Abstract
In this article, we introduce a rapid and simple fabrication method to realize a 3-dimensional (3-D) microfluidic channel with a near-perfect circular cross section. This new concept of fabrication method is defined by metal wire removal process, where the metal wire such as a thin soldering wire for the 3-D circular shape is commercially available. For the microfluidic channel mold, PDMS (polydimethylsiloxane) was poured on several shapes such as 3-D circular, helix, and double helix shapes, of soldering wire and solidified. The soldering wire was then melted out by heating. With the two-step process, rapidly and simply fabricated 3-D circular microfluidic channels can be obtained. CPAE (endothelial cell line) cells were cultured inside the channel to evaluate the biocompatibility of the fabricated microfluidic channel. Our method will be very useful in making various circular shapes of 3-D microfluidic devices that need multi-depth and round corners inside the channel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Three-dimensional (3-D) microfluidic systems have been sought for their usefulness in many applications, biomaterial patterning (Chiu et al. 2000), micro-channel mixing (Stroock et al. 2002; Liu et al. 2000), biosystem analysis (Burns et al. 1998), and optical manipulation (Schueller et al. 1999). While a number of studies on 3-D channels involve bonding of two layers fabricated by softlithography (Anderson et al. 2000), the most common strategies are through aligning, stacking, and sealing of 2-D microfluidic channels using photolithography and rapid prototyping (Duffy et al. 1998). Although these methods allow smooth topographical features, kinks are unavoidable due to misalignment between adjacent layers at the interface. Moreover, the stacking method is also time-consuming, as many layers are required to achieve 3-D microfluidic channels. Another method for fabricating the 3-D system is to combine 2-D systems with flexible tubings. However, their connecting channels are long and it is difficult to develop compact designs with this method. For these problems encountered regarding rapid prototyping, new fabrication techniques such as contact liquid photolithographic polymerization (CLIPP) (Haraldsson et al. 2006) or use of SU-8 film lamination (Abgrall et al. 2006) techniques have been suggested. Even though many solutions were introduced regarding 3-D channels fabrication, alignment and bonding issues remain a problem to be solved. To reduce such labor, attempts for an automated fabrication procedures were developed (Kim et al. 2005).
Other known procedures for 3-D microfluidic channels fabrication include stereolithography (Ikuta et al. 1994), laser-chemical 3-D writing (Bloomstein and Ehrlich 1992), and modular assembly (Gonzalez et al. 1998). Yet, these methods are not ideally convenient and are not capable of acquiring certain types of structures.
Another major issue involving conventional 3-D microfluidic channel is the shape of the cross section. Since most channels are fabricated by photolithography, their cross-sectional shapes are in rectangular form. Recently, pseudo 3-D channels, which are topologically equivalent to planar channels, are generated by bending corresponding planar channels in PDMS out of the plane into 3-D shapes (Wu et al. 2003). However, these methods still have a rectangular cross-sectional shape. Unlike rectangular channels, 3-D microfluidic channels with circular cross sections are perfectly symmetrical thus leading to a uniform velocity profile in the cross-sectional direction (Gerhart 1992).
Microfluidic channels with several shapes of cross section were developed using liquid PDMS film (Lee et al. 2007), injecting liquid solder into microfluidic channels (Siegel et al. 2007). They could be very useful in various microfluidic devices that need round corners and circular channels. Especially, the round channel is considered a good candidate for an artificial capillary vessel when a vascular structure is needed in tissue scaffolding. The round shape of microfluidic channel is suitable for imitating natural vein since round channel can diffuse radial directions of nutrition and gases uniformly.
Other fabrication approaches to achieve a circular cross section channel include bell-shaped cross section through rapid prototyping method or etching processes (Futai et al. 2004; Grosse et al. 2001). However, perfect circular channels are difficult to achieve even if etching, bonding, and aligning processes. A MEMS fabrication method using fused silica and femtosecond laser irradiation has also been suggested (Maselli et al. 2006). Using astigmatically shaped beam, microchannels with circular cross section and high aspect ratio were achieved. In addition, using melted photoresist (Wang et al. 2007), optical-fibers as sacrificial mold (Yang et al. 2004), and electroplating methods (Yi et al. 2005) have been used. However, these processes still require bonding and alignment step or complicated devices such as laser or etchants.
Recently, novel methods for fabricating 2-D and 3-D microchannel patterns in a flexible platform of PDMS have been developed. A slender nylon thread formed into different 2-D and 3-D shapes is used as a template (Verma et al. 2006) and microwire-molding (Jia et al. 2008) is embedded inside a block of cross-linked PDMS.
In light of fact that the previous studies have been concentrated on fabricating either microfluidic channels with 3-D features or channels with circular cross-sectional shapes, we propose a rapid and simple method that can achieve both features in one single channel using metal wire removal process. Our microfluidic channel fabricated using metal wire removal process has advantages of both 3-D microfluidic channel and circular cross-sectional shape. Moreover, this method can provide such an unlimited formation of the channel that complicated processes in conventional methods to make a complex shape can be overcome.
2 Materials and methods
2.1 Choice of metal wire to make PDMS mold
A commercially available soldering wire, H-712 (HOZAN Co., Seoul, Korea), was chosen as the 3-D circular-shaped pattern. The wire has a diameter of 300 μm and a stannum (Sn) and plumbum (Pb), 60:40, composition material and a low eutectic point, approximately 190°C, where the wire is in liquid phase. Once the wire exceeds its melting point, it allows us to remove the wire away without damaging the PDMS mold.
PDMS (Sylgard® 184, Dow Corning, Seoul, Korea) is known to be chemically inert and can withstand a higher temperature range from −14 to 200°C. The shape of the channel is dependent on how the wire pattern is shaped. By simply bending the wire, we can easily acquire either a 2-D or a 3-D PDMS channel molding. With the knowledge that the wire has a circular tip, we readily achieve the microfluidic channel molding with a circular cross section.
2.2 Rapid prototyping of PDMS mold
The PDMS was casted over the supporting base. Two components, pre-polymer base and curing agent of Sylgard 184 were thoroughly mixed using a volume ratio of 9:1. Air bubbles were removed from the mixture of Sylgard 184 using a degassing chamber, and the clear PDMS was cured using a hotplate at 100°C for about 1 h. After curing, the PDMS mold was taken off from the supporting base and then bonded with a slide glass using corona surface treatment equipment (BD-20AV, Electro-Technic Co., IL, USA).
2.3 3-D patterning using metal wire
3-D metal wire shape can be formed through a number of different methods. It is even possible to bend a soldering wire manually. We made a simple channel intersecting each other at a different level. We placed a 1-mm square grid plotting paper on a scale board and used office pins to set up coordinates on it. The pins are inserted on the scale board in specific coordinates to achieve a desirable pattern. We then placed the soldering wires and applied tension to form corners where the pins are located. It is an extremely simple process compared to conventional lithographic methods as PDMS layers with different height must be fabricated in order to achieve a similar shape. However, the metal wire intersecting each other at different heights is easily realized 3-D pattern PDMS mold after metal wire removal.
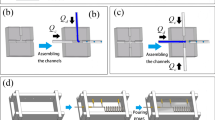
Metal wire removal process is a versatile method with which the complicated channel shapes, such as helical or double-helical structures can be made. This method can be fabricated with one simple step. The helical shape pattern is prepared by winding the soldering wire on an inflexible base rod (Figs. 1, 2). It is noticed that the radius of curvature of the pattern is dependent upon the diameter of the base rod. We used an iron pin (Office PIN 300, Peace co. Seoul, Korea) for the base and acquired the proper patterns with a curvature radius of 300 μm.
Overall fabrication scheme of 3-D circular microfluidic channel. a 3-D circular pattern is shaped using soldering wire, b a PDMS bottom layer is set up on the supporting base, c an embedded metal wire pattern in PDMS mold is casted over the supporting base, d after metal wire removed, 3-D circular microfluidic channel is achieved
2.4 Metal wire removal
The embedded metal wire in PDMS mold can be removed by heating. A convective oven was used as the heating source and it has a hot chamber. The controller and Comvac® vacuum pump, HJS245V (Max. vacuum: 660 mmHg, Gast INC, CA, USA) of the oven system can decrease the chamber pressure and allow the wire mold to seep out (Suction of the vacuum pump can be a 45 l/min at open flow.) The cured PDMS mold that the wire patterns were embedded was placed in the hot chamber and the temperature was set to 190°C. Once the temperature reached at 190°C, pressure in the chamber was lowered using the vacuum pump. The metal wire in liquid phase was forced out of the PDMS mold owing to pressure gradient and gasification. For the sake of safety, we checked the inner channel for remaining of solder by colorimetric tests using instant lead testing, PCB-0507 reagent (SME trading Co. Seoul, Korea) and washed.
2.5 PDMS coating on inner wall of microfluidic channel
After the fabrication of 3-D circular microfluidic channel, the diluted PDMS solutions were introduced in microfluidic channel for coating. This PDMS coating procedure is essential to solving major issues raised by the metal wire removal, i.e., a roughness inner surface due to imperfection of soldering wire and possible remainder of solder after the removal process caused to problematic biological application issues. These problems are simply resolved by introducing the inner wall coating of microfluidic channel using PDMS solutions diluted with serial portion of the agent of Sylgard 184. We controlled the inner diameter thickness by viscosities of the uncured PDMS solutions with curing agent, i.e., changing the diluted PDMS with concentration of the curing agent 0, 25, 50, 75, 100, 125, 150, 175, and 200%. Based on this process, we inserted the PDMS solution with curing agent of total channel volume into the inlet and drew it out of microfluidic channel from outlet using Comvac® vacuum pump, HJS245V (Max. vacuum: 660 mmHg., Gast INC, CA, USA). Suction of the vacuum pump can be a 45 l/min at open flow. So the uncured PDMS solutions are drawn out of microfluidic channel depending on viscosities of the PDMS solutions with curing agent. In this case, a dimensionless number (Reynolds No.) of the fluid flow, Re = 60000 (Nominal viscosity 50 cSt in PDMS Sylgard 184). It should be noted that a fraction of PDMS solutions would remain in the inner surface of microfluidic channel due to surface tension. The adhesion force between the liquid PDMS and the inner wall is larger than the molecular forces of liquid PDMS (Lee et al. 2007).
3 Results and discussions
3.1 Fabrication of the 3-D circular microfluidic channel
The metal wire removal method allows us to fabricate various channel shapes. A 3-D channel orientation, which is difficult to fabricate using existing methods, was fabricated through a rapid and simple procedure. The channel crossing each other without merging and spiral microfluidic channel can be fabricated which is difficult to achieve using conventional 3-D methods (Fig. 3a). Furthermore, with careful spacing between the two spiral wire patterns, we can form a double helix channel which cannot be realized otherwise by metal wire removal (Fig. 3b).
These fabricated channels have a circular cross section as shown in Fig. 4. This cross section of channel is near perfect circle. The diameter of the channel is approximately the same as the diameter of the removed soldering wire, and wire of 300 μm can be easily purchased.
3.2 Inner wall coating of the microfluidic channel with PDMS
Three issues can be considered in the metal wire removal. Possible remainder of solder after the removal process may lead to problematic biological application issues, rough inner surface due to imperfection of soldering wire may alter the fluid flow, and dependence of inner channel diameter to the soldering wire thickness. However, these problems can be simply resolved by introducing the inner channel wall with diluted PDMS solution.
However, for experiments where cells or bacteria are involved, biocompatibility of the channel is an imperative factor. Even though most of the solder is removed from the vacuum oven, there is a possibility that a fraction of solder is remained in the channel. This problem can be solved by adding an extra PDMS layer using the PDMS coating procedure.
As fine surface roughness is an important factor in microfluidics, the rough surface can disturb fluidic flow leading to early transition to turbulence. Moreover, the surface roughness may hinder optical observation due to its opaqueness. After eliminating the solder, it is apparent that the inner surface is too rough to be used as an ideal microfluidic channel. With the PDMS coating procedure, we can observe a clean inner wall surface as shown in Fig 5.
In addition, the PDMS coating method can be used to control the inner channel diameter. We changed the concentration of the curing agent to control the viscosity of PDMS solution to achieve different layer thicknesses. Once the microfluidic channel is coated, the coated PDMS layer on the inner surface of microfluidic channel can be observed. We captured images of cell using optical microscope (I-70; Olympus Co., Japan). Captured images were analyzed with the image-processing program, Image J (NIH, MD, USA) and measured inner diameters of circular channel. Figure 6a shows that diameters of circles are varied with respect to PDMS inner wall coating. From the initial diameter of circular channel (i.e., 300 μm), the diameter (mean ± SD%, n = 3) decreased down to 280 μm (±0.76), 260 μm (±0.76), 240 μm (±0.76), 200 μm (±0.76), 180 μm (±1), 160 μm (±1), 140 μm (±0.29), 110 μm (±0.76), 80 μm (±0.76) at each concentration of the curing agent 200, 175, 150, 125, 100, 75, 50, 25, 0%, respectively. The inner wall thickness created by coating was determined from the difference between the initial and the resulting diameter (Fig. 6b). These values (mean ± SD%, n = 3) are 10.3 μm (±0.76), 20.3 μm (±0.76), 30.3 μm (±0.76), 50.2 μm (±0.76), 60.5 μm (±1), 70.5 μm (±1), 80.2 μm (±0.29), 95.3 μm (±0.76), and 110.3 μm (±0.76).
3.3 Biocompatibility of the coated microfluidic channel
Calf pulmonary artery endothelial (CPAE) cells were cultured to evaluate the biocompatibility of the coated microfluidic channel. Before cell cultivation, it was necessary to apply surface treatments to the microfluidic channels that we had constructed, and to sterilize them. We exposed our fabricated device to corona discharge treatment, BD-20AV, to increase the hydrophilicity of the exposed surface, which improved fluidic flow and cell adhesion. After surface treatment, the device was promptly sterilized by filling with 70% ethanol and then washed with deionized water. To complete the washing, Dulbecco’s phosphate-buffered saline (DPBS, Gibco, NY, USA) was flowed through the microchamber. Once sterilization and washing were complete, the devices were filled with culture medium and stored in an incubator prior to cell seeding.
CPAE cell was obtained from the American Type Culture Collection (ATCC No. CCL-209, USA). The cell culture medium contained GIBCO® RPMI Media 1640 (Invitrogen, CA, USA) supplemented with 1% (v/v) penicillin streptomycin and 10% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco, NY, USA) and pre-warmed to 37°C. The cells were seeded in chambers filled with culture medium at a concentration of 5 × 105 cells/ml and incubated at 37°C in a humidified atmosphere containing 5% CO2. Cells were maintained in the above culture conditions before harvesting. Any cells that failed to adhere to the surface of substrate were washed off and removed during this process.
The CPAE cells were cultured for 5 days and then cell morphologies were observed in the microfluidic channel as shown in Fig. 7. Total numbers of cells were counted by hemacytometer. When cells were stained with trypan blue (T8154, Sigma–Aldrich, Korea) to determine cell viability it was observed that cells were viable indicating our channel can be used for biological applications such as microfluidic cell culture device.
4 Conclusion
A new method, to fabricate 3-D microfluidic channels with circular cross section was demonstrated. This method allows us to freely fabricate 3-D microfluidic channels with circular cross sections. Channels crossing each other in different heights and twisting microfluidic channel with a helical shape were fabricated. Furthermore, with careful spacing of two spiral soldering wire masters, we were able to form a double helix channel, which cannot be realized in otherwise our method. Three problems were encountered during the fabrication process: rough inner surface, remainder of solder after the removal process, and controlling of inner diameter thickness. However, these problems were solved by coating the inner area with diluted PDMS solution. By employing this method, we anticipate that complex microfluidic channels mimicking biological environments, such as artificial blood vessels, can be easily fabricated in the future, thus contributing to the biomimetics research area.
References
Abgrall P, Lattes C, Conedera V, Dollat X, Colin S, Gue AM (2006) Novel fabrication method of 3D microfluidic structures using lamination of SU-8 films. J Micromech Microeng 16:113–121
Anderson JR, Chiu DT, Jackman RJ, Cherniavskaya O, McDonald JC, Wu H, Whisides SH, Whitesides GM (2000) Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal Chem 72:3158–3164
Bloomstein TM, Ehrlich DJJ (1992) Laser-chemical three-dimensional writing for microelectromechanics and application to standard-cell microfluidics. Vac Sci Technol B 10:2671–2674
Burns MA, Johnson BN, Brahmasandra SN, Handique K, Webster JR, Krishnan M, Sammarco TS, Man PM, Jones D, Heldsinger D, Mastrangelo CH, Burke DT (1998) An integrated nanoliter DNA analysis device. Science 282:484–487
Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, Ingber DE, Whitesides GM (2000) Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. PNAS 97:2408–2413
Duffy DC, McDonald JC, Schueller OJA, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984
Futai N, Gu W, Takayama S (2004) Rapid prototyping of microstructures with Bell-Shaped cross-sections and its application to deformation-based microfluidic valves. Adv Mater 16:1320–1323
Gerhart PM (1992) Fundamentals of fluid mechanics, 2nd edn. Addison-Wesley, New York
Gonzalez C, Smith RL, Howitt DG, Collins SD (1998) MicroJoinery: concept, definition, and application to microsystem development. Sens Actuators A 66:315–332
Grosse A, Grewe M, Jouckhardt H (2001) Deep wet etching of fused silica glass for capillary optical leaky waveguides in microfluidic devices. J Micromech Microeng 11:257–262
Haraldsson KT, Hutchison JB, Sebra RP, Good BT, Anseth KS, Bowman CN (2006) 3D polymeric microfluidic device fabrication via contact liquid photolithographic polymerization (CLiPP). Sens Actuators B 113:454–460
Ikuta K, Hirowatari K, Ogata T (1994) Three dimensional micro integrated fluid systems (MIFS) fabricated by stereo lithography. In: Proceedings of the IEEE MEMS 94, Oiso, Japan, January, 25–28
Jia YF, Jiang JH, Ma XD, Li Y, Huang HM, Cai KB, Cai SX, Wu YP (2008) PDMS microchannel fabrication technique based on microwire-molding. Chin Sci Bull 53(24):3928–3936
Kim JY, Baek JY, Lee KA, Lee SH (2005) Automatic aligning and bonding system of PDMS layer for the fabrication of 3D microfluidic channels. Sens Actuators A 119:593–598
Lee K, Kim C, Shin KS, Lee JW, Ju BK, Kim TS, Lee SK, Kang JY (2007) Fabrication of round channels using the surface tension of PDMS and its application to a 3D serpentine mixer. J Micromech Microeng 17:1533–1541
Liu RH, Stremler MA, Sharp KV, Olsen MG, Santiago JG, Adrian RJ, Aref H, Beebe DJ (2000) Passive mixing in a three-dimensional serpentine microchannel. J MEMS 9(2):190–197
Maselli V, Osellame R, Cerullo G, Ramponi R, Laporta P (2006) Fabrication of long microchannels with circular cross section using astigmatically shaped femtosecond laser pulses and chemical etching. Appl Phys Lett 88:191107
Schueller OJA, Zhao XM, Whitesides GM, Smith SP, Prentiss M (1999) Fabrication of liquid-core waveguides by soft lithography. Adv Mater 11:37–41
Siegel AC, Bruzewicz DA, Weibel DB, Whitesides GM (2007) Microsolidics: fabrication of three-dimensional metallic microstructures in poly(dimethylsiloxane). Adv Mater 19:727–733
Stroock AD, Dertinger SKW, Ajdari A, Mezit I, Stong HA, Whitesides GM (2002) Chaotic mixer for microchannels. Science 295:647–651
Verma MKS, Majumder A, Ghatak A (2006) Embedded template-assisted fabrication of complex microchannels in PDMS and design of a microfluidic adhesive. Langmuir 22:10291–10295
Wang GJ, Ho KH, Hsu SH, Wang KP (2007) Microvessel scaffold with circular microchannels by photoresist melting. Biomed Microdevices 9:657–663
Wu H, Odom TW, Chiu DT, Whitesides GM (2003) Fabrication of complex three-dimensional microchannel systems in PDMS. JACS 125:554–559
Yang LJ, Chen YT, Kang SW, Wang YC (2004) Fabrication of SU-8 embedded microchannels with circular cross-section. Int J Mach Tool Man 44:1109–1114
Yi Y, Kang JH, Park JK (2005) Moldless electroplating for cylindrical microchannel fabrication. Electrochem Commun 7:913–917
Acknowledgments
This work was supported by the “System IC 2010”project (10030554-2008-02) of the Korea Ministry of Commerce, Industry and Energy. Facilities were kindly provided by the National Core Research Center (NCRC) for Nanomedical Technology (Grant no. R15-2004-024-00000-0) of the National Research Foundation, and the ICBIN of the Seoul R&BD program (Grant no. 10816).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, SH., Lee, CK., Kim, TJ. et al. A rapid and simple fabrication method for 3-dimensional circular microfluidic channel using metal wire removal process. Microfluid Nanofluid 9, 533–540 (2010). https://doi.org/10.1007/s10404-010-0570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-010-0570-y