axis of the native leaflets, an anatomic orientation); Fig. 4(c) the valve opens bilaterally and symmetrically with respect to the x-axis (perpendicular to the axis of the native leaflets, an anti-anatomical orientation); and Fig. 4(d) the valve opens instantaneously. In these models, the valve orifice had the same shape when the mitral valve was fully opened.
The temporal variation in the size of the valve orifice for models (a)–(c) was set to be the same and expressed simply as a linear function of the rate of ventricular volume change. Since blood is incompressible and the left ventricle is a semi-closed system with only one valve orifice open, the resulting flow rate at the orifice equals the rate of ventricular volume change. Mathematically, the temporal variation in the size of the valve orifice is given by
where A max is the area of the valve orifice when it opens fully, V′(t) (=dV(t)/dt) is the time derivative of V(t), V′max is the maximum of V′ during diastole, and C 0 is a parameter that determines the time required for the valve to fully open. In this study, C 0 was set to 0.2, such that the magnitude of the flow velocity at the orifice was not outside the physiological range. With this value of C 0, the time for mitral valve opening was 0.035 s, accounting for 4% of the entire cardiac cycle.30 These models were placed at the position of the mitral valve. For the aortic valve, we used the axisymmetric opening mode for all modes of mitral valve opening and set C 0 in (5) to 0.2 to describe the temporal change in orifice size.
Blood Flow Analysis and the Computer Simulation Procedure
Blood was treated as an incompressible Newtonian fluid with a density of 1.05×103 kg/m3 and a viscosity of 3.5×10−3 Pa·s. The blood flow was analyzed by solving the Navier–Stokes equations along with the equation of continuity:
where U is the three-dimensional velocity vector, P is the pressure, and ρ and ν are the density and kinematic viscosity of blood, respectively. The commercial computational fluid dynamics (CFD) package ANSYS/FLOTRAN ver. 7.0 (distributed by Cybernet Systems, Tokyo, Japan), which implements a finite element method, was used as the solver. The boundary conditions were
The flow simulation commenced at the onset of diastole, for which a quiescent flow state was assumed. The geometry of the left ventricle at the onset of diastole was obtained by reducing the volume of the model shown in Fig. 1 by 80 cm3. After advancing a time step and specifying dV/dt, we calculated the moving velocity of the ventricular wall using Eqs. (1)–(4). The geometry of the left ventricle model was then updated and divided into finite elements. Physical quantities, i.e., the velocity and pressure at new nodal points, were interpolated to the new nodal points using the Lagrange interpolation. Blood flow was then calculated given the boundary conditions specified by Eq. (8). This process was repeated for five cardiac cycles until a periodic cyclic flow pattern was obtained.
Schematic drawings of the modes of valve opening as seen from the top of the left ventricle: (a) axisymmetric opening, (b) anteroposterior opening (parallel to the x-axis), (c) bilateral opening (parallel to the y-axis), and (d) instantaneous opening. Note that valve models (a)–(c) are fully opened at t=0.035 s after the onset of diastole.
RESULTS
Transmitral Flow Velocity Profile
Regardless of the mode of mitral valve opening, blood flowed into the ventricular cavity and filled it when the ventricle started to expand. Figure 5 illustrates the velocity profiles of the transmitral flow at t=0.04 s, just after the mitral valve orifice had opened fully. As the figure shows, there was marked variation in the velocity profile of the transmitral flow between the different modes of mitral valve opening. In the axisymmetric opening mode, the velocity profile was almost axisymmetric and parabolic (Fig. 5(a)). In contrast, in the anteroposterior opening mode, the velocity profile formed a triangular-prism shape lying transversally in the anteroposterior direction (Fig. 5(b)). A similar profile, but lying in the bilateral direction, was seen with bilateral opening (Fig. 5(c)). A flat velocity profile was found for the instantaneous opening mode (Fig. 5(d)). These results indicated that the shape of the mitral valve orifice strongly affected the transmitral velocity profile, even if the valve opened within a very short period of time after the onset of diastole.
Figure 6 shows the velocity profiles of transmitral flow just before the mitral valve was about to close at the end of early diastole (t=0.36 s). In each case, the transmitral velocity profile was similar to the one seen just after the mitral valve orifice had opened fully. Therefore, the mode of mitral valve opening affected the velocity profile not only during the phase of valve opening, but even after the mitral valve had opened fully.
Intraventricular Blood Flow
The hemodynamics within the ventricular cavity during diastole are shown in Figs. 7–10, which plot the instantaneous streamlines in the long-axis plane of the ventricle. The times shown in each figure refer to the time elapsed from the onset of diastole. For the axisymmetric opening mode, a clockwise-rotating vortex formed under the aortic valve at an early stage of diastole. With expansion of the left ventricle, this vortex enlarged longitudinally toward the ventricular apex and expanded circumferentially to the posterior, becoming an annular vortex about the long axis of the left ventricle. In the long-axis plane of the ventricle, the annular vortex appeared as a pair of vortexes that sandwiched the ventricular filling flow on the long axis of the left ventricle (Fig. 7, t=0.2–0.4 s). In this case, the anterior vortex was comparable in size to the posterior vortex. Although the vortex persisted until the end of diastole (Fig. 7, t=0.5 s), it disappeared when blood was ejected from the aortic valve during systole (Fig. 7, t=0.6–0.7 s). The flow pattern with anteroposterior opening was similar to that in the case of axisymmetric opening throughout the cardiac cycle. During diastole, blood entering from the mitral valve flowed along the long axis of the ventricle and an annular vortex formed around it (Fig. 8, t=0.4–0.5 s). During systole, blood was ejected from the aortic valve orifice smoothly (Fig. 8, t=0.6 s) and no vortex was observed at t=0.8 s. Even in the bilateral opening mode, an annular vortex arose in diastole. Nevertheless, the posterior part of this annular vortex (posterior vortex) was larger than the anterior part (Fig. 9, t=0.4–0.5 s). Furthermore, the posterior vortex stalled near the ventricular apex in systole (Fig. 9, t=0.6–0.7 s) and remained stalled until the beginning of diastole (Fig. 9, t=0 s). In contrast, in instantaneous opening mode, there was a very large posterior annular vortex (Fig. 10, t = 0.4–0.5 s), which shifted blood inflow from the ventricular long axis to the anterior side.
Efficiency of Ventricular Ejection
Particles were tracked in the obtained flow field to examine the effect of the mode of mitral valve opening on the efficiency of ventricular ejection. Twenty-six thousand particles were distributed equidistantly in three dimensions in the left ventricle at the end of diastole. The particles were traced until they left through either the aortic (ejection) or mitral (regurgitation) orifices. Euler integration was used to compute the flow paths. The number of particles remaining at the end of systole in each cardiac cycle is plotted in Fig. 11, normalized to the number in the first cardiac cycle. Here, the ordinate is logarithmic. In all cases, the number of particles was halved by the end of the second cardiac cycle. After the third cardiac cycle, there was a difference in the number of particles that had not been ejected. The graph shows that the efficiency of ventricular ejection was worst with instantaneous opening mode. In bilateral opening mode, the particles were not ejected as efficiently as in anteroposterior and axisymmetric opening modes.
DISCUSSION
As is evident from our results, the intraventricular hemodynamics showed a distinct pattern depending on the mode of mitral valve opening. Since the imposed movement of the left ventricular wall was the same in all cases, any differences in the intraventricular flow field were attributable to the velocity profile of the transmitral flow. Although the mitral valve opened fully within 0.035 s and the shape of the mitral valve orifice was the same after the valve had opened fully, there was a difference in the velocity profile of the transmitral flow. According to our results, the framework of the velocity profile for the transmitral flow was built during the phase of mitral valve opening and was characterized by the mode of valve opening. After the mitral valve opened completely, the transmitral velocity profile developed while maintaining its topological features. This phenomenon could be explained in terms of fluid mechanics. During the phase of mitral valve opening, the fluid momentum develops a spatial distribution corresponding to the velocity profile at the mitral valve orifice. Although blood is allowed to flow through the entire mitral orifice once the valve has opened fully, fluid elements introduced from a higher momentum part of the mitral valve orifice can enter the ventricular cavity more effortlessly. Since the distribution of fluid momentum is unchanged qualitatively until the valve starts to close, the transmitral flow maintains the features of the velocity profile established during valve opening.
In all cases, the flow evolution resulted in the development of an annular vortex around the ventricular filling flow. However, the appearance of the vortex differed with the mode of mitral valve opening. The formation of this annular vortex is a key feature of the intraventricular diastolic flow, as commonly observed in computational,7 , 18 , 21 , 22 , 29 , 31 , 32 experimental,5 , 28 and clinical8 , 14 , 15 studies. Previous computational11 , 16 , 21 , 35 and clinical14 studies have shown that the intraventricular annular vortex is asymmetrically large in its anterior part when there is no heart or valve failure. In our simulation, the posterior vortex was larger than the anterior vortex in all cases because the left ventricle model used represented a posteriorly dilated cardiomyopathy that had room for the posterior vortex to grow. Nevertheless, the size of the anterior vortex was comparable to that of the posterior vortex when the valve orifice opened axisymmetrically or anteroposteriorly.
The fluidic advantages of an intraventricular vortex have been reported. It has been postulated that the vortex helps to mix blood in the ventricular cavity and displaces blood that would stagnate and clot otherwise, reducing the risk of thrombus formation.27 In particular, since mechanical heart valves are highly thrombogenic, a small amount of stagnation in the left ventricle could be fatal. According to Kilner et al.,13 , 14 the vortex under the aortic valve (anterior vortex) plays an important role in helping to redirect blood inflow preferentially toward the ventricular outflow tract, such that blood is ejected into the aorta smoothly with little loss of momentum. Therefore, the vortex under the aortic valve should be very large to redirect inflow toward the aortic valve, rather than away from it. This was clearly reflected in our results, when the efficiency of ventricular ejection was compared. The efficiency was excellent in axisymmetric and anteroposterior opening modes. In these two cases, the anterior vortex was large, which contributed to fluid mixing in the ventricular cavity and in recruiting fluid elements on the posterior side for ventricular ejection. Conversely, instantaneous opening mode was the least efficient. The posterior part of the vortex was larger, which trapped fluid particles so that they did not flow out during systole. These results address the importance of the mode of mitral valve opening from the viewpoint of intraventricular vortex dynamics.
Recently, artificial heart valves have been actively developed. In reality, the valve orifice opens almost instantaneously, and consequently the fluid mechanics within the ventricle do not receive as much attention as those near the valve when evaluating valve performance. However, our results show that the intraventricular flow dynamics, including ventricular ejection, were dramatically affected by the mode of mitral valve opening. This implies that even if the shape of the valve orifice is the same when it opens fully, the vortex pattern in the ventricle may change if the orifice shape differs during the process of valve opening. Therefore, we suggest that the opening mode of the valve orifice should also be emphasized in the design of artificial valves.
The limitations of our study must be noted. The geometry of the left ventricle model was idealized by neglecting luminal irregularities and attachments, such as papillary muscles and chordae tendinae. The movement of the mitral annulus could disturb flow near the valve. Nevertheless, we speculate that these effects are confined locally and do not result in major changes in intraventricular flow. Our simulation does not include isovolumetric expansion and contraction phases because CFD always requires a pressure boundary condition or a traction-free condition at some nodal points. If included, these phases contribute to settling flow disturbances within the ventricular cavity. The most crucial aspect of our simulation that limits extension of the discussion to the design of artificial valves favored by fluid mechanics is the absence of valve structure. As Akutsu’s group showed,2 – 4 small differences in valvular design can generate marked differences in the downstream flow field. According to Gao et al.10 and Wang et al.,34 hinge designs yield small vortices, stagnant flows, and turbulent shear stresses that cause thrombogenesis or thromboembolic events. The presence of valve leaflets might intensity vortices, since a shear layer develops on the leaflets, although the valve leaflets are not essential requirements for developing diastolic vortices.25 If these mechanical structures of the valves are simulated, the variation in the intraventricular flow field induced by different opening modes of the valve is more pronounced, or the resulting flow fields near the valve might change. However, we emphasize that in addition to the structural design of the valve, the opening mode of the valve also influences the flow dynamics downstream. This conclusion is unchanged even if these aforementioned factors are considered.
CONCLUSION
We examined the effect of the mode of mitral valve opening on the hemodynamics within the left ventricle using computational fluid dynamics. We demonstrated that the mode of mitral valve opening influenced the transmitral velocity profile, not only during opening, but also throughout diastole, although valve opening accounted for only 4% of the cardiac cycle. The velocity profile of the transmitral flow also affected the flow field in the ventricular cavity throughout the cardiac cycle and consequently the efficiency of ventricular ejection. Therefore, it is important to consider the mode of valve opening when designing artificial valves.
REFERENCES
Aazami, M., and H. J. Schafers. Advances in heart valve surgery. J. Interv. Cardiol. 16:535–541, 2003.
Akutsu, T., and D. Higuchi. Effect of the mechanical prosthetic mono and bi-leaflet heart valve orientation on the flow field inside the simulated ventricle. J. Artif. Organs 3:126–135, 2000.
Akutsu, T., and D. Higuchi. Flow analysis of the bileaflet mechanical prosthetic heart valves using laser Doppler anemometer: Effect of the valve designs and installed orientations to the flow inside the simulated ventricle. J. Artif. Organs 4:113–112, 2001.
Akutsu, T., and T. Masuda. Three-dimensional flow analysis of a mechanical bileaflet mitral prosthesis. J. Artif. Organs 6:112–123, 2003.
Bellhouse, B. J. Fluid mechanics of a model mitral valve and left ventricle. Cardiovasc. Res. 6:199–210, 1972.
Butterfield, M., D. J. Wheatley, D. F. Williams, and J. Fisher. A new design for polyurethane heart valves. J. Heart Valve Dis. 10:105–110, 2001.
Domenichini, F., G. Pedrizzetti, and B. Baccani. Three-dimensional filling flow into a model left ventricle. J. Fluid Mech. 539:179–198, 2005.
Ebbers, T., L. Wigstrom, A. F. Bolger, B. Wranne, and M. Karlsson. Noninvasive measurement of time-varying three-dimensional relative pressure fields within the human heart. J. Biomech. Eng. 124:288–293, 2002.
Ellis, J. T., T. M. Wick, and A. P. Yoganathan. Prosthesis-induced hemolysis: mechanisms and quantification of shear stress. J. Heart Valve Dis. 7:376–386, 1998.
Gao, Z. B., N. Hosein, F. F. Dai, and N. H. Hwang. Pressure and flow fields in the hinge region of bileaflet mechanical heart valves. J. Heart Valve Dis. 8:197–205, 1999.
Iwase, H., H. Liu, S. Fujimoto, and R. Himeno. Computational modeling of left ventricle dynamics and flow based on ultrasonographic data. JSME Int. J. Ser. C 46:1321–1329, 2003.
Keren, G. Functional mitral regurgitation: Physiologic and clinical significance in congestive cardiomyopathy. Isr. J. Med. Sci. 27:105–108, 1991.
Kilner, P. J., M. Y. Henein, and D. G. Gibson. Our tortuous heart in dynamic mode—an echocardiographic study of mitral flow and movement in exercising subjects. Heart Vessels 12:103–110, 1997.
Kilner, P. J., G. Z. Yang, A. J. Wilkes, R. H. Mohiaddin, D. N. Firmin, and M. H. Yacoub. Asymmetric redirection of flow through the heart. Nature 404:759–761, 2000.
Kim, W. Y., P. G. Walker, E. M. Pedersen, J. K. Poulsen, S. Oyre, K. Houlind, and A. P. Yoganathan. Left ventricular blood flow patterns in normal subjects: A quantitative analysis by three-dimensional magnetic resonance velocity mapping. J. Am. Coll. Cardiol. 26:224–238, 1995.
Long, Q., R. Merrifield, G. Z. Yang, P. J. Kilner, D. N. Firmin, and X. Y. Xu. The influence of inflow boundary conditions on intra left ventricle flow predictions. J. Biomech. Eng. 125:922–927, 2003.
Manning, K. B., V. Kini, A. A. Fontaine, S. Deutsch, and J. M. Tarbell. Regurgitant flow field characteristics of the St. Jude bileaflet mechanical heart valve under physiologic pulsatile flow using particle image velocimetry. Artif. Organs 27:840–846, 2003.
McQueen, D. M., and C. S. Peskin. Heart simulation by an immersed boundary method with formal second-order accuracy and reduced numerical viscosity In: edited by Aref, H., and J. W. Philips. Mechanics for a New Millennium, Dordrechet, The Netherlands: Kluwer Academic Publishers, pp. 429–444, 2000.
Nakamura, M., S. Wada, T. Mikami, A. Kitabatake, and T. Karino. Relationship between intraventricular flow patterns and the shapes of the aliasing area in color M-mode Doppler echocardiograms—A CFD Study with an axisymmetric model of the LV. JSME Int. J. Ser. C 44:1013–1020, 2001.
Nakamura, M., S. Wada, T. Mikami, A. Kitabatake, and T. Karino. A computational fluid mechanical study on the effects of opening and closing of the mitral orifice on a transmitral flow velocity profile and an early diastolic intraventricular flow. JSME Int. J. Ser. C 45:913–922, 2002.
Nakamura, M., S. Wada, T. Mikami, A. Kitabatake, and T. Karino. Computational study on the evolution of a vortical flow in a human left ventricle during early diastole. Biomech. Model. Mechanobiol. 2:59–72, 2003.
Nakamura, M., S. Wada, T. Mikami, A. Kitabatake, T. Karino, and T. Yamaguchi. The effect of flow disturbances remaining at the beginning of diastole on intraventricular diastolic flow and color M-mode Doppler echocardiogram. Med. Biol. Eng. Comput. 42:509–515, 2004.
Paul, R., O. Marseille, E. Hintze, L. Huber, H. Schima, H. Reul, and G. Rau. In vitro thrombogenicity testing of artificial organs. Int. J. Artif. Organs 21:548–552, 1998.
Pierrakos, O., P. Vlachos, and D. Telionis. Time-resolved DPIV analysis of vortex dynamics in a left ventricular model through bileaflet mechanical and porcine heart valve prostheses. J. Biomech. Eng. 126:714–726, 2004.
Saber, N. R., N. B. Wood, A. D. Gosman, R. D. Merrifield, G. Z. Yang, C. L. Charrier, P. D. Gatehouse, and D. N. Firmin. Progress towards patient-specific computational flow modeling of the left heart via combination of magnetic resonance imaging with computational fluid dynamics. Ann. Biomed. Eng. 31:42–52, 2003.
Sezai, A., M. Shiono, Y. Orime, H. Hata, S. Yagi, N. Negishi, and Y. Sezai. Evaluation of valve sound and its effects on ATS prosthetic valves in patients’ quality of life. Ann. Thorac. Surg. 69:507–512, 2000.
Shortland, A. P., R. A. Black, J. C. Jarvis, and S. Salmons. Factors influencing vortex development in a model of a skeletal muscle ventricle. Artif. Organs 20:1026–1033, 1996.
Steen, T., and S. Steen. Filling of a model left ventricle studied by colour M mode Doppler. Cardiovasc. Res. 28:1821–1827, 1994.
Taylor, T. W., and T. Yamaguchi. Flow patterns in three-dimensional left ventricular systolic and diastolic flows determined from computational fluid dynamics. Biorheology 32:61–71, 1995.
Tsakiris, A. G., D. A. Gordon, Y. Mathieu, and I. Lipton. Motion of both mitral valve leaflets: A cineroentgenographic study in intact dogs. J. Appl. Physiol. 39:359–366, 1975.
Vierendeels, J. A., K. Riemslagh, E. Dick, and P. R. Verdonck. Computer simulation of intraventricular flow and pressure gradients during diastole. J. Biomed. Eng. 122:667–674, 2000.
Vierendeels, J. A., E. Dick, and P. R. Verdonck. Hydrodynamics of color M-mode Doppler flow wave propagation velocity V(p): A computer study. J. Am. Soc. Echocardiogr. 15:219–224, 2002.
Walker, D. K., A. M. Brendzel, and L. N. Scotten. The new St. Jude Medical regent mechanical heart valve: laboratory measurements of hydrodynamic performance. J. Heart Valve Dis. 8:687–696, 1999.
Wang, J., H. Yao, C. J. Lim, Y. Zhao, T. J. Yeo, and N. H. Hwang. Computational fluid dynamics study of a protruded-hinge bileaflet mechanical heart valve. J. Heart Valve Dis. 10:254–262, 2001.
Watanabe, H., S. Sugiura, and T. Hisada. Finite element analysis on the relationship between left ventricular pump function and fiber structure within the wall. JSME Int. J. Ser. C 46:330–1339, 2003.
ACKNOWLEDGMENTS
This work was supported by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists (No. 06787). It was also funded by Grants-in-Aid of Scientific Research No. 15086204 and 17300138, the “Revolutionary Simulation Software (RSS21)” project, supported by the next-generation IT program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Grants-in-Aid of Scientific Research from the MEXT and JSPS Scientific Research in Priority Areas (768) “Biomechanics at Micro- and Nanoscale Levels,” and Scientific Research (A) No.16200031, “Mechanism of the formation, destruction, and movement of thrombi responsible for ischemia of vital organs.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, M., Wada, S. & Yamaguchi, T. Influence of the Opening Mode of the Mitral Valve Orifice on Intraventricular Hemodynamics. Ann Biomed Eng 34, 927–935 (2006). https://doi.org/10.1007/s10439-006-9127-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9127-3