Abstract
The analysis of the faunal remains from Middle Stone Age deposits of Magubike rockshelter was undertaken to contribute to the modern human behavior debate. Multivariate taphonomic analyses implicate hominins as the key taphonomic agent in the accumulation and modification of the faunal assemblage. Results also show they mainly foraged on large-sized animals as the key sources of meat and marrow. Small-sized animals were also exploited to maximize the foraging net return rates, but only in relatively small quantities. There is evidence that they transported and systematically processed fleshed or relatively complete carcasses. The majority of the cut-marked elements are the high-meat and marrow-bearing bones. The percentages of cut and percussion marks on long bone midshafts fall within the range of variations documented in experimental simulations that sought to replicate hominin primary (early) access to fully fleshed carcasses. There is also evidence that carnivores were scavenging bone fragments discarded by hominins.
Résumé
Les restes fauniques des dépôts de l’âge moyen de la pierre de l’abri sous roche de Magubike ont été analysés dans le cadre du concept général de manifestations du débat sur le comportement humain moderne. Les analyses taphonomiques multivariées impliquent les hominines comme l’agent taphonomique clé dans l’accumulation et la modification de l’assemblage faunique. Les résultats montrent également qu’ils se nourrissent principalement d’animaux de grande taille comme principales sources de viande et de moelle. Des animaux de petite taille ont également été exploités pour maximiser les taux de retour nets de nourriture, mais uniquement en quantités relativement faibles. Il est également prouvé qu’ils ont transporté et traité systématiquement des carcasses complètes ou relativement complètes. La majorité des éléments marqués par la coupe sont les os riches en viande et en moelle. Les pourcentages de marques de coupe et de marques de percussion sur les arbres intermédiaires des os longs se situent dans la plage de variation documentée par les simulations expérimentales qui reproduisent l’accès primaire (précoce) des hominines aux carcasses entièrement charnues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The origin, spread, and adaptations of anatomically modern humans (i.e., Homo sapiens) during the Pleistocene are hotly debated topics. The Middle and Upper Pleistocene period saw the emergence of H. sapiens and cultural advancements that gave them food foraging competitive advantages over the Neanderthals and other archaic hominin populations in Eurasia (Cann et al. 1987; Hovers and Kuhn 2006; Hublin and Richards 2009; McBrearty and Brooks 2000; Shea 2009). In Africa, most of the Middle and Upper Pleistocene is associated with the archaeological period known as the Middle Stone Age (MSA) that dates between 300 and 30 kya.
Hunting proficiency has been a major intriguing issue in the study of the MSA hominin foraging economy (Clark and Speth 2013; Klein 2009). Effective hunting of large-sized animals is one of the defining characteristics of modern human behavior (Henshilwood and Marean 2003; Klein 2001; Marean 1998; Marean and Assefa 1999; Milo 1998). For over four decades, scholars have debated the comparable level of hunting proficiency and meat acquisition mode during the MSA and the Later Stone Age (see Assefa 2002; Marean and Assefa 1999). The current understanding heavily relies on models developed from cave sites on the southern African coast. In those contexts, MSA hominins are frequently characterized as less efficient at hunting large-sized, dangerous, and prime-adult prey and exploiting the aquatic and avian resources (Klein 1975, 1976, 1977, 1989, 2001). They are also portrayed as passive scavengers of the carnivore-defleshed carcasses for scraps of meat and bone marrow (Binford 1981, 1984). Hence, some scholars have argued that MSA hominins were not behaviorally modern because they had limited predatory ability due to their hunting technology limitations. Some archaeologists have also passionately argued that effective hunting of ungulates, as well as the exploitation of the aquatic and avian resources, is a hallmark of the Later Stone Age (LSA) modern humans who developed projectile weaponry that permitted them to hunt prey from a safe distance (Klein and Cruz-Uribe 1984, 1996, 2000; Klein et al. 2004; Steele and Klein 2009). However, some archaeologists hold the opposite view that MSA hominins were effective hunters (see Assefa 2006; Clark 2009; Dusseldorp 2010; Faith 2008, 2011; Marean and Assefa 2005; Marean et al. 2000; Milo 1998; Thompson 2008).
The Eastern African region is a critical area for studying the transition from the Middle to the Later Stone Age. The region has produced some of the oldest remains of Homo sapiens, dated to around 200 kya, and aspects of their technological development in the stone tool industries (Grine 2016; Morgan and Renne 2008). The fossil record includes fragmentary cranium and postcranial bones from Omo Kibish 1 in southern Ethiopia dated to 196 kya and Herto in the Middle Awash region of Ethiopia dated to 160–154 kya (McDougall et al. 2005; White et al. 2003). There is also the Singa cranium from Sudan dated to around 133 kya (McDermott et al. 1996). The oldest H. sapiens remains in Africa are currently from Jebel Irhoud in Morocco, dated to 315 kya (Hublin et al. 2017).
There is evidence that several glacial cycles enabled some areas of East Africa to support H. sapiens populations (Basell 2008; Blome et al. 2012; Cohen et al. 2007; Maslin et al. 2014; Scholz et al. 2007), and diverse populations of large, medium, and small-sized mammalian species (Estes 1991). Hence, MSA sites are present in relatively large numbers and in a broad range of ecological settings across time and space. These include rockshelters, caves, and open-air sites, which are well distributed along the Eastern African Rift Valley, forested areas, tropical coastal areas, grasslands, lake margins, and river valleys (Basell 2008; Blinkhorn and Grove 2018; Clark 1988; Tryon and Faith 2013). The lithic technology at these sites has been widely discussed, but studies of the MSA faunal assemblages that include detailed zooarchaeological and taphonomic analyses are few and far between. This gap is mostly attributed to the absence of long intact archaeological sites rich in well-preserved organic materials at most MSA sites (Assefa 2006; Marean and Assefa 2005; Steele and Klein 2009). Recent archaeological excavations at Magubike rockshelter in southern Tanzania have unearthed a large faunal assemblage that provides an opportunity to investigate the foraging economy of the MSA hominins. In this paper, we present a comprehensive zooarchaeological and taphonomic study of the assemblage recovered in 2012. The roles of hominins and carnivores are underscored, and the order (sequence) of access, either to the fully fleshed or partially fleshed carcasses, is discussed.
Magubike Rockshelter
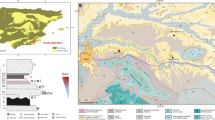
Magubike is a large granitic rockshelter located approximately 40 km west of Iringa town at 1,541 m above sea level (Fig. 1). The rockshelter takes its name from a nearby village and is registered in the Tanzanian archaeological database as HxJf-01. The second author (PRW) recorded the site in 2005, and excavations were conducted in 2006, 2008, and 2012. There are stratified archaeological deposits in different areas of the rockshelter spanning from the Iron Age (IA) through MSA, much like Mumba and Nasera rockshelters in northern Tanzania, and Enkapune Ya Muto and Panga Ya Saidi in Kenya (Ambrose 1998; Mehlman 1989; Shipton et al. 2018). The site is located in an area mostly characterized by escarpments, scattered rock outcrops, and highlands, ranging from 1,200 to 2,700 m above sea level. The area receives an annual rainfall of 600–1,000 mm and forms the Kalenga River watershed, one of the Great Ruaha River’s tributaries. Natural springs supply fresh water all year round. This ecological setting would have supported a wide range of animal species and was ideal for hunting (Behrensmeyer 1987; Lupo and O’Connell 2002; O’Connell et al. 1992).
Stratigraphy and Chronology
There are up to 2 m of archaeological deposits inside the main rockshelter (Fig. 2). The uppermost levels (0–50 cm below the surface) mainly contain Iron Age (IA) and historical period deposits. The sediments are characterized by poorly to moderately sorted silt, sand, and almost gravel-sized clasts, with dark colors that resulted from anthropogenic activities. Characteristic artifacts include a few iron implements, tuyeres, iron slags, potsherd fragments, beads, charcoal, land snails, microliths, and a few faunal remains, mainly of the domesticated species (cattle, sheep, and goats). Ostrich eggshell fragments have yielded a radiocarbon date of 4,477±32 (uncalibrated BP) for the IA deposits (Miller and Willoughby 2014). Optically stimulated luminescence (OSL) dates also fall within the known IA time range in East Africa (see Werner and Willoughby 2017 for the details). It is not yet clear whether there are LSA elements inside the shelter. Werner and Willoughby (2017) have suggested that LSA materials are either mixed with IA or have been washed out by water erosion. It has also been hypothesized that Magubike was probably deserted during the LSA period for reasons not yet explicitly known (Masele 2017, p. 152, 384). But, radiocarbon dates of ostrich eggshell fragments and beads give dates that fit well with the LSA timeframe (see Miller and Willoughby 2014). The LSA occupation phase is indiscernible stratigraphically inside the rockshelter but present in the back (test pit 1) and outside of the main shelter (test pits 4 and 5). This is likely a reflection of differences in activities at the site. Unfortunately, no chronometric dates are available for these deposits because of the absence of organic materials.
The MSA deposits in the main part of the site extend from 50 to 200 cm below the surface, and then the sediments become sterile. The deposits are characterized by moderate to poorly sorted silty and gravel to cobble-sized clasts, whose proportions increase with depth. Soil colors range from red-brown to dark-brown. The deposits include thousands of lithics, fossilized animal bones, land snail shells, and a few ostrich eggshell beads. These have also yielded fossilized Homo sapiens teeth in association with the stone tools and animal bones (Miller et al. 2020; Willoughby 2012; Willoughby et al. 2018). The teeth represent the oldest hominin remains recovered in Tanzania outside the northern region. The new radiocarbon dates on two ostrich eggshell beads from the MSA deposits are close to or older than 50 kya (Miller and Willoughby 2014). Optically stimulated luminescence (OSL) dating has yielded an age estimate of 64 kya, which confirms greater antiquity for the MSA deposits. Electron spin resonance (ESR) dates on the animal teeth and Achatina shells suggest the MSA deposits likely ranged between 250 and 50 kya (Werner and Willoughby 2017).
Materials and Methods
Seven test pits were opened in the main chamber of the rockshelter and were excavated by 10-cm arbitrary levels. The test pits varied in size and depth, with test pit 6–9 being 1 m × 1 m, whereas test pits 10 and 11 were 0.35 cm × 1 m, and test pit 12 measured 1 × 1.35 m (Fig. 2). The smaller size of these three units was because they intersected the 2006 test pits (2 and 3). Excavated sediments were dry-sieved using 5-mm meshes. The excavation of test pit 6 was suspended at 50 cm due to the appearance of a large boulder, but the other units were excavated to the bedrock.
The Faunal Sample and Analysis
A total of 6,568 faunal specimens were analyzed. The majority had massive traces of concretions, potentially resulting from constant dampness of the sediments under the overhang or repeated episodes of wetting and drying in a sheltered environment. Specimens were immersed in dilute acetic acid (5%) for between one and three hours. The dirt and concretions often dissolve in the acid and improve the detection of taphonomic signatures. All specimens were later washed in clean water to buffer the salts and were then left to air-dry slowly. However, some specimens retained a thin coating of concretions after the cleaning process. Similar problems have been reported by Mary E. Prendergast at Sonai rockshelter in northern Tanzania (Prendergast 2008, p. 238–239), Rivka Rabinovich and Erella Hovers for the Amud Cave faunal assemblage in Israel (Rabinovich and Hovers 2004, p. 290), and Sofia E. Carro and Jorge Martínez-Moreno (2014, p. 24) for the faunal assemblage from Cova del Gegant in Spain.
Specimens were identified to the most discrete level possible (e.g., femur or humerus) and, where possible, to species. Long bone midshaft fragments were mostly identified based on their shaft thickness, section shape, and medullary surface properties (Barba and Domínguez-Rodrigo 2005; Pickering et al. 2006). Finer taxonomic determinations were mostly achieved with a comparative osteological collection housed in the National Museum of Tanzania. Where the attribution to species was not possible, specimens were assigned to animal size classes as defined by Bunn (1982) and further analytically grouped together as small- (sizes 1 and 2) and large-sized (sizes 3 and 4) carcasses. Following Domínguez-Rodrigo (1997, 1999) and Yravedra and Domínguez-Rodrigo (2009), skeletal parts of each class or species were grouped into five broad anatomical segments: cranial (i.e., horn, skull, mandible, and dentition), axial (vertebra, rib, pelvis, and scapula), and the appendicular limb bones. The appendicular limb bones were further sub-divided into the upper (humerus and femur), intermediate (radius and tibia), and lower limb bones (metacarpal and metatarsal).
Quantification of abundance calculated for each skeletal part includes the number of identified specimens (NISP), minimum number of elements (MNE), minimum number of individuals (MNI), and minimum animal unit (MAU). Considering the animal size class involved, symmetry, portion, and age, MNE value was generated for each skeletal part with the abundant side (left or right) then taken as MNI. Limb bone shaft fragments were also considered in the MNE estimates (Bunn and Kroll 1986; Marean et al. 2000, 2004; Pickering et al. 2003, 2006). For unpaired elements, the MNE value was divided by the number of times it occurs in a complete carcass to arrive at the MNI count. The coefficient was rounded up to the next whole number. Skeletal part abundance (%MAU) is correlated against bone mineral density (BMD2) values provided by Lam et al. (1999) to determine the extent of attrition the faunal assemblage underwent. To evaluate the transport decisions of carcass parts made by foragers, skeletal part abundance (%MAU) is examined in relation to standardized food utility index (SFUI) values (Metcalfe and Jones 1988). Following Marean and Cleghorn’s (2003) and Faith and Gordon’s (2007) protocols, the high-survival elements, including the appendicular limb bones and cranial elements (skull and mandible), are considered in the evaluation of selective transport of carcass parts. These survive the rigor of carnivore ravaging and density-mediated attrition in post-discard better than most axial elements (Marean et al. 1992; Marean and Spencer 1991). Based on optimal foraging theory, the likely transport strategy is typically reflected in the distribution of specimens across classes of the high-survival elements (Cleghorn and Marean 2004; Marean and Cleghorn 2003). The Shannon evenness index was then calculated: E = −∑pi*lnpi/lnS where pi represents the standardized proportion of specimens for the ith element (MAU), S is the total number of elements present in the sample per type, and E is the evenness index value. In situations where the transport of carcass parts is selective, the evenness value always drops, and when less selective, it always increases. Evenness values of > 0.9 and < 0.9 broadly reflect even and uneven distributions of the high-survival elements, respectively (Faith and Gordon 2007).
For each specimen, the state of preservation was evaluated, and the study of bone weathering follows Behrensmeyer’s (1978) scheme. Trampling was also assessed following Reynard and Henshilwood (2018). The analysis of surface modifications was primarily achieved by using a binocular stereomicroscope with a high-intensity oblique light source at a magnification of up to ×45. Types of modification, frequency, distribution, and orientation to the long axis were recorded. Quantification of surface modifications on appendicular limb bone fragments, measuring ≥ 2 cm, follows Domínguez-Rodrigo’s (1997, 1999) protocol and is based on NISP. To get a satisfactory answer to the question, “who was scavenging from whom,” surface modifications on long bone midshafts are compared to several datasets generated via naturalistic and experimental simulations of carcasses consumed by hominins and carnivores, replicating primary (early) or secondary (late) access. It should be noted that primary access by either hominin or carnivore implies procurement of fully fleshed carcasses through active hunting. In contrast, secondary access by either hominin or carnivore denotes passive scavenging. In hominin’s first access foraging scenarios, cut marks and percussion marks on midshafts frequently cluster between 10% and 30% depending on the animal size involved and processing intensity (see Domínguez-Rodrigo et al. 2014; Lupo and O’Connell 2002; Marean et al. 2000; Pante et al. 2012). Tooth marks frequently range between 5% and 15%, when carnivores had secondary access to defleshed and demarrowed bone fragments (Blumenschine 1995; Capaldo 1997, 1998; Lupo and O’Connell 2002; Selvaggio 1994). Results are also compared to the new estimates of bone surface modifications reported by Domínguez-Rodrigo and Barba (2006, 2007), from the reanalysis of the FLK 22 Zinj faunal assemblage from Olduvai Gorge (Tanzania), to expand the interpretive framework.
Fragmentation patterns were assessed using different methods. Each long bone fragment was recorded as an estimate of the remaining length by 25% intervals (Bunn and Kroll 1986). Following Bunn’s (1982) guideline, the degree of completeness of shaft circumferences was recorded (type 1 retains < 50%, type 2 retains > 75%, and type 3 preserves 100%). The ratio of the sum of type 2 and 3 shafts to the type 1 shafts often ranges between 0.10 and 0.44, with the hominin-created assemblages clustering at the lower end of the range (Bunn 1983). A morphological study of the fractured ends of shafts was also carried out, based on Villa and Mahieu (1991), to establish the stage at which they were fractured. Fracture angles (as oblique, right, and intermediate), fracture outlines (curved, transverse, and intermediate), and fracture edges (smooth or jagged) were coded. Only explicit proximal and distal fractures were recorded. Specimens with recent fracture and all fragment ends that were unclassified, unbroken, or broken through the thick cancellous bones were ignored.
Results
This section covers the faunal analysis results based on the following subheadings: state of preservation of the faunal remains, skeletal part representation, mortality profile, taxonomic composition and diversity, hominin-induced surface modifications, and nonhominin-induced surface modifications.
State of Preservation of the Faunal Remains
The majority of specimens were fragmented. Few were complete and mainly included isolated teeth, tarsals, and carpals. Specimens had an average maximum length of 2.4 cm (Fig. 3). About 42% of the total sample consisted of fragments measuring ≤ 2 cm. Bone specimens measuring > 5 cm account for only < 3% of the assemblage. In contrast, most specimens recovered from hyena dens often measure > 5 cm in maximum length (Brain 1981, p. 53). About 67.5% (n = 4,227) of specimens scored for cortical surface preservation displayed well-preserved surfaces, and 6.2% (n = 387) were moderate. The remaining 26.31% (n = 1,650) were poorly preserved. As for identified skeletal parts, about 82.12% (NISP = 2,618) had well-preserved surfaces. The rest were moderately (4.74%, NISP = 151) or poorly preserved (13.14%, NISP = 419). Specimens also exhibited different bone weathering stages when classified using Behrensmeyer’s (1978) scheme. About 69% of the total sample was recorded in stage 2, which indicated they were deposited rapidly. The remaining 31% belonged to stages 3 to 5 and had cracking and flaking, typical of subaerial bone weathering.
A total of 503 specimens (8.77% of the total sample) had rounded edges. Of the identified skeletal parts, very few pieces exhibited edge-rounding (NISP = 145, 4.59%), which can be attributed to the effects of anthropogenic activities, tumbling, and trampling (Fernández-Jalvo and Andrews 2016; Reynard and Henshilwood 2018). High intensity of burning in the rockshelter also characteristically made bones friable and encouraged edge-damages (Stiner et al. 1995). A considerable proportion of specimens had cemented concretions on cortical surfaces and in the marrow cavities. A total of 2,248 specimens (34.23% of the total sample) exhibited concretions of different degrees of coverage. A few specimens (n = 33) were heavily encrusted and masked any possibility of detecting bone surface modifications. Nonetheless, the proportion of concretion-affected specimens from MSA deposits is low, compared to that reported by Collins (2009, p. 143) from the same site, whereby approximately 90% (548/612) were affected. The difference may be attributed to the differential spatial distribution of bone fragments recovered and the sample sizes studied.
Skeletal Part Representation
A total of 3,436 specimens were confidently identified to skeletal parts. The small- and large-sized animals correspond to 111 and 115 MNI, respectively. All five major anatomical segments of the small- and large-sized animals are also represented (Tables 1 and 2). Irrespective of the animal size class involved, 36.85% of the skeletal part representation (NISP = 1,250) is from the upper appendicular bones, followed closely by axial elements (36.43%, NISP = 1,236). The cranial bones and lower limb bones are only represented by a combined percentage of approximately 27% (NISP = 907).
Rib shaft fragments make up almost 30% of the total NISP. Tibia fragments (NISP = 637, 18.54%) that are easy to identify to skeletal parts, based on the angulations on the shaft, are represented in a high proportion (Barba and Domínguez-Rodrigo 2005; Pickering et al. 2006). The femur (NISP = 383, 11.15%) and metatarsal (NISP = 309, 8.99%) are also represented in relatively high proportions, followed distantly by the radius and metacarpal (see Table 1). Teeth survive postdepositional attrition better because of their compactness, dense-enamel, and dentine composition, but they only make up 7.3% (251) of the NISP. A combined percentage of 3.3% (NISP = 113) represents the phalanges. Carpals and tarsals are very rare. Other elements were identified in small proportions.
Long bone midshaft fragments are the most ubiquitous, but their epiphyseal ends are less frequent. The ratio of the epiphyses (n = 76) to the midshaft fragments (n = 1,580), excluding the complete bones, is 0.048, suggesting the presence of postdepositional destructive taphonomic processes. Moderately positive and statistically significant correlation coefficients between %MAU and density values, involving all elements, are recorded (Spearman’s rho: small-sized, rs = 0.423, p = 0.013, and large-sized carcasses, rs = 0.514, p = <0.010). The results indicate the original skeletal part abundance has been significantly altered by density-mediated attrition post-discard.
A plot between %MAU and SFUI values that considers all elements yielded weak positive but statistically insignificant correlation coefficients (Spearman’s rho: small-sized, rs = 0.015, p = 0.957, and large-sized carcasses, rs = 0.013, p = 0.961). The high-survival elements gave strong positive correlation coefficients for both animal size classes (Spearman’s rho: small-sized, rs = 0.500, p = 0.207, and large-sized carcasses, rs = 0.786, p = 0.021). Of note, the correlation for the large-sized animal subsample is strongly statistically significant. The results suggest selective hominin transport of high-meat and marrow-yielding bones to the rockshelter for further processing and consumption. The coefficient of determination r2 values for the small-sized (0.25) and large-sized (0.62) carcass subsamples support the inference. The small- and large-sized carcass subsamples yielded evenness indices of 0.75 and 0.82, respectively, which denote an uneven distribution of the high-survival elements. Following Faith and Gordon’s (2007) guideline, the values denote an unbiased transport strategy associated with long-distance transportation of carcass parts. The uneven values may also be the product of post-discard destructive processes, including carnivore ravaging and density-mediated attrition. However, the evenness indices do not appear to mirror the impact of sample sizes considered (MNE = 390 and MNE = 459 for small- and large-sized carcasses, respectively) and the associated bias (Faith and Gordon 2007, p. 875–877).
Mortality Profile
Mortality profile gives a very general age estimation of individuals at death. Only 99 specimens had one of their epiphyseal portions preserved. Of these, 87 specimens had their proximal or distal epiphyseal ends fused. Using full epiphyseal fusion as a threshold for maturity, about 15.4% are juveniles for the small-sized animals, by NISP counts. For the large-sized animal subsample, which provides high yields, in terms of meat and marrow, only 10% are juveniles (Table 3). Epiphyseal fusion based on MNI counts also demonstrates the same broad age structure of being heavily dominated by the prime-adults. However, the proportions of prime-adults are not consistent with those drawn from a living population in which the proportion is usually below 20% (Owen-Smith 2006). Accordingly, mortality estimates should be treated with caution.
Taxonomic Composition and Diversity
Bovids are the most numerous mammal class identified, nearly 97% of the NISP. Identified taxa include impala (Aepycerous melampus), Thomson’s gazelle (Eudorcas thomsonii), buffalo (Syncerus caffer), topi (Damaliscus korrigum), and Grant’s gazelle (Nanger granti). Warthog (Phacochoerus africanus) comes in a distant second, only 1.66% of the NISP. The other mammals, including hare (Lepus capensis), dik dik (Madoqua kirkii), and zebra (Equus burchellii), are less than 1% of the NISP (Table 4).
Carnivore remains are mainly represented by a single left and two right mandibular fragments with premolars and molars confidently identified to spotted hyena (Crocuta crocuta) with a total MNI of 2. Carnivore tooth marks are also documented on bone fragments, which suggest they resided or sporadically used the rockshelter as a den or other type of refuge. Nevertheless, the ratio of the carnivore to ungulate suggests they did not intensively use the rockshelter for denning (Cruz-Uribe 1991; Pickering 2002). The presence of hyena remains can be considered at best to be accidental as they are habitually attracted to human habitations to scavenge food refuse and may have died there naturally (Bunn et al. 1991; Bartram 1993; Lupo 1995; Lupo and O’Connell 2002). Rodents (Thryonomys swinderianus) are represented mainly by their dentition, with a total MNI of 2. Remains of medium-sized birds were also identified (Table 4).
Hominin-Induced Surface Modifications
A total of 330 specimens were cut-marked, accounting for 7.72% of the identified skeletal parts (teeth excluded). The small- and large-sized subsamples with cut marks are 3.99% and 10.27%, respectively, of the total assemblage. The rib, tibia, metatarsal, and femur have the highest number of specimens with cut marks (Table 5). The cut mark distribution suggests step-by-step episodes of carcass processing: skinning, dismembering, and defleshing. The marks on mandibles are ascribed to the skinning operations (Binford 1981). Two mandibles, belonging to large-sized animals, preserved cut marks on their inner and outer faces. These marks most likely resulted from the retrieval of the tongue (Nilssen 2000). Skinning is also inferred from the cut marks inflicted on the cranial and metapodials. Cut marks on long bone midshaft fragments are consistent with the filleting of the large muscle mass (Galán and Domínguez-Rodrigo 2013). Overall, cut marks are more abundant on lower limb bones by approximately 12% and on cranial bones by 11.83%, which is to be expected as skinning is usually a necessary step preceding the butchery process (Binford 1981; Lyman 1994; Nilssen 2000). The intermediate bones come next in terms of the number of specimens bearing cut marks (7.7%), closely followed by the upper limb bones (7.18%). The axial bones preserve relatively fewer cut-marked specimens (5.34%).
The number of cut-marked bones appears to vary with animal size. As shown in Fig. 4, the proportions of cut marks on small-sized carcasses subsample are comparatively low and do not exceed 5% across the major anatomical segments. Small-sized animals often do not require exhaustive processing at the kill sites (Yellen 1991). Of note, the intermediate limb bones with 4.9% followed closely by cranial bones with 4.55%, bear the highest proportions. The upper limb bones and the axial elements have relatively lower proportions of 3.5% and 2.85%, respectively. For the large-sized carcasses, cranial bones accounted for 18.37% and, the lower limb bones 13.94% of the cut-marked specimens (Fig. 4). Large-sized animals often require intensive processing at kill sites before transport (Oliver 1993; Lupo and O’Connell 2002). The upper limb bones have slightly more specimens bearing cut marks (13.48%) than the intermediate limb bones (10.34%). The tibiotarsus of a medium-sized bird also exhibited cut marks on its distal part, likely resulting from disarticulation (Val et al. 2016, p. 118).
A total of 703 specimens bearing percussion marks were identified (Table 5). Notches are the most frequent type registered (n = 660), indicative of deliberate opening of the long bone medullary cavities to extract bone marrow (Blumenschine 1995; Blumenschine and Selvaggio 1988; Pickering and Egeland 2006). Other types identified included striae fields (n = 33) and percussion pits (n = 10). Mandibles with a small marrow cavity were also cracked open to extract marrow (Table 5). Almost all the long bones are fractured and are represented by fragments with < 25% of their original shaft length. Among the long bone samples, only three survived intact. Type 1 shafts occur in a high proportion, which suggests the assemblage is unbiased by selective retention of specimens (Marean et al. 2000, 2004). Types 2 and 3 shafts are less frequent. The ratio of types 2 and 3 shafts to type 1 shaft is 0.09, which falls outside the lower end of the range. Bone fracture morphology analysis demonstrates the prevalence of the oblique angles (77.82%), curved or V-shaped outlines (77.65%), and smooth edges (77.84%), demonstrating that the majority were fractured while still fresh. Right angles and transverse outline fractures that potentially resulted from sedimentation compaction or rockfall do not exceed 20%. Burning within the rockshelter may have also hastened skeletal part breakage and, in turn, amplified diagenetic breakage (Stiner et al. 1995). A total of 97 specimens were recorded with evidence of burning at different stages (Table 6). Burnt bones account for about 1.6% of the total NISP. The average maximum length of burnt specimens is 1.97 cm. Burning is more prevalent on long bone midshaft fragments (n = 26) and less frequently on their epiphyseal ends (n = 2). Traces of burning were noted on the interior and exterior surfaces, suggesting that burning occurred during roasting and after the meat had been stripped off the bones. A medium-sized bird humerus also exhibited slightly (brown) to calcined (white) burning traces (Table 6).
Nonhominin-Induced Surface Modifications
Carnivore damage mainly included tooth scores (n = 31) and pits (n = 19). Spotted hyenas (Crocuta crocuta) are responsible based on the dimensions of the tooth marking inflicted, according to the study by Domínguez-Rodrigo et al. (2012). The overall proportion of tooth marks on identified skeletal parts is noticeably very low (NISP = 34, < 1.5% [teeth excluded]). Ribs displayed the highest incidences of tooth marking, followed by tibia (Table 7). The humerus, radius, femur, pelvis, first phalanx, and metapodials were also tooth-marked but at relatively low frequencies (between 1 and 3 specimens each). As shown in Fig. 5, the proportions of bones with tooth marks do not exceed 2.5% across the major anatomical segments. They appear to be preferentially (but generally not in high proportions) inflicted on the intermediate (1.47%) and upper limb bones (1.39%). The lower limb bones only bear 0.7% of the total tooth-marked elements. A total of 30 specimens (< 1% of the total NISP) exhibited rodent gnaw marks on the edges and protuberances of bones (Table 7).
Biochemical marks that resemble tooth marks were noted on a total of 25 specimens (Table 7). Following Domínguez-Rodrigo and Barba’s (2006, 2007) descriptions, none of the biomechanical marks could be mistaken for tooth marks. Two specimens had traces of root marks noted as thin channels with a zigzag form and a U-shaped cross-section, suggesting that plants proliferated inside the rockshelter at some point (Binford 1981). Traces of trampling were also recorded on a total of eight specimens.
Discussion
Zooarchaeological and taphonomic results show that MSA hominins exploited different taxa, comprising mainly large-sized, prime-aged adult ungulates, and they preferentially transported their high-meat and marrow bearing bones. Small-sized animals were also exploited to maximize the foraging net return rates, but only in small quantities. Consumption of hare (Lepus capensis) is indicated by bone fracture morphologies and traces of burning. The procurement of these fast-moving prey undoubtedly needed considerable technological investment in snares or nets (Lupo and Schmitt 2005; Wadley 2010) or the use of stone-tipped arrows (Lombard and Phillipson 2010). Birds probably were procured and consumed as attested by the butchery and burning traces on their bones. Sibudu is another MSA site, which has yielded evidence of hominin exploitation of birds in the form of definite cut marks and burnt bones from its Pre-Still Bay, Still Bay, Howiesons Poort, and Post Howiesons Poort layers (Val et al. 2016, p. 111–115).
Only eight percent of the identified specimens have cut marks, and all five major anatomical segments bear cut marks, suggesting the occupants of the rockshelter processed fully fleshed carcasses efficiently (see Fig. 4). The marks are more abundant on the high-meat-bearing upper appendicular limb bones of large-sized animals (see Tables 5 and 8; Fig. 4). Of the total cut-marked specimens, 122 were preferentially preserved on long bone midshaft sections (Table 8). The midshafts of the metacarpal and metatarsal are the most cut-marked. The prevalence of cut marks is mostly attributed to the process of removing the attached meat remnants in preparation for hammerstone breakage to retrieve bone marrow (Bunn and Kroll 1986; Domínguez-Rodrigo 1997, 2002). The lower limb bone midshaft fragments have more cut marks than the upper and intermediate limb bones combined, which may suggest MSA hominins processed none-meaty bearing bones (Table 8). However, a combined percentage of cut marks on the upper and intermediate limb bones is higher for the large-sized carcasses (sizes 3–4). The intermediate bones are also more cut-marked than the upper limb bones for the small-sized carcasses, a pattern that may suggest hominins scavenged nutritionally depleted carcasses (Binford et al. 1988; Domínguez-Rodrigo 1999; Domínguez-Rodrigo and Barba 2005, 2007; Gidna et al. 2014). The fact that the intermediate bones are identified almost three times more often than the upper limb bones can partly explain the observed pattern (see Tables 1 and 2). Nevertheless, the upper limb bones involving all animal size classes (sizes 1–4) and large-sized (sizes 3–4) carcasses bear relatively higher proportions of cut-marked midshaft fragments than the intermediate limb bones. These suggest that MSA hominins butchered fleshed or complete carcasses (Bunn 1982; Bunn and Kroll 1986; Domínguez-Rodrigo 1997; Domínguez-Rodrigo and Barba 2007).
The proportions of cut marks on long bone midshaft fragments are compared to known/published simulations to determine the order/sequence to the carcasses (fully or partially fleshed). The 7.4% of cut marks on long bone midshaft fragments involving all animal size classes falls slightly outside the range of variation documented in experimental simulations modeling hominin primary (early) access to fully fleshed carcasses (Tables 8 and 11, Fig. 6). This proportion of cut-marked long bone midshaft fragments is slightly lower than all assemblage mean percentages reported by Blumenschine (1988) and Pante et al. (2012) but falls within the corresponding 95% confidence limits in their hominin-only simulation study. It is also lower and outside the 95% confidence limits for the values of 16.6% and 47.5% obtained, respectively, in hominin-only simulations by Capaldo (1997, 1998) and Domínguez-Rodrigo (1997). Likewise, it is lower than the 23.6% and outside the corresponding 95% confidence limits for the faunal assemblage butchered by the Hadza (Lupo and O’Connell 2002). It is also lower than the 13.5% but also outside the corresponding 95% confidence limits in Capaldo’s hominin-to-carnivore simulations (Capaldo 1997, 1998). Likewise, it is below 15.5% for the FLK 22 Zinj faunal assemblage (Tables 8 and 11, Fig. 6).
For the small-sized carcasses subsample, 3.44% of cut marks on long bone midshaft fragments fall outside the range. It is far below the 10.3% of the comparable animal size class subsample provided by Blumenschine (1988) and Pante et al. (2012), but within the corresponding 95% confidence limits in their hominin-only and hominin-to-carnivore simulations. Likewise, it is notably lower than the 11.9% for a comparable animal size class subsample for the FLK 22 Zinj assemblage (Tables 8 and 11, Fig. 6).
For the case of the large-sized carcasses subsample, 11% of cut marks on long bone midshaft fragments fall within the range (Tables 8 and 11, Fig. 6). However, this proportion is below the 20.1%, and in the middle of the corresponding 95% confidence limits, reported by Blumenschine (1988) and Pante et al. (2012) for a comparable animal size class subsample in their hominin-only simulations. It is slightly lower than the 12.2% but falls in the middle of 95% confidence limits for comparable animal size class in their hominin-to-carnivore simulations. Of note, it falls very close to the 11.7% of all animal size classes (sizes 1–4) in the hominin-only simulations, suggesting consistency with the general model of hominin primary (early) access to the fully fleshed carcasses. However, it is slightly lower than the new cut marks estimate of 17.2% for a comparable animal size class for the FLK 22 Zinj assemblage (Tables 8 and 11, Fig. 6).
Percussion marks on long bones were consistently present on midshaft sections (n = 466). Of all the skeletal elements, femur and tibia of both large- and small-sized carcasses have the highest occurrence of percussion marks (30%) (Table 9). Percussion marks concentrate on the upper and intermediate limb bones, 30.55% and 29.35%, respectively. About 25.8% of the lower limb bones bear percussion marks (Tables 9 and 11). About 28.12% of the long bone midshaft fragments belonging to all animal size classes preserved percussion marks (Tables 9 and 11). This proportion falls within the reported range of variation where bones were broken to extract the marrow. But, it is slightly higher than those reported by Blumenschine (1995), Capaldo (1997, 1998), and Pante et al. (2012) in their simulations for hammerstone-only and hammerstone-to-carnivore. However, the proportion of percussion marks in MSA Magubike is below the 49.2% result in the hammerstone-only simulations provided by Marean et al. (2000) and below the 33.7% result in the hammerstone-to-carnivore simulations by Marean et al. (2000). However, it exceeds the 24.5% proportions of percussion-marked bones in the FLK 22 Zinj assemblage (Tables 9 and 11).
For small-sized carcasses, 25.48% of the long bone midshaft fragments have hammerstone marks. It is slightly below those provided by Blumenschine (1995) and Pante et al. (2012). However, it is relatively lower than the 37.3% of the comparable animal size class subsample for the FLK 22 Zinj assemblage (Tables 9 and 11). About 30.5% of the large-sized carcasses bear hammerstone marks in contrast to 20.4% in the FLK 22 Zinj assemblage (Tables 9 and 11). This proportion of hammerstone-marked long bones in MSA Magubike suggests that the large-sized animal long bones were intensively harvested for marrow. However, it is slightly lower than the 33.5% figure for a comparable animal size class with hammerstone-only but well above the percentage for the hammerstone-to-carnivore simulations reported by Blumenschine (1995), Capaldo (1997, 1998), and Pante et al. (2012).
The proportion of the total bones with tooth marks does not exceed 2%, a markedly low percentage of the assemblage when compared to the faunal remains exclusively consumed by bone-crunching carnivores (Blumenschine 1995; Blumenschine and Marean 1993; Capaldo 1995; Domínguez-Rodrigo 1997; Marean et al. 2000; Pante et al. 2012; Selvaggio 1994). Only a total of 19 long bone fragments preserved tooth marks on their midshaft sections. Overall, the midshaft sections of the humerus (4.08%) are more tooth-marked than the rest, which have relatively low proportions of < 2%. The proportions of tooth-marked long bone midshaft fragments involving all animal size classes and tooth marks on small- and large-sized carcasses are markedly very low (< 1.3%). They are also notably lower than the new estimates of tooth marks for the FLK 22 Zinj assemblage (see Tables 10 and 11, Fig. 7). Tooth mark proportions on long bone midshafts in the Magubike faunal assemblage only can be explained by the 95% confidence limit of 0.2±10 for a small-sized carcasses subsample reported by Blumenschine (1995, p. 33) in his hammerstone-to-carnivore experimental simulations in the Serengeti and Ngorongoro ecosystems in Tanzania.
The “hot zones,” as defined by Domínguez-Rodrigo and Barba (2007, p. 82) and Gidna et al. (2014, p. 177), refer to the upper and intermediate limb bones which frequently offer marginal foraging returns to the next prospective scavenger in the context of post-carnivore consumption. In the potential scenarios of the MSA hominin scavenging the carcasses initially defleshed and abandoned by carnivores at Magubike, these elements are not expected to bear cut marks in high proportions. However, when the proportions of cut marks are compared to tooth marks, a clear pattern comes out. In general, they are more cut-marked than tooth-marked, respectively showing 5.79% vs. 1.16% on the upper limb bones and 5.08% vs.1.40% on the intermediate limb bones. For the small-sized carcasses subsample, the proportions of cut marks and tooth marks are, respectively, 2.0% and 1.0% on the upper limb bones, and 3.65% vs. 1.15% on the intermediate limb bones. The difference between cut marks and tooth marks is relatively higher for large-sized carcasses, respectively, showing 9.05% vs. 1.29% on the upper limb bones and 7.89% and 1.88% on the intermediate limb bones (see Tables 8 and 10 for the details).
Conclusion
The faunal assemblage studied is highly fragmented but relatively well preserved because of the rockshelter environment (Bergsvik and Skeates 2012). The results indicate that density-mediated attrition significantly biased the original skeletal part abundance. Bone weathering profiles show that the majority fall below stage 2, and specimens in high weathering profiles are very few. This study demonstrates that MSA hominins played the primary role in modifying and accumulating the faunal assemblage and that carnivores played a marginal role. The proportion of cut marks on long bone midshafts suggests they enjoyed primary (early) access to fully fleshed carcasses. In contrast, MSA hominins at Magubike had no economic motivation to inflict cut marks on completely meatless parts of the long bones. Such an attempt would only damage the cutting edges of the stone tools (Bunn 2001; Pickering and Domínguez-Rodrigo 2006). Likewise, high proportions of percussion marks strongly indicate MSA hominins extracted marrow from intact long bones of both small- and large-sized animals, which they transported into the rockshelter. Carnivores could not have been responsible for transporting long bone fragments into the rockshelter or cracking them for consumption (see Tables 9 and 11). The high proportion of green fractures, type 1 shafts, coupled with very strong positive and statistically significant correlation coefficients between %MAU of the marrow-bearing bones against their corresponding unsaturated marrow index (UMI) values generated by Morin (2007), confirm regular hominin marrow extraction at the site (Table 12).
The findings support the view that MSA hominins at Magubike effectively hunted the large-sized ungulates, aggressive prey, and prime-adult individuals. The passive scavenging of carnivore-ravaged carcasses was not a fundamental part of their adaptive behavioral repertoire. Results from several other MSA faunal assemblages in sub-Saharan African sites, such as Klasies River, Sibudu, Blombos, Die Kelders Cave 1, Porc-Epic Cave, and Loiyangalani, have also confirmed MSA hominins’ effective hunting capacities (Assefa 2006; Clark 2009; Dusseldorp 2010; Faith 2008, 2011; Marean et al. 2000; Masele 2017; Milo 1998; Thompson 2008). Given the overall low proportions of tooth marks, carnivore secondary (late) access to the defleshed and demarrowed bone fragments likely followed the temporary abandonment of the rockshelter. The low proportions may also be related to roasting, which potentially made discarded bone fragments less attractive to bone-crunching carnivores (Bartram 1993; Lupo 1995). Furthermore, it can be argued that the possible continuous presence of hominins at the site reduced the amounts of “scavengeable” bones available for carnivores post-discard.
References
Ambrose, S. H. (1998). Chronology of the Later Stone Age and food production in East Africa. Journal of Archaeological Science, 25, 377–392.
Assefa, Z. (2002). Investigation of faunal remains from Pock-Epic: A Middle Stone Age in southeastern, Ethiopia. Ph.D. dissertation. State University of New York, Stony Brook.
Assefa, Z. (2006). Faunal remains from Porc-Epic: Paleoecological and zooarchaeological investigations from a Middle Stone Age site in southeastern Ethiopia. Journal of Human Evolution, 56(1), 1–26.
Barba, R., & Domínguez-Rodrigo, M. (2005). The taphonomic relevance of the analysis of bovid long limb bone shaft features and their application to element identification: Study of bone thickness and morphology of the medullary cavity. Journal of Taphonomy, 3, 29–42.
Bartram, L. E. (1993). An ethnoarchaeological analysis of Kua San (Botswana) bone food refuse. Ph.D. dissertation. University of Wisconsin, Madison.
Basell, L. S. (2008). Middle Stone Age (MSA) site distributions in Eastern Africa and their relationship to Quaternary environmental change, refugia and the evolution of Homo sapiens. Quaternary Science Reviews, 27, 2484–2498.
Behrensmeyer, A. K. (1978). Taphonomic and ecologic information from bone weathering. Paleobiology, 4(2), 150–162.
Behrensmeyer, A. K. (1987). Taphonomy and hunting. In M. H. Nitecki & D. V. Nitecki (Eds.), The evolution of human hunting (pp. 423–450). New York: Plenum Press.
Bergsvik, K. A., & Skeates, R. (Eds.). (2012). Caves in context: The cultural significance of caves and rockshelters in Europe. Oxford: Oxbow Books.
Binford, L. R. (1981). Bone: Ancient men and modern myths. New York: Academic Press.
Binford, L. R. (1984). Faunal remains from Klasies River Mouth. New York: Academic Press.
Binford, L. R., Mills, M. G. L., & Stone, N. M. (1988). Hyena scavenging behavior and its implications for the interpretation of faunal assemblages from FLK 22 (the Zinj Floor) at Olduvai Gorge. Journal of Anthropological Archaeology, 7, 99–135.
Blinkhorn, J., & Grove, M. (2018). The structure of the Middle Stone Age of Eastern Africa. Quaternary Science Reviews, 195, 1–20.
Blome, M. W., Cohen, A. S., Tryon, T. A., Brooks, A. S., & Russell, J. (2012). The environmental context for the origins of modern human diversity: A synthesis of regional variability in African climate 150,000-30,000 years ago. Journal of Human Evolution, 62, 563–592.
Blumenschine, R. J. (1988). An experimental model of the timing of hominid and carnivore influence on archaeological bone assemblages. Journal of Archaeological Science, 15, 483–502.
Blumenschine, R. J. (1995). Percussion marks, tooth marks, and experimental determinations of the timing of hominid and carnivore access to long bones at FLK Zinjanthropus, Olduvai Gorge, Tanzania. Journal of Human Evolution, 29, 21–51.
Blumenschine, R. J., & Marean, C. W. (1993). A carnivore’s view of archaeological bone assemblages. In J. Hudson (Ed.), From bones to behaviour: Ethnoarchaeological and experimental contributions to the interpretation of faunal remains (pp. 273–300). Carbondale: Center for Archaeological Investigations, University of Southern Illinois.
Blumenschine, R. J., & Selvaggio, M. M. (1988). Percussion marks on bone surfaces as a new diagnostic of hominid behavior. Nature, 333, 763–765.
Brain, C. K. (1981). The hunters or the hunted: An introduction to African cave taphonomy. Chicago: University of Chicago Press.
Bunn, H. T. (1982). Meat-eating and human evolution: Studies on the diet and subsistence Patterns of Plio-Pleistocene hominids in East Africa. Ph.D. dissertation. University of California, Berkeley.
Bunn, H. T. (1983). Comparative analysis of modern bone assemblages from a San hunter-gatherer camp in the Kalahari Desert. In J. Clutton-Brook & C. Grison (Eds.), Animals and archaeology: Hunters and their prey (pp. 143-148). Oxford: B.A.R. International Series 163.
Bunn, H. T. (2001). Hunting, power scavenging, and butchering by Hadza foragers and by Plio-Pleistocene Homo. In C. B. Stanford & H. T. Bunn (Eds.), Meat-eating and human evolution (pp. 199–218). Oxford: Oxford University Press.
Bunn, H. T., & Kroll, E. M. (1986). Systematic butchery by Plio/Pleistocene hominids at Olduvai Gorge, Tanzania. Current Anthropology, 27, 431–452.
Bunn, H. T., Bartram, L. E., & Kroll, E. M. (1991). Variability in camp structure and food refuse patterning at Kua San camps. In E. M. Kroll & T. D. Price (Eds.), The interpretation of spatial patterns in Stone Age archaeological sites (pp. 77–148). New York: Plenum Press.
Cann, R. L., Stoneking, M., & Wilson, A. C. (1987). Mitochondrial DNA and human evolution. Nature, 325, 31–36.
Capaldo, S. D. (1995). Inferring hominid and carnivore behavior from dual-patterned archaeological assemblages. Ph.D. dissertation. Rutgers University, New Brunswick.
Capaldo, S. D. (1997). Experimental determinations of carcass processing by Plio-Pleistocene hominids and carnivores at FLK 22 (Zinjanthropus), Olduvai Gorge, Tanzania. Journal of Human Evolution, 33, 555–597.
Capaldo, S. D. (1998). Simulating the formation of dual-patterned archaeofaunal assemblages with experimental control samples. Journal of Archaeological Science, 25(4), 311–330.
Carro, S. C., & Martínez, J. (2014). Who let the hyenas out? Taphonomic analysis of the faunal assemblage from GL-1 of Cova del Gegant (Sitges, Spain). Quaternary International, 330, 19–35.
Clark, J. D. (1988). The Middle Stone Age of East Africa and the beginnings of regional identity. Journal of World Prehistory, 2(3), 235–305.
Clark, J. L. (2009). Testing models on the emergence and nature of modern human behavior: Middle Stone Age fauna from Sibudu cave (South Africa). Ph.D. dissertation. University of Michigan, Ann Arbor.
Clark, J. L., & Speth, J. D. (Eds.), (2013). Zooarchaeology and modern human origins: Human hunting behavior during the Later Pleistocene. New York: Springer.
Cleghorn, N., & Marean, C. W. (2004). Distinguishing selective transport and in situ attrition: A critical review of analytical approaches. Journal of Taphonomy, 2(2), 43–67.
Cohen, A. S., Stone, J. R., Beuning, K. R. M., Park, L. E., Reinthal, P. N., Dettman, D., Scholz, C. A., Johnson, T. C., King, J. W., Talbot, M. R., Brown, E. T., & Ivory, S. J. (2007). Ecological consequences of early late Pleistocene megadroughts in tropical Africa. Proceedings of the National Academy of Sciences, 104(42), 16422–16427.
Collins, B. R. (2009). An initial zooarchaeological analysis of Magubike and Mlambalasi: Two archaeological sites from the Iringa region of southern Tanzania. M.A. thesis. University of Alberta, Edmonton.
Cruz-Uribe, K. (1991). Distinguishing hyena from hominid bone accumulations. Journal of Field Archaeology, 18, 467–486.
Domínguez-Rodrigo, M. (1997). Testing meat-eating in early hominids: An analysis of butchery marks on defleshed carcasses. Journal of Human Evolution, 12(3), 169–182.
Domínguez-Rodrigo, M. (1999). Flesh availability and bone modifications in carcasses consumed by lions: Palaeoecological relevance in hominid foraging patterns. Palaeogeography Palaeoclimatology Palaeoecology, 149, 373–388.
Domínguez-Rodrigo, M. (2002). Hunting and scavenging by early humans: The state of the debate. Journal of World Prehistory, 16(1), 1–54.
Domínguez-Rodrigo, M., & Barba, R. (2005). A study of cut marks on small-sized carcasses and its application to the cut-marked bones from small mammals at the FLK Zinj site. Journal of Taphonomy, 3(3), 121–134.
Domínguez-Rodrigo, M., & Barba, R. (2006). New estimates of tooth mark and percussion mark frequencies at the FLK Zinj site: The carnivore-hominid-carnivore hypothesis falsified. Journal of Human Evolution, 50, 170–194.
Domínguez-Rodrigo, M., & Barba, R. (2007). The behavioral meaning of cut marks at the FLK Zinj level: The carnivore-hominid-carnivore hypothesis falsified (II). In M. Domínguez-Rodrigo, R. Barba, & C. P. Egeland (Eds.), Deconstructing Olduvai: A taphonomic study of the Bed I sites (pp. 75–100). New York: Springer.
Domínguez-Rodrigo, M., Gidna, A., Yravedra, J., & Musiba, C. A. (2012). A comparative neotaphonomic study of felids, hyenids and canids: An analogical framework based on long bone modification patterns. Journal of Taphonomy, 10(3-4), 147–164.
Domínguez-Rodrigo, M., Bunn, H. T., & Yravedra, J. (2014). A critical re-evaluation of bone surface modification models for inferring fossil hominin and carnivore interactions through a multivariate approach: Application to the FLK Zinj archaeofaunal assemblage (Olduvai Gorge, Tanzania). Quaternary International, 322-323, 32–43.
Dusseldorp, G. L. (2010). Prey choice during the South African Middle Stone Age: Avoiding dangerous prey or maximizing returns? African Archaeological Review, 27, 107–133.
Estes, R. D. (1991). Behavior guide to African mammals. Berkeley: University of California Press.
Faith, J. T. (2008). Eland, buffalo, and wild pigs: Were Middle Stone Age humans ineffective hunters? Journal of Human Evolution, 55, 24–36.
Faith, J. T. (2011). Ungulate biogeography, statistical methods, and the proficiency of Middle Stone Age hunters. Journal of Human Evolution, 60, 315–317.
Faith, J. T., & Gordon, A. D. (2007). Skeletal element abundances in archaeofaunal assemblages: Economic utility, sample size, and assessment of carcass transport strategies. Journal of Archaeological Science, 34, 872–882.
Fernández-Jalvo, Y., & Andrews, P. (2016). Atlas of taphonomic identifications: 1001+ images of fossil and recent mammal bone modification. New York: Springer.
Galán, A. B., & Domínguez-Rodrigo, M. (2013). An experimental study of the anatomical distribution of cut marks created by filleting and disarticulation of long bone ends. Archaeometry, 55, 1132–1149.
Gidna, A., Kisui, A. B., & Domínguez-Rodrigo, M. (2014). An ecological neo-taphonomic study of carcass consumption in Tarangire National Park (Tanzania) and its relevance for human evolutionary biology. Quaternary International, 322-323, 167–180.
Grine, F. E. (2016). The Late Quaternary hominins of Africa: The skeletal evidence from MIS 6-2. In S. C. Jones & B. A. Stewart (Eds.), Africa from MIS 6-2: Population dynamics and paleoenvironments (pp. 323–382). New York: Springer.
Henshilwood, C. S., & Marean, C. W. (2003). The origin of modern human behaviour: Critique of the models and their test implications. Current Anthropology, 44(5), 627–651.
Hovers, E., & Kuhn, S. L. (Eds.). (2006). Transitions before the transition: Evolution and stability in the Middle Paleolithic and Middle Stone Age. New York: Springer.
Hublin, J.-J., & Richards, M. P. (Eds.). (2009). The evolution of hominin diets: Integrating approaches to the study of Paleolithic subsistence. New York: Springer.
Hublin, J.-J., Ben-Ncer, A., Bailey, S. E., Freidline, S. E., Neubauer, S., Skinner, M. M., Bergmann, I., Cabec, A. L., Benazzi, S., Harvati, K., & Gunz, P. (2017). New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature, 546, 286–292.
Klein, R. G. (1975). Middle Stone Age man-animal relationships in Southern Africa: Evidence from Die Kelders and Klasies River Mouth. Science, 190(4211), 265–267.
Klein, R. G. (1976). The mammalian fauna of the Klasies River Mouth Sites, Southern Cape Province, South Africa. The South African Archaeological Bulletin, 31(123/124), 75–98.
Klein, R. G. (1977). Mammalian fauna from the Middle and Later Stone Age (Later Pleistocene) levels of Border Cave, Natal Province, South Africa. The South African Archaeological Bulletin, 32(125), 14–27.
Klein, R. G. (1989). Why does skeletal part representation differ between smaller and larger bovids at Klasies River Mouth and other archeological sites? Journal of Archaeological Science, 6, 363–381.
Klein, R. G. (2001). Southern Africa and modern human origins. Journal of Anthropological Research, 57, 1–16.
Klein, R. G. (2009). The human career: Human biological and cultural origins (3rd ed.). Chicago: University of Chicago Press.
Klein, R. G., & Cruz-Uribe, K. (1984). The analysis of animal bones from archaeological sites. Chicago: University of Chicago Press.
Klein, R. G., & Cruz-Uribe, K. (1996). Exploitation of large bovids and seals at Middle and Later Stone Age sites in South Africa. Journal of Human Evolution, 31, 315–334.
Klein, R. G., & Cruz-Uribe, K. (2000). Middle and Later Stone Age large mammal and tortoise remains from Die Kelders Cave 1, Western Cape Province, South Africa. Journal of Human Evolution, 38, 169–195.
Klein, R. G., Avery, G., Cruz-Uribe, K., Halkett, D., Parkington, J. E., Steele, T., Volman, T. P., & Yates, R. (2004). The Ysterfontein 1 Middle Stone Age site, South Africa, and early human exploitation of coastal resources. Proceedings of the National Academy of Sciences, 101(16), 5708–5715.
Lam, Y. M., Chen, X., & Pearson, O. M. (1999). Intertaxonomic variability in patterns of bone density and the differential representation of bovid, cervid, and equid elements in the archaeological record. American Antiquity, 64(2), 343–362.
Lombard, M., & Phillipson, L. (2010). Indicators of bow and arrow and stone-tipped arrow use 64,000 years Ago in KwaZulu-Natal, South Africa. Antiquity, 84(325), 635–645.
Lupo, K. D. (1995). Hadza bone assemblages and hyena attrition: An ethnographic example of the influence of cooking and mode of discard on the intensity of scavenger ravaging. Journal of Anthropological Archaeology, 14, 288–314.
Lupo, K. D., & O’Connell, J. F. (2002). Cut and tooth mark distributions on large animal bones: Ethnoarchaeological data from the Hadza and their implications for current ideas about early human carnivory. Journal of Archaeological Science, 29, 85–109.
Lupo, K. D., & Schmitt, D. N. (2005). Small prey hunting technology and zooarchaeological measures of taxonomic diversity and abundance: Ethnoarchaeological evidence from Central African forest foragers. Journal of Anthropological Archaeology, 24, 335–353.
Lyman, R. L. (1994). Vertebrate taphonomy. Cambridge: Cambridge University Press.
Marean, C. W. (1998). A critique of the evidence for scavenging by Neanderthals and early modern humans: New data from Kobeh Cave (Zagros Mountains, Iran) and Die Kelders Cave 1 Layer 10 (South Africa). Journal of Human Evolution, 35, 111–136.
Marean, C. W., & Assefa, Z. (1999). Zooarchaeological evidence for the faunal exploitation behavior of Neanderthals and early modern humans. Evolutionary Anthropology, 8, 22–37.
Marean, C. W., & Assefa, Z. (2005). The Middle and Upper Pleistocene African record for the biological and behavioral origins of modern humans. In A. B. Stahl (Ed.), African archaeology (pp. 93–129). Oxford: Blackwell Publishing Limited.
Marean, C. W., & Cleghorn, N. (2003). Large mammal skeletal transport: Applying foraging theory in a complex taphonomic system. Journal of Taphonomy, 1, 15–42.
Marean, C. W., & Spencer, L. M. (1991). Impact of carnivore ravaging on zooarchaeological measures of element abundance. American Antiquity, 56(4), 645–658.
Marean, C. W., Spencer, L. M., Blumenschine, R. J., & Capaldo, S. D. (1992). Captive hyaena bone choice and destruction, the schlepp effect and Olduvai Archaeofaunas. Journal of Archaeological Science, 19, 101–121.
Marean, C. W., Abe, Y., Frey, C. J., & Randall, R. C. (2000). Zooarchaeological and taphonomic analysis of the Die Kelders Cave 1 Layers10 and 11 Middle Stone Age larger mammal fauna. Journal of Human Evolution, 38, 197–233.
Marean, C. W., Dominguez-Rodrigo, M., & Pickering, T. R. (2004). Skeletal element equifinality in zooarchaeology begins with method: The evolution and status of the “shaft critique”. Journal of Taphonomy, 2(2), 69–98.
Masele, F. (2017). Middle Stone Age hominin foraging ecology in Tanzania: An archaeozoological study from the Loiyangalani open-air site and Magubike rockshelter. Ph.D. thesis, University of Alberta, Edmonton.
Maslin, M. A., Brierley, C. M., Milner, A. M., Shultz, S., Trauth, M. H., & Wilson, K. E. (2014). East African climate pulses and early human evolution. Quaternary Science Reviews, 101, 1–17.
McBrearty, S., & Brooks, A. S. (2000). The revolution that wasn’t: A new interpretation of the origin of modern human behavior. Journal of Human Evolution, 39, 452–563.
McDermott, F., Stringer, C., Grün, R., Williams, C. T., Din, V. K., & Hawkesworth, C. J. (1996). New late Pleistocene Uranium-thorium and ESR dates for the Singa hominid (Sudan). Journal of Human Evolution, 31, 507–516.
McDougall, I., Brown, F. H., & Fleagle, J. G. (2005). Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature, 433, 733–736.
Mehlman, M. J. (1989). Later Quaternary archaeology sequences in northern Tanzania. Ph.D. dissertation, University of Illinois, Urbana-Champaign.
Metcalfe, D., & Jones, K. T. (1988). A reconsideration of animal body-part utility indices. American Antiquity, 53(3), 486–504.
Miller, J. M., & Willoughby, P. R. (2014). Radiometrically dated ostrich eggshell beads from the Middle and Later Stone Age of Magubike rockshelter, southern Tanzania. Journal of Human Evolution, 74, 118–122.
Miller, J. M., Werner, J. J., Biittner, K. M., & Willoughby, P. R. (2020). Fourteen years of Archaeological and Heritage Research in the Iringa Region, Tanzania. African Archaeological Review, 37, 271–292.
Milo, R. G. (1998). Evidence for hominid predation at Klasies River Mouth, South Africa, and its implications for the behavior of early modern humans. Journal of Archaeological Science, 25, 99–133.
Morgan, L. E., & Renne, P. R. (2008). Diachronous dawn of Africa’s Middle Stone Age: New 40Ar/39Ar ages from the Ethiopian Rift. Geology, 36(12), 967–970.
Morin, E. (2007). Fat composition and Nunamiut decision-making: A new look at the marrow and bone grease indices. Journal of Archaeological Science, 34, 69–82.
Nilssen, P. J. (2000). An actualistic butchery study in South Africa and its implications for the reconstructing hominid strategies of carcass acquisition and butchery in the Upper Pleistocene and Plio-Pleistocene. Ph.D. dissertation. University of Cape Town, South Africa.
O’Connell, J. F., Hawkes, K., & Blurton-Jones, N. G. (1992). Patterns in the distribution, site structure and assemblage composition of Hadza kill-butchering sites. Journal of Archaeological Science, 19, 319–345.
Oliver, J. S. (1993). Carcass processing by the Hadza: Bone breakage from butchery to consumption. In J. Hudson (Ed.), From bones to behaviour: Ethnoarchaeological and experimental contributions to the interpretation of faunal remains (pp. 200–227). Carbondale: Center for Archaeological Investigations, University of Southern Illinois.
Owen-Smith, N. (2006). Demographic determination of the shape of the density dependence for three African ungulate populations. Ecological Monographs, 76(1), 93–109.
Pante, M. C., Blumenschine, R. J., Capaldo, S. D., & Scott, R. S. (2012). Validation of bone surface modification models for inferring fossil hominin and carnivore feeding interactions, with reapplication to FLK 22, Olduvai Gorge, Tanzania. Journal of Human Evolution, 63, 395–407.
Pickering, T. R. (2002). Reconsideration of criteria for differentiating faunal assemblages accumulated by hyenas and hominids. International Journal of Osteoarchaeology, 12, 127–141.
Pickering, T. R., & Domínguez-Rodrigo, M. (2006). The acquisition and use of large mammals carcass by Oldowan homininds in eastern and southern Africa: A selected review and assessment. In N. Toth & K. Schick (Eds.), The Oldowan: Case studies into the Earliest Stone Age (pp. 113–128). Bloomington: Stone Age Institute Press.
Pickering, T. R., & Egeland, C. P. (2006). Experimental patterns of hammerstone percussion damage on bones: Implications for inferences of carcass processing by humans. Journal of Archaeological Science, 33, 459–469.
Pickering, T. R., Marean, C. W., & Dominguez-Rodrigo, M. (2003). Importance of limb bone shaft fragments in zooarchaeology: A response to “On in situ attrition and vertebrate body part profiles” (2002) by M.C. Stiner. Journal of Archaeological Science, 30, 1469–1482.
Pickering, T. R., Egeland, C. P., Schnell, A. G., Osborne, D. L., & Enk, J. (2006). Success in identification of experimentally fragmented limb bone shafts: Implications for estimates of skeletal element abundance in archaeofaunas. Journal of Taphonomy, 4(2), 97–108.
Prendergast, M. E. (2008). Forager variability and transitions to food production in secondary settings: Kansyore and Pastoral Neolithic Economies in East Africa. Ph.D. dissertation. Harvard University, Cambridge.
Rabinovich, R., & Hovers, E. (2004). Faunal assemblages from Amud cave: Preliminary results and interpretations. International Journal of Osteoarchaeology, 14, 287–306.
Reynard, J. P., & Henshilwood, C. S. (2018). Using trampling modification to infer occupational intensity during the Still Bay at Blombos Cave, southern Cape, South Africa. African Archaeological Review, 35, 1–19.
Scholz, C. A., Johnson, T. C., Cohen, A. S., King, J. W., Peck, J. A., Overpeck, J. T., Talbot, M. R., Brown, E. T., Kalindekafe, L., Amoako, P. Y. O., Lyons, R. P., Timothy, M., Shanahan, T. M., Castañeda, I. S., Heil, C. W., Forman, S. L., McHargue, L. R., Beuning, K. R., Gomez, J., & Pierson, J. (2007). East African megadroughts between 135 and 75 thousand years ago and bearing on early-modern human origins. Proceedings of the National Academy of Sciences, 104(42), 16416–16421.
Selvaggio, M. M. (1994). Carnivore tooth marks and stone tool butchery marks on scavenged bones: Archaeological implications. Journal of Human Evolution, 27, 215–228.
Shea, J. J. (2009). The impact of projectile weaponry on Late Pleistocene hominin evolution. In J-J. Hublin & M. P. Richards (Eds.), The evolution of hominin diets: Integrating approaches to the study of Paleolithic subsistence (pp. 189-199). New York: Springer.
Shipton, C., Roberts, P., Archer, W., Armitage, S. J., Bita, C., Blinkhorn, J., Courtney-Mustaphi, C., Crowther, A., Curtis, R., d’Errico, F., Douka, K., Faulkner, P., Groucutt, H. S., Helm, R., Herries, A. I. R., Jembe, S., Kourampas, N., Lee-Thorp, J., Marchant, R., Mercader, J., Marti, A. P., Prendergast, M. E., Rowson, B., Tengeza, A., Tibesasa, R., White, T. S., Petraglia, M. D., & Boivin, N. (2018). 78,000-year-old record of Middle and Later Stone Age innovation in an East African tropical forest. Nature Communications, 9, 1832.
Steele, T. E., & Klein, R. G. (2009). Late Pleistocene subsistence strategies and resource intensification in Africa. In J.-J. Hublin & M. P. Richards (Eds.), The evolution of hominin diets: Integrating approaches to the study of Paleolithic subsistence (pp. 113–126). New York: Springer.
Stiner, M. C., Kuhn, S. L., Weiner, S., & Bar-Yosef, O. (1995). Differential burning, re-crystallization, and fragmentation of archaeological bone. Journal of Archaeological Science, 22, 223–237.
Thompson, J. C. (2008). Zooarchaeological tests for modern human behavior at Blombos Cave and Pinnacle Point Cave 13B, Southwestern Cape, South Africa. Ph.D. dissertation. Arizona State University, Tempe.
Tryon, C. A., & Faith, T. J. (2013). Variability in the Middle Stone Age of Eastern Africa. Current Anthropology, 54, S234–S254.
Val, A., de la Pena, P., & Wadley, L. (2016). Direct evidence for human exploitation of birds in the Middle Stone Age of South Africa: The example of Sibudu Cave, KwaZulu-Natal. Journal of Human Evolution, 99, 107–123.
Villa, P., & Mahieu, E. (1991). Breakage patterns of human long bones. Journal of Human Evolution, 21, 27–48.
Wadley, L. (2010). Were snares and traps used in the Middle Stone Age and does it matter? A review and a case study from Sibudu, South Africa. Journal of Human Evolution, 58, 179–192.
Werner, J. J., & Willoughby, P. R. (2017). Middle Stone Age technology and cultural evolution at Magubike rockshelter, southern Tanzania. African Archaeological Review, 34(2), 249–273.
White, T. D., Asfaw, B., DeGusta, D., Gilbert, H., Richards, G. D., Suwa, G., & Howell, F. C. (2003). Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature, 423, 742–747.
Willoughby, P. R. (2012). The Middle and Later Stone Age in the Iringa Region of Southern Tanzania. Quaternary International, 270, 103–118.
Willoughby, P. R., Compton, T., Bello, S. M., Bushozi, P. M., Skinner, A. R., & Stringer, C. B. (2018). Middle Stone Age human teeth from Magubike rockshelter, Iringa Region, Tanzania. PLoS One, 13(7), e0200530.
Yellen, J. E. (1991). Small mammals: !Kung San utilization and the production of faunal assemblages. Journal of Anthropological Archaeology, 10, 1–26.
Yravedra, J., & Domínguez-Rodrigo, M. (2009). The shaft-based methodological approach to the quantification of long limb bones and its relevance to understanding hominid subsistence in the Pleistocene: Application to four Paleolithic sites. Journal of Quaternary Science, 24(1), 85–96.
Acknowledgements
The Tanzanian Commission on Science and Technology (COSTECH) and the Antiquities Division, Ministry of Natural Resources and Tourism of Tanzania authorized the excavations of Magubike rockshelter. The research was part of the first author’s (FM) Ph.D. thesis at the University of Alberta, Edmonton, Canada. We are deeply grateful to the archaeology students from the University of Dar es Salaam for their help in the excavations. We also thank the anonymous reviewers whose comments contributed to improving this paper.
Funding
Financial supports from the University of Dar es Salaam and the University of Alberta awarded to FM partly made it possible to study the faunal assemblage. Financial support from the Social Sciences and Humanities Research Council (SSHRC) Standard Research Grants awarded to Pamela Rae Willoughby is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masele, F., Willoughby, P.R. Zooarchaeology of the Middle Stone Age in Magubike Rockshelter, Iringa Region, Tanzania. Afr Archaeol Rev 38, 275–295 (2021). https://doi.org/10.1007/s10437-021-09429-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10437-021-09429-7