Abstract
The osteoinductive activity induced by recombinant human BMP-2 (rhBMP-2) blunts proportionately as the recipient ages. In order to compensate for this bluntness administration of fibroblast growth factor-2 (FGF-2) has been considered. The aim of this study was to determine whether FGF-2 administration augments osteoinductive activity caused by rhBMP-2 and to evaluate the effect of aging on bone formation induced by coadministration of rhBMP-2 and FGF-2. Sixty-four Wistar strain male rats of 8-week-old (prepubertal) and 16-week-old (postpubertal) received bone defects bilaterally in the parietal bone and the defects were filled by a polylactic acid polyglycolic acid copolymer/gelatin sponge (PGS) impregnated with rhBMP-2 plus 0 ng, 25 ng, and 250 ng FGF-2 (n=10 in each). At 2 weeks after grafting, the new bone volume seemed to be larger in the rhBMP-2+FGF-2 groups than in the rhBMP-2 alone group. At 4 weeks, the new bone formation was linked to the adjacent original bone. In the prepubertal rats, all newly formed bone was similarly calcified. In the postpubertal rats, only the rhBMP-2+25 ng FGF-2 group showed this higher degree of calcification. At 2 weeks, alkaline phosphatase (ALP) activity in the rhBMP-2+25 ng FGF-2 group was significantly (p<0.05) larger than that in the rhBMP-2 group in both prepubertal and postpubertal rats. This result shows that low-dose administration of FGF-2 enhanced the degree of calcification and ALP activity in the rhBMP-2 grafting site especially in the postpubertal rats. Therefore, FGF-2 would be a candidate to compensate for the reduction of osteoinductive activity of rhBMP-2 with aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Bone morphogenetic protein (BMP) was first described by Urist32 and its therapeutic potential for bone formation has been widely recognized since then. BMPs are a group of related osteoinductive proteins that share sequence similarities with the transforming growth factor-β (TGF-β) superfamily.36 Recombinant human BMP-2 (rhBMP-2) is known as one of the most osteoinductive BMP agents, and its efficacy and safety have been validated by a number of animal studies.16, 18,19, 31,33 Therefore, rhBMP-2 may be an effective substitute for autogenous bone in bone grafting into defects.
Numerous reports from animal and cell culture models indicate that bone formation is impaired with aging, and an imbalance between formation and resorption thus leads to bone loss.28,29 These age-related changes not only affect bone metabolism and regeneration under normal conditions but also influence pathological conditions such as in fracture or alveolar bone repair.2 After BMP graft implantation, a progression of cellular events occurs that closely resembles the endochondral bone formation during development or adult fracture repair.32 rhBMP-2 is effective in recruiting cells to the implant and stimulating their differentiation, however, aging may reduce its bone inducing activity by altering the ability of an implant to be properly vascularized.4,8,13,22
Recently, in order to enhance the osteoinductive activity of rhBMP-2, a growth factor such as fibroblast growth factor-2 (FGF-2) has been considered as a candidate. FGF-2 has a strong proliferative effect on endothelial cells, osteocytes, and chondrocytes.7,11 FGF-2 exerts its pro-angiogenic activity by interacting with various endothelial cell surface receptors.25 Also, cross-talk among FGFs, VEGFs, and inflammatory cytokines may play a role in the modulation of angiogenesis in different pathological conditions, including bone healing.25 With this in mind and because the reduction of rhBMP-2-induced osteoinductive activity in aged animals has been related to a reduction of vascularization,22 a proper understanding of the combined action of rhBMP-2 and FGF-2 can presumably provide us a better insight into possible clinical application of rhBMP-2 for bone grafting both in the younger and elder humans.
Because rhBMP-2 is too diffusible, its application for clinical use requires a suitable carrier to retain it at the vicinity of the grafting site. Polylactic acid polyglycolic acid copolymer/gelatin sponge (PGS)12,40 is a promising carrier material, i.e., it has the potential to absorb BMP, it is a porous structure which allows cells to migrate into it, and it has enough firmness against soft tissue pressure.12
The present study was designed to determine whether FGF-2 administration augments osteoinductive activity caused by rhBMP-2 in prepubertal and postpubertal bone. Rats were used as an animal model, and PGS was used as a carrier for rhBMP-2 and FGF-2. The process of bone formation in grafted rat skull defects was observed histologically and the newly formed bone was analyzed radiographically and biochemically.
MATERIALS AND METHODS
Animals
Sixty-four Wistar strain male rats were used for this study; 8-week-old (prepubertal; n=34) and 16-week-old (postpubertal; n=30) rats. The animals were kept in cages with a constant ambient temperature of 22°C and fed a solid diet and water ad libitum. The protocol of the experiment was approved by the Animal Care and Use Committee at Hiroshima University.
Preparation of Grafts
rhBMP-2 was provided by Yamanouchi Pharmaceutical Co. (Tokyo, Japan). It was dissolved in LF6 buffer containing 5 mM glutamic acid, 5 mM NaCl, 2.5% glycine, 0.5% sucrose, and 0.01% polysorbate 80. PGS12, 41 provided by Yamanouchi Pharmaceutical Co. (Tokyo, Japan) was used as a carrier for rhBMP-2 and FGF-2. PGS consisted of 40% polyglycolic acid (PGA), 40% polylactic acid (PLA) and 20% gelatin. PGS was cut into flattened cylinders (diameter: 3.5 mm; thickness: 1.0 mm) to fill the defect. FGF-2 (Product No. F0291; 16.4 kDa) was purchased from Sigma Co. (Saint Louis, MI, USA). Firstly, PGS (2 mg) was impregnated with rhBMP-2 (0.1 mg/ml) and stored at −80°C. With respect to FGF-2, freeze-dried samples of 25 and 250 ng were dissolved in phosphate buffer saline (PBS) with the same volume as the PGS (resultant concentrations: 2.6 and 26.0 ng/mm3, respectively) and stored at −80°C. Immediately before use, the frozen FGF-2 solution was dissolved and impregnated with the PGS with rhBMP-2. The concentrations of the two growth factors were determined according to previous studies.17, 35,39, 42
Surgical Procedure
All rats were anesthetized intraperitoneally with sodium pentobarbital at a dose of 50 mg/kg body weight. Under infiltrating anesthesia with 0.4 ml of 2% xylocaine hydrochloride, an anteroposteriorly directed incision of about 10-mm long was made into the scalp. Periosteum of the skullcap was ablated, and a full-thickness of standardized trephine defect with a diameter of 3.5 mm was made bilaterally in the parietal bone by a low speed trephine burr (Fig. 1). Extreme care was taken to avoid injury to the dura mater. For each age group, rats were randomly divided into three subgroups, rhBMP-2 alone, rhBMP-2+25 ng FGF-2, and rhBMP-2+250 ng FGF-2. The artificial defects on both sides were implanted by PGS impregnated with rhBMP-2 plus 0 ng, 25 ng, and 250 ng FGF-2 (n=10 in each). Total six subgroups consisted of three prepubertal and three postpubertal subgroups. After implantation, the ablated periosteum and skin were replaced and sutured, respectively. In order to protect against infections, all rats were intraperitoneally administrated with tetracycline (Sigma Aldrich Japan, Tokyo, Japan) at a dose of 30 mg/kg.
In each subgroup five rats were killed at 2 weeks, and the other five at 4 weeks after the implantation. The grafted bone defects were dissected together with the surrounding bone and tissue, and examined radiographically and biochemically (n=4 in each time) and histologically (n=1 in each time). Histological evaluation was performed in each animal at both sites; hence the total amount of histological examinations was 12 (6 subgroups×2 sites).
The remaining four 8-week-old rats served as control group in order to confirm the possibility of natural healing in the artificial bone defect. After preparation of the defects, no implantation with grafting materials was conducted and control rats survived for 4 weeks.
Histological Evaluation
The tissues were fixed with 10% buffered formaldehyde overnight at 4°C, demineralized with 14% EDTA for 3 weeks, and then embedded in paraffin. Serial sections of 7 μm thickness were made in the sagittal plane, and stained with hematoxylin and eosin. These sections were observed with a microscope (BX-50; Olympus Optical, Tokyo, Japan).
Radiographic and Biochemical Evaluation
From the dissected tissues, the central part of the implants was resected by a cylindrical trephine bur (inside diameter: 2.7 mm) cooled with physiological saline. First, the resected implants were radiographically evaluated (n=4 in each) with a rat/mouse cephalometric apparatus (conditions used: 20 kVp, 1.4 s; RM60: Asahi Roentgen Industry Co., Kyoto, Japan). The implants were analyzed together with a graduated aluminum stepwedge (thickness: 192–1152 μm). The optical densities of the radiographic images of the grafts and the six steps on the aluminum stepwedge were measured by means of Scion image software (Scion Co., Baltimore, USA). The optical density values for each aluminum step were plotted as a function of the aluminum thickness in mm to create a regression curve using Statview (Abacus Concepts Inc., Berkeley, USA). By means of the regression curve, the equivalent aluminum thickness of each sample was calculated and used to evaluate the degree of calcification for the implants.5 The average value of both sides was used as the mean value of each rat.
After taking a radiograph, the resected grafts were examined biochemically. First they were crushed in an achate mortar under liquid nitrogen, and lysed with 0.2% Triton-X 100 (Katayama chemical Industries, Osaka Japan). After stirring, they were centrifuged at 10,000 rpm for 5 min, and the supernatants were used for the enzyme assay. Alkaline phosphatase (ALP) activity was assayed with disodium p-nitrophenyl-phosphate hexahydrate (Wako Pure Chemical Industries, Osaka, Japan) used as a substrate, according to the method described previously.24 The amount of p-nitrophenol produced was spectrophotometrically measured with a microplate reader (Model 550, Japan Biorad Lab., Tokyo, Japan) at 405 nm and ALP activity was calculated with reference to known density standards. The average value of both sides was calculated and used as the mean value of each rat.
Statistical Analysis
For each subgroup, mean and standard deviation (SD) were calculated for each measured value, i.e., the equivalent aluminum thickness (mm) and the ALP activity (nmol/implant). Numerical data were analyzed by ANOVA, using age, FGF-2 dose, or time implanted as independent variables with Statview (Abacus Concepts Inc., Berkeley, USA). When a significant f-value was obtained, Fisher's protected least significant difference (LSD) test was performed. The level of significance was set at p < 0.05.
RESULTS
Histological Examination
The control animals showed a narrow margin of new bone formation around the edge of the defect on 4 weeks (Fig. 2). Comparing to the original parietal bone, no cases exhibited complete closure of new bone across the 3.5 mm defect. The defects were filled with fibrous connective tissue.
Two weeks after implantation, small islands of osteoblasts and adipocytes scattered in the newly formed bone in both the prepubertal and postpubertal rats (Fig. 3). In the rhBMP-2+FGF-2 groups, the new bone volume seemed to be larger than that in the rhBMP-2 group without FGF-2. No difference in volume of newly formed bone was observed between prepubertal and postpubertal rats.
Photomicrographs of the grafting site at 2 weeks in 8-week-old and 16-week-old rats. 8-week-old rats: A) rhBMP-2 group, B) rhBMP-2+25 ng FGF-2 group, C) rhBMP-2+250 ng FGF-2 group. 16-week-old rats: D) rhBMP-2 group, E) rhBMP-2+25 ng FGF-2 group, F) rhBMP-2+250 ng FGF-2 group. Arrow and arrowhead indicate osteoblast and adipocyte, respectively. Scale bars indicate 100 μm. N: newly formed bone. Hematoxylin and eosin staining.
At 4 weeks after grafting (Fig. 4), in the prepubertal rats the new bone formation seemed to progress further than at 2 weeks, and the new bone mass appeared to be linked to the adjacent original bone. Comparing the three groups with different amounts of FGF-2, the new bone volume seemed to be larger in the rhBMP-2+25 ng FGF-2 group than in the other two groups.
Photomicrographs of the grafting site at 4 weeks in 8-week-old and 16-week-old rats. 8-week-old rats: A) rhBMP-2 group, B) rhBMP-2+25 ng FGF-2 group, C) rhBMP-2+250 ng FGF-2 group. 16-week-old rats: D) rhBMP-2 group, E) rhBMP-2+25 ng FGF-2 group, F) rhBMP-2+250 ng FGF-2 group. Arrowhead indicates bone marrow. Scale bars indicate 100 μm. N: newly formed bone. Hematoxylin and eosin staining.
In the postpubertal rats, after 4 weeks the new bone formation had slightly progressed compared with that after 2 weeks. However, in the rhBMP-2 and rhBMP-2+250 ng FGF-2 groups, the volume of the newly formed bone seemed to be markedly smaller than in the similarly treated prepubertal rats. However, the new bone formation in the rhBMP-2+25 ng FGF-2 group was similar to that in the prepubertal rats.
Radiographic Evaluation
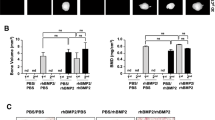
The aluminum equivalent thickness of nontreated parietal bone in the prepubertal and postpubertal rats was approximately 0.5 mm. This was taken as a reference. In the prepubertal rats, the aluminum equivalent thickness at 2 weeks was 0.21 mm (SD 0.02), 0.29 mm (SD 0.02), and 0.29 mm (SD 0.03) in the grafts with rhBMP-2, rhBMP-2+25 ng FGF-2, and rhBMP-2+250 ng FGF-2, respectively (Fig. 5). The aluminum equivalent thicknesses in the rhBMP-2+FGF-2 groups were significantly (p < 0.01) larger than that in the rhBMP-2 group. On the contrary, the aluminum equivalent thicknesses of the grafts in the postpubertal rats were almost similar, irrespective of the amount of FGF-2 (rhBMP-2: 0.16±0.01 mm; rhBMP-2+25 ng FGF-2: 0.16±0.01 mm; rhBMP-2+250 ng FGF-2: 0.18±0.01 mm), and they were significantly (p < 0.01 or p < 0.05) smaller than those in the prepubertal rats.
After 4 weeks, the aluminum equivalent thickness of the grafts was larger than after 2 weeks. In the prepubertal rats, the values were 0.38 mm (SD 0.02), 0.40 mm (SD 0.03), and 0.39 mm (SD 0.02) in the rhBMP-2, rhBMP-2+25 ng FGF-2, and rhBMP-2+250 ng FGF-2 groups, respectively, and did not differ significantly. In the postpubertal rats, the rhBMP-2+25 ng FGF-2 group showed a significantly (p < 0.01 or p < 0.05) higher value (0.37±0.05 mm) of the aluminum equivalent thickness compared to the rhBMP-2 (0.29±0.04 mm) and rhBMP-2+250 ng FGF-2 groups (0.21±0.05 mm), which was almost equal to those in the prepubertal groups. The aluminum equivalent thickness in the rhBMP-2+250 ng FGF-2 group was significantly (p < 0.05) smaller than that in the rhBMP-2 group.
Biochemical Evaluation
In both prepubertal and postpubertal rats, ALP activity at 2 weeks (Fig. 6) was significantly (p < 0.05) larger in the rhBMP-2+FGF-2 groups (prepubertal: 5.86±0.04 nmol in rhBMP-2+25 ng FGF-2, 5.85±0.04 nmol in rhBMP-2+250 ng FGF-2; postpubertal: 5.84±0.02 nmol in rhBMP-2+25 ng FGF-2, 5.83±0.03 nmol in rhBMP-2+250 ng FGF-2) than that in the rhBMP-2 group (prepubertal: 5.79±0.01 nmol; postpubertal: 5.76±0.08 nmol). There was no significant difference of ALP activity between the prepubertal and postpubertal rats at 2 weeks. At 4 weeks, ALP activity was significantly (p<0.01) less than at 2 weeks. In both prepubertal and postpubertal rats, ALP activity was significantly (p<0.01) larger in the rhBMP-2 group (prepubertal: 5.61±0.06 nmol; postpubertal: 5.70±0.05 nmol) than in the rhBMP-2 and FGF-2 groups (prepubertal: 5.55±0.03 nmol in rhBMP-2+25 ng FGF-2, 5.55±0.05 nmol in rhBMP-2+250 ng FGF-2; postpubertal: 5.61±0.06 nmol in rhBMP-2+25 ng FGF-2, 5.62±0.04 nmol in rhBMP-2+250 ng FGF-2). The postpubertal rats showed significantly (p<0.01 or p < 0.05) larger ALP activity than the prepubertal rats at 4 weeks.
DISCUSSION
Numerous cytokines are reported to induce bone formation in vivo. Among these cytokines, rhBMP-2 is one of the strongest bone inducers in bone allografts. It enhances the migration of osteoblast progenitor cells, the proliferation of mesenchymal cells, and their differentiation into chondrogenic and osteogenic cells.2 As a result, rhBMP-2 primarily augments the differentiation of mesenchymal precursor cells into cartilage- and bone-forming cells.36 For example, the nonosteogenic mouse pluripotent cell line has a potential ability to differentiate into osteoblastic cells under the control of rhBMP-2.14 Furthermore, rhBMP-2 induces differentiation of osteoblast progenitor cells into mature osteoblasts with the ability to synthesize osteocalcin.38 It has been recognized that the osteoinductive activity of rhBMP-2 blunts with age.4, 8,22 In order to compensate for this bluntness, a growth factor such as FGF-2 has been considered as a candidate because FGF-2 has not only a strong proliferative effect on various cells but also a physiological effect on angiogenesis in vivo.7, 10,25 Angiogenesis is a vital process for early bone formation and remodeling, and plays a key role in various physiological and pathological conditions, including embryonic development, wound repair, and inflammation.6 Numerous inducers of angiogenesis have been identified, including the vascular endothelial growth factor (VEGF) family, transforming growth factors (TGF-α and -β), and FGF family.25 Of these, FGF requires the activation of the VEGF/VEGFR system for promoting angiogenesis.25 Clinically, FGF-2 has already been used for wound healing and regeneration of periodontal and bone tissues.21, 37 Furthermore, coadministration of osteogenic rhBMP-2 and FGF-2 may result in more advanced bone formation in a bone-healing model, such as cranial bone defect, because cytokines use independent signal transduction pathways in bone and suture development.27 Therefore, we investigated the effect of coadministration of rhBMP-2 and FGF-2 in graft material.
In this study, 8-week-old and 16-week-old rats were used as prepubertal and postpubertal experimental animals, respectively. It has been demonstrated that there is a rapid decline in bone forming ability in growing rats from 3 weeks to 3 months of age and a further decline in bone forming ability from 3 to 16 months.8 According to the classification of animal life span, the former rats are a model for the growth period before puberty and the latter rats for the mature period after puberty. The age-difference of osteoinductive ability induced by coadministraion of rhBMP-2 and FGF-2 in this study is assumed to be of importance for indicating its possibilities for clinical application in patients of advanced age.
In the present study, we found a dose-dependent effect on osteoinductive activity by the combination of rhBMP-2 and FGF-2 in the postpubertal group. Here a low dose of FGF-2 had a stimulatory effect on bone formation and after 4 weeks the degree of calcification was nearly equal to that of the prepubertal rats. On the contrary, a high dose of FGF-2 had an inhibitory effect on bone formation although this effect was not always found. Previously, FGF-2 has revealed a dose-dependent effect on bone formation. Kimoto et al. 17 indicated that bone formation in parietal bone defects was promoted by 1 ng and 10 ng FGF-2 and was inhibited by 100 ng FGF-2. Zellin and Linde42 reported that 10 ng and 100 ng FGF-2 had a stimulative effect on bone formation in mandibular defects and that 1000 ng FGF-2 had an inhibitory effect; Fujimura et al. 9 showed a stimulative effect of 400 ng FGF-2. In addition, in vitro experiments have shown that high-dose administration of FGF-2 causes apoptosis of mature osteoblasts. The administration of low-dose FGF-2 is considered to enhance the rhBMP-2 promoted mitogenic activity and differentiation of the osteogenic precursor cells from mesenchymal cells.9 Akita et al. 1 investigated in vivo calvarial bone defect healing using human mesenchymal stem cells and they revealed that human-derived mesenchymal stem cells are augmented by coadministration of two osteogenic cytokine treatments via presumably independent signaling pathways. Combining these findings, we speculate that the mesenchymal stem cells derived from the bone adjacent to the grafting defect are stimulated by rhBMP-2 and FGF-2, resulting in promotion of bone formation in the grafting site.
Although our result could not identify the effect of FGF-2 on osteoclasts, the lower bone volume associated with high-dose FGF-2 may be concerned with a catabolic effect of high-dose FGF-2. In chondrocyte cultures, FGF-2 causes a dose-dependent inhibition of ALP activity and calcium content.15 Therefore, the administration of high doses of FGF-2 for 4 weeks may inhibit the osteoinductive activity of rhBMP-2. If the optimal carriers are used, rhBMP-2 and FGF-2 remain in the carrier for 3 weeks and 2 weeks, respectively.41 The decline of the bone volume increase from 2 to 4 weeks after implantation of the graft may therefore be caused by a long time release of FGF-2.
In this study, FGF-2 also affected the ALP activity in rhBMP-2 graft of the prepubertal and postpubertal rats. At 2 weeks after implantation, the ALP activity was enhanced in grafted regions enriched with FGF-2 in either rat. At 4 weeks after graft implantation, the ALP activity was less in rhBMP-2 + FGF-2 groups than in the rhBMP-2 group. Reddi26 reported that ALP activity showed a maximum level at nearly 2 weeks after rhBMP-2 grafting whereas the calcification was maximized after a delay of a few weeks. The possible explanation for ALP activity stimulation by coadministration of rhBMP-2 and FGF-2 is as follows: 1) rhBMP-2 and FGF-2 induce mesenchymal stem cells, 2) FGF-2 enhances the cell proliferation, and 3) rhBMP-2 strongly stimulates the cell differentiation to osteoblasts.
Osteoblasts and adipocytes originate from a common progenitor, which arises from bone marrow mesenchymal stem cells. Aging causes a decrease in commitment of mesenchymal stem cells to the osteoblast lineage and an increase in the commitment to the adipocyte lineage.20 As a result, the rate and quantity of new bone formation in the rhBMP-2 grafting site were reduced with aging. It was speculated that the decreased response may be in part due to a decrease in the responsiveness of these target cells to rhBMP-2. Meanwhile, although FGF-2 promotes cartilage and bone repair, FGF-2 alone does not induce ectopic bone and/or cartilage formation.9 However, FGF-2 increases the calcium content of demineralized bone-matrix implants34 that contain a factor that acts on a specific bone marrow cell population by increasing the proliferation of active cells or inducing the differentiation of dormant cells.3 Therefore, our result that ALP activity and bone formation in the postpubertal group were upregulated by coadministration of rhBMP-2 and 25 ng FGF-2 is presumably due to enhancement of mesenchymal stem cells with respect to both their intrinsic differentiation potential and production of signaling molecules, which contributes to the formation of a specific marrow microenvironment necessary for maintenance of bone homeostasis.
The importance of the delivery system was noted in previous studies.18,19,30 The delivery system releases and localizes the BMP, ensuring interaction with mesenchymal cells that can differentiate into chondrocytes or osteoblasts. Previously several bioabsorbable carriers for rhBMP-2 were developed and tested, e.g., demineralized bone matrix,19, 31 biophasic calcium phosphate (BCP),17, 28 absorbable collagen sponge (ACS),33 and PGS.12, 40 Most carrier materials for rhBMP-2 showed some advantages and disadvantages and it has not yet been determined what the ideal delivery system for rhBMP-2 is. For example, collagen sponge performed well as a carrier for bone formation, but it has risks associated with transplantation of allogeneic and xenogeneic tissues.23 BCP that will biodegrade in register with bone formation may have limited clinical utility. The combination of rhBMP-2, atelopeptide type I collagen, and porous hydroxyapatite is suggested to be advantageous for clinical application in reconstructing bone defects. The PGS carrier used in this study possesses some therapeutic advantages as a carrier for rhBMP-2 and FGF-2, since it is 1) easy to apply, 2) able to retain rhBMP-2 and FGF-2 at a bone regeneration site for a period of time sufficient to induce bone formation, 3) able to provide space for bone regeneration due to its stiffness, and 4) noncytotoxic. Furthermore, PGS retained an appropriate amount of rhBMP-2 at the orthotopically implanted site for at least 3 weeks enough to induce bone regeneration,41 which suggested that PGS has sufficient ability in controlling the release of rhBMP-2. These characteristics are almost consistent with those that a successful rhBMP-2 carrier needs.40
In conclusion, low-dose administration of FGF-2 enhanced the degree of calcification and ALP activity in the rhBMP-2 grafting site especially in the postpubertal rats. Therefore, FGF-2 would be one of the candidates to improve the osteoinductive activity of rhBMP-2 with aging. Further study is required to clarify the mechanism of interaction of rhBMP-2 and FGF-2 on the grafts.
REFERENCES
Akita, S., M. Fukui, H. Nakagawa, T. Fujii, and K. Akino. Cranil bone defect healing is accelerated by mesenchymal stem cells induced by coadministration of bone morphogenetic protein-2 and basic fibroblast growth factor. Wound Repair Regen. 12:252–259, 2004.
Almer, M. H. Age factor in human alveolar bone repair. J. Oral Implantol. 19:138–142, 1993.
Becerra, J., J. A. Andrades, D. C. Ertl, N. Sorgente, and M. E. Nimni. Demineralized bone matrix mediates differentiation of bone marrow stromal cells in vitro: Effect of age of cell donor. J. Bone Miner. Res. 11:1703–1714, 1996.
Bessho, K., and T. Iizuka. Changes in bone inducing activity of bone morphogenetic protein with aging. Ann. Chir. Gynaecol. 207:S49–S53, 1993.
Biovin, G., and C. A. Baud. Microradiographic methods for calcified tissues. In: Methods of Calcified Tissue Preparation, edited by G. R. Dickson. Amsterdam: Elsevier Science, 1984, pp. 391–412.
Carmeliet, P., and R. K. Jain. Angiogenesis in cancer and other diseases. Nature 407:249–257, 2000.
Connolly, D. T., B. L. Stoddard, N. K. Harakas, and J. Feder. Human fibroblast-derived growth factor is a mitogen and chemoattractant for endothelial cells. Biochem. Biophys. Res. Commun. 144:705–712, 1987.
Fleet, J. C., K. Cashman, K. Cox, and V. Rosen. The effects of aging on the bone inductive activity of recombinant human bone morphogenetic protein-2. Endocrinology 137:4605–4610, 1996.
Fujimura, K., K. Bessho, Y. Okubo, K. Kusumoto, N. Segami, and T. Iizuka. The effect of fibroblast growth factor-2 on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rat muscle. Arch. Oral Biol. 47:577–584, 2002.
Globus, R. K., P. Patterson-Buckendahl, and D. Gospodarowicz. Regulation of bovine bone cell proliferation by fibroblast growth factor and transforming growth factor β. Endocrinology 123:98–105, 1988.
Gospodarowicz, D., N. Ferrara, L. Schweigerer, and G. Neufeld. Structural characterization and biological functions of fibroblast growth factor. Endocrinol. Rev. 8:95–114, 1987.
Higuchi, T., A. Kinoshita, K. Takahashi, S. Oda, and I. Ishikawa. Bone regeneration by recombinant human bone morphogenetic protein-2 in rat mandibular defects. An experimental model of defect filling. J. Periodontol. 70:1026–1031, 1999.
Jergesen, H. E., J. Chua, R. T. Kao, and L. B. Kalsau. Age effects on bone induction by demineralized bone powder. Clin. Orthop. Relat. Res. 268:253–259, 1991.
Katagiri, T., A. Yamaguchi, T. Ikeda, S. Yoshiki, J. M. Wozney, V. Rosen, E. A. Wang, H. Tanaka, S. Omura, and T. Suda. The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphgenetic protein-2. Biochem. Biophys. Res. Commum. 15(172):295–299, 1990.
Kato, Y., and M. Iwamoto. Fibroblast growth factor is inhibitor of chondrocyte terminal differentiation. J. Biol. Chem. 265:5903–5909, 1990.
Kenley, R., L. Marden, T. Turek, L. Jin, E. Roz, and J. O. Holloinger. Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-2). J. Biomed. Mater. Res. 28:1139–1147, 1994.
Kimoto, T., R. Hosokawa, T. Kubo, M. Maeda, A. Sano, and Y. Akagawa. Continuous administration of basic fibroblast growth factor (FGF-2) accelerates bone induction on rat calvaria—an application of a new drug delivery system. J. Dent. Res. 77:1965–1969, 1998.
Marden, L. J., J. O. Hollinger, A. Chaudhari, T. Turek, R. G. Schaub, and E. Ron. Recombinant human bone morphogenetic protein-2 is superior to demineralized bone matrix in repairing craniotomy defects in rats. J. Biomed. Mater. Res. 28:1127–1138, 1994.
Mayer, M., J. Hollinger, E. Ron, and J. Wozney. Maxillary alveolar cleft repair in dogs using recombinant human bone morphogenetic protein-2 and polymer carrier. Plast. Reconstr. Surg. 98:247–259, 1996.
Moerman, E. J., K. Teng, D. A. Lipschitz, and B. Lecka-Czernik. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3:379–389, 2004.
Murakami, S., S. Takayama, M. Kitamura, Y. Shimabukuro, K. Tanagi, K. Ikezawa, T. Saho, T. Nozaki, and H. Okada. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. J. Periodontal. Res. 38:97–103, 2003.
Nagai, N., C. L. Qin, H. Nagatsuka, M. Inoue, and Y. Ishiwari. Age effects on ectopic bone formation induced by purified bone morphogenetic protein. Int. J. Oral Maxillofac. Surg. 28:143–150, 1998.
Nemzek, J. A., S. P. Arnoczky, and C. L. Swenson. Retroviral transmission by the transplantation of connective-tissue allografts. An experimental study. J. Bone Joint Surg. Am. 76:1036–1041, 1994.
Ohno, S., T. Doi, K. Fujimoto, C. Ijuin, N. Tanaka, K. Tanimoto, K. Honda, M. Nakahara, Y. Kato, and K. Tanne. RGD-CAP (betaig-h3) exerts a negative regulatory function on mineralization in the human periodontal ligament. J. Dent. Res. 81:822–825, 2002.
Presta, M., P. Dell'Era, S. Mitola, E. Moroni, R. Ronca, and M. Rusnati. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16:159–178, 2005.
Reddi, A. H. Cell biology and biochemistry of endochondral bone development. Coll. Res. 81:822–825, 1981.
Rice, D. P., T. Aberg, Y. Chan, Z. Tang, P. J. Kettunen, L. Pakarinen, R. E. Maxson, and I. Thesloff. Integration of FGF and TWIST in calvarial bone and suture development. Development 127:1845–1855, 2000.
Shih, M. S., M. A. Cook, C. A. Spence, S. Palnitkar, H. McElroy, and A. M. Parfitt. Relationship between bone formation rate and osteoblast surface on different subdivisions of the endosteal envelope in aging & osteoporosis. Bone 14:519–521, 1993.
Sontag, W. Age-dependent morphometric alterations in the distal femora of male and female rats. Bone 13:297–310, 1992.
Tan, H., A. Ransick, H. Wu, S. Dobias, Y. H. Liu, and R. Maxson. Disruption of primary mesenchyme cell patterning by misregulated ectodermal expression of SpMsx in sea urchin embryos. Dev. Biol. 201:230–246, 1998.
Toriumi, D. M., H. S. Kotler, D. P. Luxeberg, M. E. Holtrop, and E. A. Wang. Mandibular reconstruction with a recombinant bone inducing factor. Functional, histologic, and biomechanical evaluation. Arch. Otolaryngol. Head Neck Surg. 117:1101–1112, 1991.
Urist, M. R. Bone formation by autoinduction. Science 150:893–899, 1965.
Wada, K., A. Niimi, K. Watanabe, T. Sawai, and M. Ueda. Maxillary sinus floor augmentation in rabbits: A comparative histologic–histomorphometric study between rhBMP-2 and autogenous bone. Int. J. Periodont. Restorative Dent. 21:253–263, 2001.
Wang, E. A., V. Rosen, J. S. D'Alessandro, M. Bauduy, P. Cordes, T. Harada, D. I. Israel, R. M. Hewick, K. M. Kerns, P. Lapan, D. P. Luxenberg, D. Mcquaid, I. K. Moutsatsos, J. Nove, and J. M. Wozney. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. U.S.A. 87:2220–2224, 1990.
Wang, J.-S., and P. Aspenberg. Basic fibroblast growth factor and bone induction in rats. Acta Orthop. Scand. 64:557–561, 1993.
Wozney, J. M. The potential role of bone morphogenetic proteins in periodontal reconstruction. J. Periodontol. 66:506–510, 1995.
Yamada, K., Y. Tabata, K. Yamamoto, S. Miyamoto, I. Nagata, H. Kikuchi, and Y. Ikada. Potential efficacy of basic fibroblast growth factor incorporated in biodegradable hydrogels for skull bone regeneration. J. Neurosurg. 86:871–875, 1997.
Yamaguchi, A. Regulation of differentiation pathway of skeletal mesenchymal cells in cell lines by transforming growth factor-beta superfamily. Semin. Cell Biol. 6:165–173, 1995.
Yamaji, K., A. Matsumoto, and H. Kato. Aging effect on bone formation induced by recombinant human BMP-2 combined with polylactat-polyactate-copolymaer/gelatine sponge complexes at palatal subperiosteal sites in rats. J. Jpn. Soc. Periodontol. 41:380–391, 1999.
Yokota, S., T. Uchida, S. Kokubo, K. Aoyama, S. Fukushima, K. Nozaki, T. Takahashi, R. Fujimoto, R. Sonohara, M. Yoshida, S. Higuchi, S. Yokohama, and T. Sonobe. Release of recombinant human bone morphogenetic protein 2 from a newly developed carrier. Int. J. Pharm. 30:57–66, 2003.
Yokota, S., S. Sonohara, M. Yoshida, M. Murai, S. Shimokawa, R. Fujimoto, S. Fukushima, S. Kokubo, K. Nozaki, K. Takahashi, T. Uchida, S. Yokohama, and T. Sonobe. A new recombinant human bone morphogenetic protein-2 carrier for bone regeneration. Int. J. Pharm. 223:69–79, 2001.
Zellin, G., and A. Linde. Effects of recombinant human fibroblast growth factor-2 on osteogenic cell populations during orthopic osteogenesis in vivo. Bone 26:161–168, 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, E., Ishino, Y., Sasaki, A. et al. Fibroblast Growth Factor-2 Augments Recombinant Human Bone Morphogenetic Protein-2-Induced Osteoinductive Activity. Ann Biomed Eng 34, 717–725 (2006). https://doi.org/10.1007/s10439-006-9092-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9092-x