Abstract
Microscale bioparticle analysis has advanced significantly providing advantages over bench-scale studies such as the use of a reduced amount of sample and reagents, higher sensitivity, faster response, and portability. Insulator-based dielectrophoresis (iDEP) is a microscale technique where particles are driven by polarization effects under a non-uniform electrical field created by the inclusion of insulators between two electrodes. iDEP possesses attractive advantages over traditional electrode-based dielectrophoresis since there is no electrode degradation and manufacture of the device is simpler and economical. This novel and powerful technique has been applied successfully in the manipulation of macromolecules and cells. In this study, differences in dielectric properties (cell membrane conductivity) of viable and non-viable microalgae, Selenastrum capricornutum, were employed to concentrate and separate a mixture of live and dead cells. A microchannel, manufactured in glass and containing an array of cylindrical insulating posts, was employed to dielectrophoretically immobilize and concentrate the mixture of cells employing direct current electric fields. Experiments showed that live cells exhibited a stronger dielectrophoretic response than dead cells, which allowed cell differentiation. Separation and enrichment of viable and non-viable microalgae was achieved in 35 s with a concentration yield of 10.36 and 15.87 times the initial cell concentration, respectively. These results demonstrate the use of iDEP as a technique for rapid assessment of microalgae viability; unveiling the potential of this powerful technique for environmental applications on lab-on-a-chip platforms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, an increasing interest on microscale bioseparation systems has been observed. Numerous fields of knowledge including the environmental, clinical, and pharmaceutical have beneficiated from advances in microanalytical systems. Miniaturization has brought significant advantages; since the use of small sample volumes allows for faster analysis, reduced costs, greater sensitivity, and portability (Whitesides 2006). As a result, the development of bioparticles separation protocols coupled with electrokinetic phenomena in microfluidic systems has expanded significantly in the last decade.
Dielectrophoresis (DEP) is one of the most important microanalytical techniques, since the manipulation, separation, and concentration of macromolecules, such as virus, cell organelles, bacteria, yeast, and parasites has been successfully performed in a single step (Gascoyne et al. 2002; Lapizco-Encinas and Rito-Palomares 2007; Moncada-Hernández and Lapizco-Encinas 2010; Voldman 2006). DEP is a non-destructive, non-invasive, electrokinetic transport mechanism with great potential for the separation and manipulation of bioparticles in microfluidics devices. DEP, first described in 1951 by Pohl (1951, 1978), is defined as the movement of particles due to polarization effects in a non-uniform electrical field. Particles having a higher polarizability than their immersion medium exhibit positive DEP: motion towards regions of greater field intensity. In contrast, particles having a lower polarizability than that of the immersion medium exhibit negative DEP: motion away from regions of high electric field intensity. DEP can occur in alternating current (AC) or direct current (DC) electric fields. The most common approach to produce non-uniform electric fields is to apply AC electric fields employing microelectrodes arrays (Li and Bashir 2002; Markx et al. 1994; Medoro et al. 2002; Pohl 1978; Rousselet et al. 1998). However, electrode-based DEP has some drawbacks when compared to iDEP microdevices, such as high electrode cost, complex fabrication, and functionality loss due to fouling effects; commonly observed when handling biological samples (Li and Bashir 2002; Markx et al. 1994; Medoro et al. 2002; Rousselet et al. 1998). Majority of the electrode-based systems are two dimensional since electrodes are usually placed on the bottom of the microchannel/chamber, that is, particle flowing far away from the electrodes will not be affected by the DEP force. In contrast, insulator-based devices function as three dimensional systems allowing higher throughput since deeper channels can be employed. On the other hand, using DC electric fields, instead of AC, allows fluid to be driven by electrokinetic forces (electroosmosis) which eliminates the need of micropumps, thus, simplifying the system and increasing portability. For miniaturization purposes, these features bring great advantages.
It has been demonstrated that DEP can be carried out successfully employing arrays of insulating structures (Cummings and Singh 2003; Washizu and Kurosawa 1990). Insulator-based DEP (iDEP) requires only two electrodes that straddle an array of insulating structures. When an electric field is applied across an insulating structures array, these structures are responsible for creating regions of higher and lower field strength, i.e., dielectrophoretic traps are generated (Cummings and Singh 2003; Kang et al. 2008). iDEP systems do not lose their functionality despite of fouling effects, which makes them more suitable for biological applications. In addition, iDEP systems can be fabricated from a variety of materials, including plastics (Davalos et al. 2008; Sabounchi et al. 2008b; Simmons et al. 2006), leading to inexpensive and simpler systems with increasing potential for high throughput applications. Effective concentration and fractionation using iDEP for biological particles in a non-destructive manner has been reported (Barrett et al. 2005; Chen et al. 2009; Cho et al. 2009; Davalos et al. 2008; Kang et al. 2009; Kang et al. 2008; Lapizco-Encinas et al. 2005; Lapizco-Encinas et al. 2004a, 2004b; Moncada-Hernández and Lapizco-Encinas 2010). Differences in cell membrane state and conductivity have an effect on the dielectrophoretic response of cells, allowing viability discrimination; as most cells lose membrane stability as they lose viability (Lapizco-Encinas et al. 2004a, 2004b; Li and Bashir 2002; Pethig and Markx 1997; Urdaneta and Smela 2007). In general, when a cell losses viability its membrane is compromised and becomes permeable allowing high conductive particles from the cell cytoplasm to penetrate this barrier, which leads to a significant increase of 3–4 orders of magnitude in membrane conductivity (Li and Bashir 2002; Pethig and Markx 1997; Urdaneta and Smela 2007). Insulator-based DEP can exploit this dielectric difference to selectively trap and, therefore, achieve separation of viable and non-viable cells (Lapizco-Encinas et al. 2004a, 2004b).
Assessment of microalgae viability is of great significance due to the ubiquity of these organisms in nature and multiple industrial applications. For example, microalgae are associated with the production of toxins in water blooms, they are used as test organisms in ecotoxicology assays, and they belong to the organisms responsible for wastewater treatment (Capasso et al. 2003; Graham and Wilcox 2000; Li and Song 2007; Markelova et al. 2000; Pouneva 1997; Raja et al. 2008; Sato et al. 2004; USEPA 1996). Therefore, to accurately determine the presence and viability of microalgae cells is of great importance after an algaecide application or to establish toxic chemical concentrations or as an indicator of efficient water reclamation. Nowadays, microalgae are receiving great attention because of their potential as a source of biofuels or CO2 mitigation system (Wu et al. 2005). However, to explore, develop, and implement these systems at the laboratory- and field-scale depends on methods that could rapidly discern cell viability in the system.
Classical methods to determine physiological state or viability of microalgae have been inferred from parameters associated with photosynthesis (ATP formation, carbon uptake, and oxygen evolution), cell morphology (motility, and membrane integrity), and cell or colony counts in culture media. At present, efforts are being made to define a more reliable and rapid method utilizing colorimetric assays and fluorescent microscopic methods based on membrane permeability, chlorophyll auto-fluorescence, and enzymatic activity of viable cells (USEPA 1996). Membrane integrity is an indicator of cell death; cells that exclude a “dead cell dye” are considered viable, and therefore fluorescent dye analysis is commonly used to assess cell viability (Bradford and Buller 2010). Other examples of fluorescent dyes employed for determining cell viability have been reported in the literature (Li and Bashir 2002; Mukhopadhyay et al. 2009; Pouneva 1997; Sato et al. 2004). This procedure (cell dyeing to assess viability) is a common microbiology assay. The study of the cell’s dielectric properties as a function of its physiological state and membrane conductivity could serve as the basis for protocols that can rapidly determine the viability of a microorganism (Sato et al. 2004; USEPA 1996), thus, eliminating the need for time-consuming procedures, expensive equipment, and reagents. It is suggested that DEP response of cells could be used as a direct mean to separate and assess viability of microalgae accordingly to changes in the cell membrane.
Considering the need for a technique or method that could rapidly evaluate the physiological state of algal cultures, this study presents the application of iDEP to fractionate and concentrate viable and non-viable Selenastrum capricornutum cells, a unicellular eukaryote green alga commonly used in ecotoxicology tests (USEPA 1996). S. capricornutum (UTEX 1648) liquid cultures showed unicellular crescent-shaped cells with an average cell dimension of 10 μm long and 2 μm wide. However, it has been reported that cells can occur in clusters, not entangled, as a transient form after reproduction (Garrett 2004). S. capricornutum cell wall is composed of a single smooth layer, and the cytoplasmic space is occupied by a large chloroplast (Garrett 2004). As reported in the literature, several approaches have been made to develop small-scale toxicity tests that could simultaneously quantify active growing cells (Sato et al. 2004; USEPA 1996; Voldman 2006). Conventional methods to determine viability are recommended for homogeneous population of cells where no differences in membrane properties are expected. These methods quantify DNA fluorescence after exposure of the cells to DNA-intercalating dyes such as SYTOX®, not taking into consideration cells that have lost their DNA to the action of degradative enzymes (Pohl 1951). A viability test for S. capricornutum using DEP was introduced by Hübner et al. (2003), where AC electric fields were used to separate live from dead cells to detect the presence of CuSO4 in water samples. Other uses of DEP on algae include the continuous separation of different species, distinguishing autotrophic and heterotrophic organisms, and the differentiation of algae from yeast (Pohl and Kaler 1979; Wu et al. 2005). This study has the potential to be a first step toward the development of this type of device. Cell differences are determined on membrane properties, and dyes are no longer required once the technique has been tested and adapted to the sample.
In this study, a portable system consisting of a glass microchannel with cylindrical insulating posts was employed to dielectrophoretically immobilize and concentrate microalga cells employing DC electric fields. Experiments were carried out using live and dead microalga cells, by varying the electric field to observe differences in their dielectrophoretic responses. Results from this study showed that both live and dead microalga cells exhibited negative dielectrophoretic behavior when exposed to DC electric fields; however, live cells exhibited a stronger dielectrophoretic response than dead cells. In addition, simultaneous enrichment and separation of live and dead cells was obtained, achieving concentration enrichments of 10.36 and 15.86 times the inlet concentration, respectively. Successful immobilization and concentration of microalga cells exhibiting viability differences were achieved, demonstrating the great potential of iDEP as a technique for rapid microalgae viability assessment carried out in a miniaturized and portable system. In this study, portable equipment (power supply and microscope) was employed facilitating carrying out analysis on site, which is critical for many environmental applications. Microscope and fluorescent labeling were needed for proof of concept of the employed technique, but once the system is characterized nor the microscope or the fluorescent dyes will be required. Regarding the power supply, there are important efforts by different research groups in the development of miniaturized power sources (Cheng 2000; Hoagland 1999). Previous studies with similar iDEP systems have been reported for the discrimination of live and dead bacteria (Lapizco-Encinas et al. 2004a, 2004b; Pysher and Hayes 2007). However, dielectrophoretic differentiation was achieved only within the microchannel, which is useful for proof of concept applications, without resulting in a real separation, since cells were not selectively concentrated and eluted. To the best knowledge of the authors, this is the first report on microalgae viability assessment using iDEP, considering that microalgae posses more complex structures than bacteria. Moreover, simultaneous concentration and separation were carried out by selectively eluting one cell type at a time as a concentrated plug of cells, achieving concentration enrichments employing a dielectropherogram. Feasibility of the technique was demonstrated unveiling the potential for future coupling in lab-on-a-chip platforms.
2 Theory of dielectrophoresis
The dielectrophoretic force acting on an isolated spherical particle can be represented as:
where ε m is the permittivity of the suspending medium, r p is the radius of the particle, E the local electric field and Re (f CM) it is the real part of the Clausius–Mossotti (CM) factor or polarizability factor. When low frequency electric fields are used (frequency ≤ 100 kHz), the CM factor can be approximated in terms of real conductivities as (Cummings and Singh 2003; Simmons et al. 2006; Washizu and Kurosawa 1990):
From Eqs. 1 and 2, it is possible to observe that the dielectrophoretic force exerted on a particle can be positive or negative depending on the sign of the CM factor. In addition, the dielectrophoretic force depends on the intensity of the applied electric field, the particle size and dielectric properties of the particle, and suspending medium (Gascoyne and Vykoukal 2002). In the case of a mixture of live and dead alga cells of the same species culture, it is expected that size differences are negligible, and that differences in membrane conductivity are high enough to achieve dielectrophoretic differentiation. Furthermore, operating conditions can be varied to manipulate the dielectrophoretic force to achieve separation and concentration of similar particles. The dielectrophoretic force is of second order with respect to the applied electric fields (Eq. 1); at low applied electric fields, low electric fields gradients are produced, leading to negligible dielectrophoretic force. Therefore, the applied electric field has to be high enough for DEP to become significant and immobilize particles.
When iDEP is applied employing DC electric fields, other electrokinetic transport mechanisms are present: electrophoresis (EP) and electroosmotic flow (EOF) (Cummings and Singh 2003). Electrokinetics (EK) is the superposition of EP and electroosmosis. According to that, there are two regimes of iDEP: streaming and trapping DEP. Streaming DEP occurs when the dielectrophoretic force overcomes diffusion but does not overcome the EK flow. Trapping DEP is the reversible immobilization of particles in dielectrophoretic traps and it occurs when the DEP force overcomes diffusion and EK flow (as well as pressure-driven flow if present) (Cummings 2003; Cummings and Singh 2003).
It has been reported that the majority of cells exhibit negative dielectrophoretic behavior when exposed to DC electric fields (Kang et al. 2008; Lapizco-Encinas et al. 2004a, 2004b; Moncada-Hernández and Lapizco-Encinas 2010; Pysher and Hayes 2007). Under DC electric fields, the dielectrophoretic response of the cells is dominated by the properties of their cell membrane (Suehiro et al. 2003a, 2003b; Urdaneta and Smela 2007; Wanichapichart et al. 2002). Cell membranes conductivities are usually very low, in the range of nS/cm (Suehiro et al. 2003a, 2003b; Urdaneta and Smela 2007; Wanichapichart et al. 2002), while suspending medium conductivities are in the range of μS/cm, even when plain bidistilled water is employed, thus, leading to negative magnitudes for the CM factor.
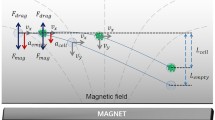
Figure 1a shows a representation of the forces involved in the system, EK and DEP. Since the surface of the microdevice employed has a negative net charge, EK will be driven toward the outlet reservoir. As conductivity of viable and non-viable algae is lower than that of the suspending medium used in these experiments, DEP experience will be negative and the particles will experience repulsion from the regions of higher electric field intensity (narrow regions between posts). As mentioned in section 2, EK is of first order to the electric field applied while DEP is of second order. Therefore, for low electric fields, EK will dominate the system and particles will flow freely from the inlet to the outlet reservoir (streaming DEP) (Cummings 2003); nevertheless, when the electric field is significantly increased DEP will overcome EK and the particles will be immobilized (trapping DEP) (Cummings 2003). The magnitude of the electric field required to pass from the streaming regime to the trapping regime will be different for live and dead microalgae, due to their differences in cell membrane conductivity that directly affects the CM factor.
Schematic representation of the experimental design. a Representation of the forces involved in the system, iDEP shown as a repulsion force from areas of high field intensity and EK force is always toward the negative electrode; b non-uniformity of the electric field with the inclusion of insulating posts with the following dimensions: A—diameter of 440 or 470 μm, B—center-to-center distance of 520 or 510 μm, and depth of 20 μm; c microdevice employed for iDEP experiments, the voltage is applied between the inlet and outlet reservoirs. The first column of posts at both ends of the array has a “dove-tailed” geometry to prevent particles from colliding against the first row of posts and plugging the system
3 Experimental
3.1 Microalgae sample preparation
Selenastrum capricornutum (UTEX 1648) was cultured in 50 ml Falcon tubes in Modified Bold 3 N medium, pH 6.2 (Andersen 2005). Culture conditions were 24°C, continuous illumination with a fluorescent lamp (100 μmol m−2 s−1) and an air atmosphere. A sample, on the mid logarithmic growth phase, was divided into two equal parts to obtain viable and non-viable algae from the same culture. After centrifugation at 3000 rpm for 3 min, both pellets were resuspended in 500 μl of bidistilled water to reach a concentration of 1.79 × 107 cell/ml. The non-viable sample was obtained by heating the cell solution at 70°C for 30 min. Then, cells were stained for 15 min with 0.5 μM final concentration of SYTOX® Green dye (Invitrogen, Carlsbad, CA, USA), ex/em 504/523 nm, which can only penetrate cells with damaged membranes and intercalates with its DNA. Viable cells were not dyed since the autofluorescence of chlorophyll a (ex/em 430/663 nm) was used to visually differentiate these cells from the heat-treated sample. This dual-fluorescence viability method can be used to discriminate live from dead cells since SYTOX® and chlorophyll a emission spectra do not overlap (Sato et al. 2004), as seen in the 4 section of this article.
3.2 Microdevice description
Experiments were carried out using microdevices fabricated from two D263 borosilicate glass substrates (ε = 6.7 at 1 MHz), where the top substrate contains reservoirs and the bottom substrate holds the channels and insulating cylindrical posts. Microdevices were 10.16 mm long, 2 mm wide, and 20 μm deep, contained an inlet and outlet reservoir, and comprised an array of 32 cylindrical posts arranged in eight columns and four rows. The insulating posts were etched on the glass wafer employing standard wet etching techniques. DC electric fields were applied via a pair of remote electrodes consisting of two pieces of platinum wire located one at the inlet and one at the outlet of the microchannel. Two microdevices were used with slightly different posts dimensions: (i) 470 μm diameter arranged 510 μm center-to-center, and (ii) 440 μm diameter arranged 520 μm center-to-center; all posts are 20 μm tall, i.e., they transverse the entire depth of the microchannel creating a true 3-dimensional system (Fig. 1b and c). The first column of posts at both ends of the array has a “dove-tailed” geometry to prevent particles from colliding against the first row of posts and plugging the system.
3.3 Equipment
Dielectrophoretic behavior of microalga was visualized employing an inverted microscope model Axiovert 200 (Carl Zeiss, Göttingen, Germany), with an integrated camera to record pictures and videos utilizing the software AxioVision LE (Carl Zeiss, Göttingen, Germany). A 10× microscope objective was used for dielectrophoretic experiments, and a 5× objective was used for concentration enrichment experiments. The microscope is equipped with a UV lamp that was used for simultaneous excitation of both fluorescent tags in the sample (SYTOX Green and chlorophyll a), allowing discrimination of viable (red) and non-viable (green) cells. Cell morphology was determined with a digital microscope Motic® DM-B1 (Speed Fair Co, Ltd, Causeway Bay, Hong Kong).
Direct current electric fields were applied by employing a 3000 V high voltage sequencer, model HVS448 (Labsmith, Livermore, CA, USA), using 0.3048 mm diameter platinum-wire electrodes (Omega, Stamford, CT, USA) at the inlet and outlet reservoirs (Fig. 1a). Both microscopes and the high voltage sequencer required the use of a personal computer.
3.4 Dielectrophoresis experiments
Before each iDEP experiment, the microdevice was cleaned and filled with either bidistilled water with a conductivity of 2.25 μS/cm and pH 5.8, or a 1 mM KH2PO4 in bidistilled water with a conductivity of 187 μS/cm and pH 9. The pH and conductivity were measured with a multiparameter bench meter HI 255 (Hanna Instruments, Woonsocket, RI, USA). Each experiment consisted of a 50 μl microalgae sample, at a concentration of 1.7 × 107 cells/ml, which was introduced at the inlet reservoir of the microdevice. Pressure-driven flow was eliminated after electrodes were introduced into the reservoirs. Then, DC electric fields were applied, and the dielectrophoretic behavior was observed and recorded employing the previously described microscope configuration.
3.5 Cell enrichment and separation experiments
To measure cell concentration and separation of viable and non-viable microalgae cells, experiments were conducted in four stages by varying the magnitude of the applied DC electric field and measuring cell fluorescence at each stage. First stage, after the microchannel was filled with the suspending medium, a sample of live and dead microalgae cells were added, EOF was generated for 5 s by applying a field of 100 V/cm. This low applied potential was enough to generate flow inside the microchannel without dielectrophoretic concentration/trapping of the cells. Second stage, after the initial period of 5 s, the applied DC potential was increased to 2500 V/cm for a time period of 10 s, to dielectrophoretically trap and concentrate microalgae cells within the array of insulating structures; fluorescence was recorded to quantify any cells that escaped the post array. Third stage, the electric field was decreased to 800 V/cm for 10 s to release the dead cells that were concentrated at the dielectrophoretic traps and push them out of the post array employing EOF while live cells continued dielectrophoretically immobilized; the fluorescence of the exiting dead cells was measured, generating a “peak” signal. Fourth stage, the electric field was again decreased to 100 V for 10 s to release the live microalgae cells, which were pushed out from the dielectrophoretic traps by the EOF, the fluorescence of the live cells was also measured and generated a second “peak” signal.
Concentration enrichment of microalgae cells was determined by fluorescence measurements employing a software built-in house, taken from an interrogation window just at the end of the post array in the microdevice. Concentration enrichment was calculated as follows:
where C E is the concentration enrichment, F MAX is the maximum fluorescence signal obtained when the plug of concentrated cells is released, i.e., “peak” maxima. F 0 is the fluorescence signal of “non-concentrated” cells that are flowing under EOF during the first stage of the experiment. C MAX and C 0 are cell concentration maxima and cell inlet concentration, respectively. Concentration estimations are obtained by direct correlation of fluorescence measurements.
4 Results and discussion
As previously mentioned in the 2 section, viable microalgae cells posses integrity in their cell membrane which will be reflected in lower conductivities, leading to higher magnitudes for the CM factor, and, hence, higher DEP force. Figure 2 presents a comparison between the results obtained at three different electric fields when live and dead cells were treated separately. Figure 2a shows viable cells (red) weakly trapped at 500 V/cm which experienced stronger immobilization when the field was increased to 800 and 1200 V/cm, Fig. 2b and c, respectively. On the other hand, for non-viable cells (green), an electric field of 500 V/cm (Fig. 2d) was not high enough to produce any trapping, though when increased to 800 V/cm (Fig. 2e) stronger trapping was clearly achieved, which was improved at 1000 V/cm (Fig. 2f). Experiments were performed with a suspending medium of bidistilled water with conductivity of 2.25 μS/cm and pH of 5.8, and using the microdevice with insulating posts of 470 μm in diameter. To discard differences in cell size as a determinant factor for distinct dielectrophoretic response between viable and non-viable algae, 30 heat-treated and 30 non-treated cells were measured using a digital microscope. An ANOVA test using the Data Analysis Tools from Microsoft Excel® (Microsoft Corporation, Redmond, WA) showed no statistically significant evidence to conclude that the treatment affects the size of the algae (p-value of 0.3423 with a significance of 0.05).
Dielectrophoretic response of viable (red) and non-viable (green) Selenastrum capricornutum cells suspended in bidistilled water with conductivity of 2.25 μS/cm and pH of 5.8. Viable algae show weak dielectrophoretic trapping at 500 V/cm (a) and strong trapping at 800 V/cm (b) and 1200 V/cm (c); non-viable algae show negligible dielectrophoretic trapping at 500 V/cm (d), but strong trapping at 800 V/cm (e) and 1000 V/cm (f). Experiments were conducted in a microdevice with circular insulating posts of 470 μm in diameter spaced 510 μm center-to-center. (Color figure online)
Similar results were obtained when a mixture of non-treated and heat-treated algae were introduced simultaneously in the inlet reservoir using a suspending medium with a conductivity of 187 μS/cm and pH of 9 (Fig. 3). At an applied electric field of 600 V/cm, non-viable algae were dominated by EK force, and cells were flowing towards the outlet reservoir (shown as green lines), while some viable algae were immobilized by DEP (Fig. 3a). When the field was increased to 1000 V/cm viable cells exhibited strong negative dielectrophoretic trapping while non-viable cells continue to be dominated by EK (Fig. 3b). By increasing the field to 1300 V/cm, it was possible to weakly immobilize non-viable algae, while viable algae were strongly immobilized also by negative DEP (Fig. 3c). Finally, at the highest field of 1700 V/cm, both cell types were strongly trapped but in different regions of the flow since the DEP force was not equally exerted on both cell types; repulsion from the region with higher field gradient (narrow part between posts) was stronger for live algae and, therefore, cells were trapped farther away (Fig. 3d). Posts in Fig. 3 show a thick border due to a slight conical shape resulting from the wet etching process employed for the fabrication of the microdevice. These experiments were performed in the microdevice with insulator posts of 440 μm in diameter and spaced 520 μm center-to-center to improve efficiency of the device performance for handling higher cell concentrations. Characterization of DEP trapping of non-viable cells showed a trend to agglomerate when subjected to the system conditions, specially for higher cell concentration, resulting in clogging of the channel. By moving to a device with a wider aperture between posts, though higher electric fields were required, a significant reduction for plugging of the microchannel was achieved. Results shown in Fig. 3 were obtained from the video “DEP_separation_of_viable_and_non-viable_algae.wmv” where voltage changes are described and the response of the algae to the non-uniform electric field is shown. This video is attached as Supplementary Material.
Dielectrophoretic response of a mixture of viable (red) and non-viable (green) Selenastrum capricornutum cells suspended in bidistilled water with conductivity of 187 μS/cm and a pH of 9.0. a Viable algae show weak negative dielectrophoretic trapping at 600 V/cm while non-viable algae are dominated by electroosmotic flow; b dielectrophoretic response of viable algae increases and cells are strongly trapped at 1000 V/cm while non-viable cells continue to be dominated by electroosmotic flow; c at 1300 V/cm, viable cells are strongly trapped by dielectrophoresis, and non-viable cells show weak dielectrophoretic trapping; d both algae, viable and non-viable, are trapped but at different regions with an electric field of 1700 V/cm since viable algae show stronger repulsion from the regions of high field intensity (negative DEP) and separation is achieved. Experiments were conducted in a microdevice with circular insulating posts of 440 μm in diameter spaced 520 μm center-to-center. (Color figure online)
Differences on the streaming and trapping voltages for these experiments using the two microdevices can be explained by the size of the insulating structures embedded in the microchannel, since the diameter of the circular posts is different so will be the gradient of the electric field. As seen in Eq. 1, the DEP force is directly proportional to the square of this value. Using the software COMSOL Multhiphyisics ® the difference between the two microdevices was evaluated. The software solves the Laplace equation using the Lagrange element on a triangular mesh to obtain values for the parameter such as the gradient of the electric field. The results showed that by applying a potential of 500 V, maximum values reached for ∇E 2 were 2.27 × 108 and 5.25 × 108 V2/cm3 when employing circular posts of 440 and 470 μm in diameter, respectively. This is reflected on a higher magnitude of the negative DEP force on the former microdevice, and, therefore, a lower electric field was needed to achieve a trapping voltage for both types of cells (Fig. 2).
Figure 4 represents the dielectropherogram obtained by plotting fluorescence versus time of the dielectrophoretically concentrated cells leaving the array, where green (non-viable) and red (viable) fluorescence signals are shown simultaneously as acquired by the video. Non-viable algae are released first while viable cells are still trapped, as shown by an increase in the green signal detected by the software. It was noticed that non-viable cells of S. capricornutum tend to agglomerate under these conditions, and release as “chunks” of agglomerated cells. This was demonstrated by obtaining several peaks for the dead cells. Afterward, the viable algae were released giving a well-defined high-resolution peak with a concentration enrichment value higher than the one obtained for non-viable cells; since live cells were confined a longer time inside the device. The dielectropherogram allows observing how it was possible to selectively elute dead cells while retaining live cells, allowing for separation and differentiation between viable and non-viable algae.
Dielectropherogram showing simultaneous green (non-viable algae) and red (viable algae) fluorescence measurements in real time and representation of the electric field applied versus time. Values of concentration enrichment (C E) of non-viable and viable algae are presented and were calculated utilizing Eq. 3. Total process time was 35 s in a suspending medium of 1 mM KH2PO4, conductivity of 187 μS/cm, and pH of 9.0, using a microdevice with circular insulating posts of 440 μm in diameter spaced 520 μm center-to-center. (Color figure online)
Cell enrichment was determined by measuring fluorescence of the cells when they passed the post array. By applying an electric field of 2500 V/cm for 10 s, to trap the cell mixture, and then decreasing the field to 800 V/cm for 10 s, to release the non-viable algae, an increase of 10.36 times the initial concentration of heat-treated algae was achieved (Fig. 4). Then, the electric field was decreased to 100 V/cm to release the viable algae with a cell enrichment value of 15.86 times the original concentration. Enrichment values were calculated by employing Eq. 3. It has been reported that fluorescence has a non-linear dependence with cell concentration; therefore, major changes in cell concentration are required to show a slight increase in fluorescence (Du et al. 2008). Consequently, viable and non-viable microalgae enrichments values obtained in this study might be higher than reported. Separation efficiency in terms of the resolution of the peaks in Fig. 4 is good but it can be further improved. As observed in Fig. 4, there is a small peak of green signal (dead cells) overlapping with the red peak (live cells); this could be eliminated or reduced by modifying operating conditions.
Due to the high electric field applied in iDEP experimentation, Joule Heating is present. However, it is expected that cell viability is not affected by this condition. As reported by Sabounchi et al. (2008a), for a flow rate of 40 μl/min in a very similar system, temperature rose to about 55°C for an applied field of 1250 V/cm. It is possible to consider that in the present system, with a current flow rate of 41 μl/min and 2500 V/cm, temperature might have increased to around 60–65°C in the device and since the experiment duration was only 35 s it might not have been enough time to kill the cells. As described in the 3 section, a temperature of 70°C applied for 30 min was required to kill this algae species. In order to experimentally determine whether or not the electric current applied to the sample was a factor by itself that induced death of cells; a control experiment was performed using only viable microalgae. A sample of 1 ml of an active growing culture was divided into two equal parts. One part was stained with SYTOX® Green dye to ascertain initial viability of the cells. The other half was subjected to an electric field of 2500 V/cm for 35 s under the same experimental conditions as described in the more aggressive iDEP experiment (Fig. 3). Cells were recovered from the microdevice and stained with SYTOX® Green dye. Live and dead cells were assessed using a Neubauer counting chamber in both samples. The proportion of green cells to the total cells was compared between the treated and non-treated samples. Proportions were 0.0134 and 0.0097 for the former and the latter, respectively. A Bernoulli statistical analysis was carried out to determine if the two proportions were equal employing the following test statistic z (Schork and Remington 2000):
where \( \hat{p}_{1} \) and \( \hat{p}_{2} \) are the estimated proportions for the two populations calculated as:
where x 1 and x 2 are the non-viable cells, while n 1 and n 2 are the total cells counted for the two samples. The test statistic was calculated in 0.712 and it was determined that no statistically significant evidence was obtained to conclude that the iDEP treatment affected cell viability when compared to the non-treated sample (p-value of 0.4765 with a significance of 0.05).
5 Conclusions
Differentiation between viable and non-viable microalgae cells was determined employing a simple iDEP microdevice. Live and dead microalgae cells experience different dielectrophoretic responses under same system settings. Differences in cell surface conductivity produce dissimilar CM magnitudes, and, therefore, separation of viable cells can be achieved. In this study, simultaneous separation and concentration between viable and non-viable heat-treated S. capricornutum cells was demonstrated.
Negative DEP was observed during this study, since conductivity of algae is lower than that of the buffer used as suspending medium. It was demonstrated that the magnitude of the applied electric field can be manipulated to selectively trap or release only viable algae or viable and non-viable cells simultaneously. The degree of the negative DEP trapping can be also controlled with the electric field.
In order to test the efficiency of the technique, enrichment of the different types of cells was calculated with fluorescence measurements of the samples before and after iDEP concentration. After 35 s of processing time, enrichment values of 10.36 and 15.87 times the original concentration was achieved for viable and non-viable algae, respectively. It was possible to selectively elute one cell type at a time, analogous to a chromatographic separation, obtaining peaks of concentrated cells. Dead cells had a weaker response and were eluted before live cells. Although fluorescence was used in this article, the technique can be used as a label-free method once implemented since concentration of cells can be determined by a spectrophotometer or any other common analytical tool. Miniaturization of the power source and the detection techniques can be also available.
Although DC-iDEP is still a nascent method for particle manipulation, there is a great potential for this powerful technique for environmental and biotechnological applications where rapid viability assessment is crucial.
References
Andersen RA (2005) Algal culturing techniques. Elsevier Academic Press, San Diego
Barrett LM, Skulan AJ, Singh AK, Cummings EB, Fiechtner GJ (2005) Dielectrophoretic manipulation of particles and cells using insulating ridges in faceted prism microchannels. Anal Chem 77:6798–6804
Bradford JA, Buller GM (2010) Dead cell stains in flow cytometry. A comprehensive Analysis, Scientific poster, Molecular Probes-Invitrogen Detection Technologies. http://www.invitrogen.com/etc/medialib/en/filelibrary/cell_tissue_analysis/pdfs.Par.48458.File.dat/P317%20Viability%20Dye%20Poster.pdf. Accessed December 2010
Capasso JM, Cossío BR, Berl T, Rivard CJ, Jiménez C (2003) A colorimetric assay for determination of cell viability in algal cultures. Biolmol Eng 20:133–138
Chen KP, Pacheco JR, Hayes MA, Staton SJR (2009) Insulator-based dielectrophoretic separation of small particles in a sawtooth channel. Electrophoresis 30:1441–1448
Cheng BCH (2000) Miniaturizing power supply system for portable computers by improving heat dissipation therein. United States Patent 6081425, United States, 2000
Cho Y-K, Kim S, Lee K, Park C, Lee J-G, Ko C (2009) Bacteria concentration using a membrane type insulator-based dielectrophoresis in a plastic chip. Electrophoresis 30:3153–3159
Cummings EB (2003) Streaming dielectrophoresis for continuous-flow microfluidic devices. IEEE Eng Med Biol Mag 22:75–84
Cummings EB, Singh AK (2003) Dielectrophoresis in microchips containing arrays of insulating posts: theoretical and experimental results. Anal Chem 75:4724–4731
Davalos RV, McGraw GJ, Wallow TI, Morales AM, Krafcik KL, Cummings EB, Simmons BA (2008) Performance impact of dynamic surface coatings on polymeric insulator-based dielectrophoretic particle separators. Anal Bioanal Chem 390:847–855
Du J-R, Juang Y-J, Wu J-T, Wei H-H (2008) Long-range and superfast trapping of DNA molecules in an ac electrokinetic funnel. Biomicrofluidics 2:044103
Garrett DC (2004) Effects of methanol, atrazine, and copper on the ultrastructure of Pseudokirchneriella subcapitata (Selenastrum capricornutum). PhD Dissertation, University of North Texas, Denton
Gascoyne PRC, Vykoukal J (2002) Particle separation by dielectrophoresis. Electrophoresis 23:1973–1983
Gascoyne P, Mahidol C, Ruchirawat M, Satayavivad J, Watcharasit P, Becker FF (2002) Microsample preparation by dielectrophoresis: isolation of malaria. Lab Chip 2:70–75
Graham LE, Wilcox LW (2000) Algae. Prentice-Hall, Upper Saddle River, NJ
Hoagland RW (1999) Switchmode power supply miniaturization with emphasis on integrated passive components on prefired high performance ceramic substrates. PhD Dissertation, Virginia Polytechnic Institute and State University, Blacksburg
Hubner Y, Hoettges KF, Hughes MP (2003) Water quality test based on dielectrophoretic measurements of fresh water algae Selenastrum capricornutum. J Environ Monit 5:861–864
Kang Y, Li D, Kalams S, Eid J (2008) DC-Dielectrophoretic separation of biological cells by size. Biomed Microdev 10:243–249
Kang Y, Cetin B, Wu Z, Li D (2009) Continuous particle separation with localized AC-dielectrophoresis using embedded electrodes and an insulating hurdle. Electrochim Acta 54:1715–1720
Lapizco-Encinas BH, Rito-Palomares M (2007) Dielectrophoresis for the manipulation of nanobioparticles. Electrophoresis 28:4521–4538
Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y (2004a) Dielectrophoretic concentration and separation of live and dead bacteria in an array of insulators. Anal Chem 76:1571–1579
Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y (2004b) Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis 25:1695–1704
Lapizco-Encinas BH, Davalos R, Simmons BA, Cummings EB, Fintschenko Y (2005) An insulator-based (electrodeless) dielectrophoretic concentrator for microbes in water. J Microbiol Methods 62:317–326
Li H, Bashir R (2002) Dielectrophoretic separation and manipulation of live and heat-treated cells of Listeria on microfabricated devices with interdigitated electrodes. Sens Actuator B Chem 86:215–221
Li L, Song L (2007) Applicability of the MTT assay for measuring viability of cyanobacteria and algae, specifically for Microcystis aeruginosa (Chroococcales, Cyanobacteria). Phycologia 46:593–599
Markelova AG, Vladimirova MG, Kuptsova ES (2000) A comparison of cytochemical methods for the rapid evaluation of microalgal viability. Russ J Plant Physiol 47:815–819
Markx GH, Huang Y, Zhou XF, Pethig R (1994) Dielectrophoretic characterization and separation of microorganisms. Microbiology-UK 140:585–591
Medoro G, Manaresi N, Altomare L, Leonardi A, Tartagni M, Guerrieri R (2002) In: Proceedings of the 16th European conference on solid-state transducers (Eurosensors XVI), vol 1, pp 47–51, Prague, Czech Republic, September, 2002
Moncada-Hernández H, Lapizco-Encinas BH (2010) Simultaneous concentration and separation of microorganisms: insulator-based dielectrophoretic approach. Anal Bioanal Chem 396:1805–1816
Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P (2009) Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296:H1466–H1483
Pethig R, Markx GH (1997) Applications of dielectrophoresis in biotechnology. Trends Biotechnol 15:426–432
Pohl HA (1951) The motion and precipitation of suspensoids in divergent electric fields. J Appl Phys 22:869–871
Pohl HA (1978) Dielectrophoresis. Cambridge University Press, Cambridge
Pohl HA, Kaler K (1979) Continuous dielectrophoretic separation of cell mixtures. Cell Biochem Biophys 1:15–28
Pouneva I (1997) Evaluation of algal culture viability and physiological state by fluorescent microscopic methods. Bulg J Plant Physiol 23:67–76
Pysher MD, Hayes MA (2007) Electrophoretic and dielectrophoretic field gradient technique for separating bioparticles. Anal Chem 79:4552–4557
Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34:77–88
Rousselet J, Markx GH, Pethig R (1998) Separation of erythrocytes and latex beads by dielectrophoretic levitation and hyperlayer field-flow fractionation. Colloid Surf A Physicochem Eng Asp 140:209–216
Sabounchi P, Huber DE, Kanouff MP, Harris AE, Simmons BA (2008) In: Proceedings of the 12th international conference on miniaturized systems for chemistry and life sciences (MicroTAS 2008), vol 1, pp 50–52, San Diego CA, 12–16 October 2008
Sabounchi P, Morales AM, Ponce P, Lee LP, Simmons BA, Davalos R (2008b) Sample concentration and impedance detection on a microfluidic polymer chip. Biomed Microdev 10:661–670
Sato M, Murata Y, Mizusawa M, Iwahashi H, Oka S (2004) A simple and rapid dual-fluorescence viability assay for microalgae. Microbiol Cult Coll 20:53–59
Schork MA, Remington RD (2000) Statistics with applications to the biological and health sciences. Prentice Hall, Upper Saddle River, NJ
Simmons BA, McGraw GJ, Davalos RV, Fiechtner GJ, Fintschenko Y, Cummings EB (2006) The development of polymeric devices as dielectrophoretic separators and concentrators. MRS Bull 31:120–124
Suehiro J, Hamada R, Noutomi D, Shutou M, Hara M (2003a) Selective detection of viable bacteria using dielectrophoretic impedance measurement method. J Electrostat 57:157–168
Suehiro J, Zhou GB, Imamura M, Hara M (2003b) Dielectrophoretic filter for separation and recovery of biological cells in water. IEEE Trans Ind Appl 39:1514–1521
Urdaneta M, Smela E (2007) Multiple frequency dielectrophoresis. Electrophoresis 28:3145–3155
USEPA (1996) Ecological effects test guidelines: OPPTS 850.5400 Algal toxicity, tiers I and II. United States Environmental Protection Agency document EPA712-C-96-164, Office of Prevention, Pesticides and Toxic Substances, Washington, D.C
Voldman J (2006) Electrical forces for microscale cell manipulation. Ann Rev Biomed Eng 8:425–454
Wanichapichart P, Bunthawin S, Kaewpaiboon A, Kanchanapoom K (2002) Determination of cell dielectric properties using dielectrophoretic technique. ScienceAsia 28:113–119. http://www.scienceasia.org/
Washizu M, Kurosawa O (1990) Electrostatic manipulation of DNA in microfabricated structures. IEEE Trans Ind Appl 26:1165–1172
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373
Wu Y, Huang C, Wang L, Miao X, Xing W, Cheng J (2005) Electrokinetic system to determine differences of electrorotation and traveling-wave electrophoresis between autotrophic and heterotrophic algal cells. Colloid Surf A Physicochem Eng Asp 262:57–64
Acknowledgments
The authors would like to acknowledge the financial support provided by grant CONACYT-CB-2006-53603. The authors are grateful for the financial support provided by Dr. Mario M. Álvarez and Cátedras de Investigación (CAT122 and CAT142) of Tecnológico de Monterrey and support provided by CINVESTAV-Monterrey.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 10615 kb)
Rights and permissions
About this article
Cite this article
Gallo-Villanueva, R.C., Jesús-Pérez, N.M., Martínez-López, J.I. et al. Assessment of microalgae viability employing insulator-based dielectrophoresis. Microfluid Nanofluid 10, 1305–1315 (2011). https://doi.org/10.1007/s10404-010-0764-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-010-0764-3