Abstract
We present the design of a new controlled drug delivery system potential for in vitro injection of diabetics. The system incorporates some integrated circuit units and microelectromechanical system devices, such as micropump, microneedle array and microsensor. Its goal is to achieve safer and more effective drug delivery. Moreover, a valveless micropump excited by the piezoelectric actuator is designed for the drug delivery system, and a simple fabrication process is proposed. A dynamic model is developed for the valveless micropump based upon the mass conservation. To characterize the micropump, a complete electro-solid-fluid coupling model, including the diffuser/nozzle element and the piezoelectric actuator, is built using the ANSYS software. The simulation results show that the performance of micropump is in direct proportion to the stroke volume of the pump membrane and there is an optimal thickness of the piezoelectric membrane under the 500 V/mm electric field. Based on this simulation model, the effects of several important parameters such as excitation voltage, excitation frequency, pump membrane dimension, piezoelectric membrane dimension and mechanical properties on the characteristics of valveless micropump have been investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, with the rapid development of microelectromechanical system (MEMS) technologies and the emerging demand of biological and medical fields, BioMEMS (MEMS for biological applications) has made great progress. With MEMS technologies, it is now possible to fabricate small size and high performance devices or systems for processing, delivering, manipulating, analyzing, or constructing biological entities. Today, a number of BioMEMS devices and systems, such as biosensors, immunoisolation devices, drug delivery systems, microneedles and DNA analyzing systems, are designed and fabricated (Bashir 2004).
The drug delivery system has become a promising but challenging topic in BioMEMS due to its commercial and research interests in health care. It is well known that the drug delivery routes affect the therapeutic efficacy. Compared with the conventional drug delivery routes such as oral tablets or injection, the drug delivery system based on MEMS technologies is focused on the safer and more effective use of drugs (Zhang et al. 2004). The general advantage of the drug delivery system is the possibility of small feature size and mass production at low cost, which is desirable to the commercial application. In order to better administrate the drug release, the controlled drug delivery system incorporating sensing components and micropumps has become more and more attractive. These systems not only can actively deliver drugs at any time, but also can accurately control the amount of delivered drugs to reduce the side effects of drugs (Fracais and Dufour 2002). These advantages make it useful for the chemotherapy for cancer patients, insulin delivery for diabetic patients, chronically ill patients and so on (Smits 1990). A number of designs about the key components in the controlled drug delivery system have been presented over the last several years (Cao et al. 2001; Stoeber and Liepmann 2005; Yamahata et al. 2005), but little attention is paid to design and analysis of the drug delivery system.
Micropump is one of the important MEMS devices in the controlled drug delivery system, which has been studied in a very broad range including operational principle, structure design, modeling, simulation, and so on. The reciprocating micropump is one of the earliest presented micropumps, which mainly consisted of the flexible membrane propelling the flow and the microvalve directing the flow. The oscillation of membrane excited by the pump actuators propels the drug flow. Kan et al. (2005) discussed a reciprocating micropump with cantilever valves for drug delivery. The results showed that this micropump can provide the large backpressure and the high flow rate. Yih et al. (2005) investigated a drug delivery micropump with a circular-bossed membrane and a cantilever valve to improve the flow rate. The analysis and simulation showed that the circular-bossed membrane can generate a larger drawing and pumping force, which is helpful in improving the flow rate. However, the significant problems associated with this type of micropumps are: the fatigue of moving cantilever valves, the sensitivity to solid particles, high-pressure loss of valves, and low driving frequency. In order to overcome these problems, the valveless micropump with diffuser/nozzle elements, also called fixed valves, was first presented (Stemme and Stemme 1993). The valveless micropump utilizes the difference of flow resistance across the diffuser element and nozzle element to achieve flow rectification. Andersson et al. (2001) proposed a new valveless micropump for pumping of both gas and liquid, and the dependence of the pump characteristics on the properties (i.e. density, pH, and ionic strength) of the pumped medium was further investigated. According to the experiment results, they concluded that the proposed valveless micropump can pump any sample in biology and biochemistry fields. Although a lot of research efforts (Olsson et al. 1995, 1996, 1997) have been made for the valveless micropump, further studies on the relation between the micropump characteristics and the working parameters (i.e. excitation voltage, microactuator size, and working fluid) are still necessary to improve the micropump performance.
Generally, modeling of valveless micropump involves different energy transformation (i.e. electric energy, elastic energy, thermal energy, and fluid transmission) and field coupling, which triggered great interest in the analysis of coupled field (Pan et al. 2001, 2003; Ng et al. 2004). It is difficult to obtain the analytical solution for the behavior of the valveless micropump, particularly due to the effect of coupled field and dynamic flow on the micropump. Therefore, the simulation method is employed to study the performance of micropump. Olsson et al. (2000) utilized the finite element method to analyze the diffuser/nozzle elements of valveless micropump. Fan et al. (2002) investigated the flow feature in the nozzle element using the molecular dynamic (MD) simulation. Loy et al. (1999) and Morris and Foster (2000) simulated the actuator of micropump. Previous studies about the micropump were mostly focused on the simulation of the functional elements such as microvalves and actuators, and the coupling effect between the membrane and the working fluids was often neglected. In addition, the general flow rate of the medical micropump is not enough for the drug delivery system (Teymoori and Abbaspour-Sani 2005). The current research is focused on the effect of the diffuser/nozzle element on the flow rate of the valveless micropump (Olsson et al. 2000; Li et al. 2001; Singhal et al. 2004), but little work has been attempted to investigate the effect of the microactuator on the flow rate.
In this project, we design a new controlled drug delivery system for in vitro injection of diabetics, which can incorporate many MEMS devices: micropumps, microneedle arrays, and microsensors. The salient feature of this miniature system is the ability to automatically inject the suitable dosages based on patient’s physiological information so that the heart and neural complications resulting from superfluous injections can be avoided. Also, a valveless micropump driven by the piezoelectric actuator is proposed, and designed for the controlled drug delivery system. A microfabrication process is proposed for this micropump. Based on the mass conservation, the dynamic model of the valveless micropump is developed. Finally, the complete electro-solid-fluid coupled field simulation on the valveless micropump is studied by the finite element method. To understand the characteristics of valveless micropump, the effects of several critical design parameters on the performance of micropump are further investigated. This simulation is helpful in optimizing the micropump design and in evaluating the micropump behavior, and improving the system performance.
2 Description of the controlled drug delivery system
The goal of this project is to design a controlled drug delivery system for in vitro injections of diabetics, and the whole system will be fabricated using MEMS technologies. The conventional treatment of diabetes has the limitations in accurately controlling the drug concentration in the blood, reducing the pain from the daily injection of varied dose, and efficiently preventing chronic complications. Hence, there is an emerging demand in the development of a new drug delivery system for diabetes. We proposed a controlled drug delivery system, which mainly includes hollow microneedle arrays, micropumps, microsensors, and some integrated circuit (IC) units. The block diagram for the controlled drug delivery system is shown in Fig. 1. The features and functionalities of main components are summarized below.
Hollow microneedle arrays fabricated in silicon are used as an interface between the drug delivery system and the diabetics. According to different functions, the microneedle arrays are mainly divided into microneedles for sampling the blood and microneedles for releasing the drugs. The advantage of these microneedles is to reduce deep tissue damage or skin irritation so that they are suitable for long-term and frequent injections for diabetics.
Micropumps driven by the piezoelectric actuator are employed to generate moderate pressure. They are divided into the delivery micropumps that drive the drug into patients’ bodies and the sampling micropumps that extract blood samples from patients’ bodies. Another important feature of the system is to incorporate different microsensors. Glucose sensors can efficiently monitor the blood glucose concentration at fixed moments. Moreover, flow sensors are used to provide the information about the flow properties of drugs. Fluid position sensors are arranged in sample reservoirs and drug reservoirs, where blood samples and drugs are individually kept. These sensors are used to monitor the fluid position in the reservoirs.
Besides the MEMS devices mentioned above, the IC units including a central processing unit (CPU), a signal processing unit (SPU), D/A and A/D converters, a remote module, a random access memory (RAM), power supply, liquid crystal display (LCD), and keyboard, are also very important. The CPU is used to process the information acquired from different sensors and control the behavior of the micropump. Meanwhile, the A/D converters need to be integrated into the system, when the sensor information is available. The applications of D/A converters and SPU is to control the actuation mode of the micropumps. Dry cell as power supply can provide great convenience. In addition, RAM is also necessary to store the measured therapeutic information, which has a backup battery to prevent loss of data. The remote module, a wireless transportation device, will build up the link between the physicians and the patients by emitting the therapeutic information at fixed moments. To facilitate communication between the patient and the system, the LCD and the keyboard are used as the interface of the system.
The main function of this system is to deliver the drugs. When the patient presses the “Start” button on the keyboard, the process of drug delivery will happen. According to the information from the fluid position sensors, the CPU first checks if the drug in the drug reservoir is available and if the reservoir depositing the blood samples is full. The results can be displayed on the LCD for patients. Then the micropumps will extract blood samples through the microneedles. The glucose sensors will assess the glucose level in these samples. Based on the result from the glucose sensors, the controlled system will automatically inject the desired drug dose instead of the physician. Furthermore, the system has the ability of real-time adjustment of the delivery pumps to adapt to the change of glucose strength in the patient’s body. During the drug delivery, the flow is monitored by the flow sensors to prevent the large fluctuation of flow rate.
On the other hand, the system can store the patients’ physiological information and system status, which are available for the patient and the physician. The physician can receive the patients’ physiological information sent by the remote module of the system. Based on this measured information, the physician can immediately adjust the therapy plan as well as further confirm the validation of the delivery plan. In this case, the new therapeutic mechanism of continuous communication between the physician and the patient is to be built, which is helpful for the healing of patients.
3 Design and fabrication of the micropump
As stated above, the micropump is one of the key components in the controlled drug delivery system, and its performance strongly affects the behavior of the drug delivery system. In this section, we design and evaluate a new valveless micropump excited by the piezoelectric actuator. Its operational principle is based on the motion of the pump membrane and flow properties of the diffuser/nozzle element. Two-dimensional (2D) structure schematics of the micropump are shown in Fig. 2a. The general advantage of this micropump is that both liquid and gas can be pumped, and there is high reliability and low sensitivity of drug particles due to the lack of a moving valve. In addition, it can be operated at a higher excitation frequency than the micropump with a cantilever valve, which can avoid the influence of the gas bubble on the micropump (Andersson et al. 2001). However, low backpressure resistance and low efficiency are still an issue to be further investigated for micropump applications.
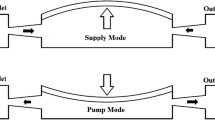
The micropump consists of three layers: glass, Si, and piezoelectric disc glued on the Si, as shown in Fig. 2b. It includes two diffuser/nozzle elements, one pump chamber and one piezoelectric disc as the actuator. The diffuser follows the direction of the increase of cross-section area, while the nozzle follows the direction of decrease of cross-section area. Two diffuser/nozzle elements have the same geometrical dimensions, and are respectively used as the inlet or the outlet of the micropump. When the pump membrane moves to increase the volume of pump chamber, the fluid is sucked into the pump across the inlet and outlet. The pump is in the supply phase. When the pump membrane moves to decrease the volume of pump chamber, the fluid is pushed out of the pump chamber. The pump is in the pumping phase. If the flow resistance in the nozzle direction is higher than that in the diffuser direction, the flow transportation is achieved from the inlet to the outlet.
In view of ease of integrating with other MEMS devices and high performance of the planar diffuser/nozzle element, the planar diffuser/nozzle element is employed, as shown in Fig. 3a. This diffuser/nozzle element has a square cross-section, with two parallel flat walls and two convergent flat walls. Figure 3b shows the basic dimension parameters of the planar diffuser/nozzle element, which involves the divergence angle θ, the diffuser length L, and the width of the narrowest part slightly rounded W 1. It has been believed that the output of micropumps depends on the geometrical dimensions and the characteristics of diffuser/nozzle element.
Silicon and glass substrates are involved in the fabrication process, as shown in Fig. 2b. Two diffuser/nozzle elements and the part of the pump chamber are made on the Si substrate. Then the Si substrate is connected to the glass substrate by using the anodic bonding. The whole structure of the pump chamber is thus formed. The process begins with the fabrication of the input port and the output port by the deep reactive ion etching (DRIE) on one side of the Si substrate. DRIE is a kind of dry etching, which has the advantage of achieving a high aspect ratio. Therefore, two identical dimension diffuser/nozzle elements and the pump chamber are formed by the second DRIE process. Similarly, the third DRIE is used on another side of the Si substrate so that the pump membrane is created on the top of the pump chamber. In the next process, the micropump is sealed by the anodic bonding between the glass substrate and the Si substrate. As a result, the piezoelectric disc is glued on the pump membrane by conductive epoxy, and external polyethylene (PE) tubes are connected to the inlet and the outlet of the micropump.
4 Analysis of the micropump
Figure 4 illustrates a model of the pump. The pressure in the pump chamber is P. P in and P out are the inlet and the outlet pressures, respectively. Q in and Q out are the volume flow through the inlet and the outlet, respectively. The pressure loss coefficient through the diffuser/nozzle elements is defined as
where ΔP is the pressure drop across the diffuser direction or the nozzle direction, ρ is the fluid density, υ is the flow mean velocity in the narrowest area of the diffuser/nozzle elements.
According to the work in the literature (Olsson et al. 1996), the pressure loss coefficient across the diffuser/nozzle element is the sum of pressure drops in three parts, namely, the sudden contraction at the entrance, the sudden expansion at the exit, the gradual expansion or contraction along the length of diffuser/nozzle element. Hence, the pressure loss coefficient K d and K n across the diffuser direction and the nozzle direction are
where A 1 and A 2 are the narrowest area and the largest area of the diffuser/nozzle element, respectively.
Substituting Eqs. (2) and (3) into Eq. (1), the flows Q d and Q n across the diffuser/nozzle directions are respectively expressed as
where ΔP d and ΔP n are the pressure drops across the diffuser/nozzle directions, respectively. They are related to the pressure in the pump chamber, the inlet pressure, and the outlet pressure.
According to the literature (White 1991), the conservation of mass is given by
where CV is the control volume, CS is the control surface, U is the velocity vector, and N is a normal vector perpendicular to the control surface. It is assumed that the fluid within the pump chamber is the control volume, as shown in Fig. 4. The conservation of mass for the valveless micropump is expressed as
Thus, the control volume of the valveless micropump V(P, t) can be written as
where V c is the volume of the pump chamber and V m is the variation of pump membrane depended on the excitation voltage.
We assume that the flow is incompressible and the chamber wall is stiff except the pump membrane. Substituting Eq. (8) into Eq. (7), the simplified governing equation of the valveless micropump can be obtained as
When the micropump is in the supply phase, the flows across the inlet and outlet are written as
When the micropump is in the pumping phase, the flows across the inlet and outlet are written as
5 Numerical analysis and simulation
The analysis of the valveless micropump is complicated by the factor that the flow is dynamic, not steady state in the micropump. Most of the current theoretical analysis about micropumps and diffuser/nozzle elements are based on the steady-state assumption, which is useful to give a principal insight about the valveless micropump. However, the performance of the valveless micropump for the dynamic flow is different from that for the steady-state flow in the actual application. Another factor complicating the micropump design is the coupling effect. There are two main couplings in the micropump design: the electromechanical coupling of piezoelectric actuator and the fluid–solid coupling between the working fluid and the pump membrane. The piezoelectric actuator consists of the piezoelectric disc and the elastic pump membrane. When an alternating voltage is imposed on the piezoelectric disc, the strain of the structure is generated due to the electromechanical conversion (reverse piezoelectric effect). The radial stain of the piezoelectric disc can cause the surface of the elastic membrane to expand or contract, and thus the vibration of the elastic membrane is generated. The vibrating membrane drives the fluid flow, and meanwhile the fluid resists the vibration of the pump membrane. In this work, considering the electromechanical coupling of the piezoelectric actuator and the effect of the fluid-membrane coupling, we build a complete simulation model of the valveless micropump including the diffuser/nozzle elements and the piezoelectric actuator.
The commercially available software ANSYS is used to model and solve the valveless micropump. According to the fabrication process, the piezoelectric disc with two electrodes is glued on the elastic pump membrane by epoxy. Since the electrode layer and the bonding layer as compared to the other layers are very thin, their effects on the piezoelectric actuator are negligible in the numerical simulation. To reduce the size of the model and to save CPU time, the simplified 2D finite element model is used in the analysis. The electromechanical-coupled field element Plane-223 with eight nodes is used to model the piezoelectric disc. The 8-node Plane-82 is chosen to model the elastic pump membrane, and the 4-node Fluid-141 is used to model the working fluid.
In the finite element model of the piezoelectric actuator, the elastic pump membrane is the interface between the solid and the fluid, whose sidewalls are clamped. The sidewalls of the piezoelectric disc are freed, and its bottom surface is clamped to the top surface of the elastic pump membrane. The sinusoidal actuating voltage is exerted on the piezoelectric disc. For simplification, it is assumed that the residual stress introduced by the fabrication process is zero inside the pump membrane. The flow is considered incompressible. To account for the effect of turbulent velocity fluctuations on the mean flow, the typical k−ε turbulence model is employed to analyze the fluid in the valveless micropump. In addition, a non-slip boundary condition is assumed on the fluid wall, and a uniform pressure outlet boundary condition is applied at the outlet of the valveless micropump. The interface flag provided by the software is applied to transfer the load between the piezoelectric actuator and the working fluid.
To further evaluate the suitability of the simplified model, we built a finite element model, to be the same as the model in the literature (Li and Chen 2003). The micropump is excited at the working voltage 50 V (zero to peak). This analysis is justified when the excitation frequency is much less than the natural frequency of the piezoelectric actuator. The piezoelectric actuator consists of a PZT-5A disc and an elastic pump membrane. The pump pressure is zero. We found that the maximum deflection at the center of the pump membrane is 0.1441 μm, and the discrepancy from the experimental result is less than 4%. This suggests that the simulation model in this work is valid.
In order to verify the computation accuracy of the finite element models, the convergence test is carried out. The increase of the element number is advantageous to obtain fine and steady results for the simulation model. However, the large number of elements means a huge cost of CPU, and a large requirement of memory (disk and RAM). Hence, it is desired to find the minimum element number, which can get satisfied result by analyzing the corresponding solutions for the finite element models with different element numbers. Figure 5 shows an example of the convergence test for the valveless micropump at zero pump pressure. The ratio of the piezoelectric membrane and the pump membrane thickness is 1.5. It is found that the convergence of the pump flow occurs when more than 5,000 fluid elements and 360 solid elements are used. In our study, the convergence tests are conducted for different geometric models of the valveless micropump before the results are discussed.
6 Results and discussion
It is well known that the pump flow depends on the excitation frequency exerted on the piezoelectric actuator and the deflection shape of the pump membrane. In this study we first investigate the natural frequency of the piezoelectric actuator. We assume that the elastic pump membrane is entirely covered by the piezoelectric disc. The piezoelectric actuator dimensions are listed in Table 1. The properties for the piezoelectric disc (PZT-5A) and the elastic pump membrane (Si) are summarized in appendix. A 3D finite element model is built for the piezoelectric actuator. The 10-node Solid-227 is chosen to model the PZT-5A disc. The same node Solid-92 is used to model the bonding layer and the Si pump membrane. Figure 6 shows the result of modal analysis. The first natural frequency is 67,007 Hz, and the second natural frequency is 141,670 Hz. The circular piezoelectric actuator bends to one direction under the first natural frequency and it has only one peak.
The simulation of the pump characteristics is carried out for the valveless micropump in the following circumstances. The design features of the diffuser/nozzle elements include: (1) its divergence angle θ is 9.4°, (2) its length L is 1.1 mm, and (3) the width W 1 of the narrowest part is 0.1 mm. The working fluid is water. According to the geometrical dimension of the piezoelectric actuator above, the 2D finite element model of the valveless micropump is built. The results can be obtained after the transient analysis. In Fig. 7, the pressure-flow characteristics of the micropump are shown for three excitation voltages, namely 80, 100 and 140 V. The frequency of the excitation voltage is 400 Hz. The pump pressure, defined as the difference of the outlet/inlet pressures, can be achieved by changing the outlet boundary condition. The result shows that the relationship between the maximum pump flow (at zero pump pressure) and the maximum pump pressure (at zero pump flow) is linear, which is in line with the experimental results demonstrated in (Olsson et al. 1995). When increasing the amplitude of the excitation voltage at the same excitation frequency, both the maximum pump flow and the maximum pump pressure increase. Figure 8 shows the cross-section deformation of the pump membrane for different excitation voltages at zero pump pressure. The membrane position means the meshed elements’ position of the pump membrane along the membrane diameter. From the deformation curve of the pump membrane, the displacement at the center of pump membrane is the largest. The stroke volume and the central displacement of the pump membrane increases when the excitation increases. Hence, the larger the stroke volume of the pump membrane is, the better is the performance of the valveless micropump.
The dependency of the maximum pump flow on the excitation voltage for different excitation frequencies is shown in Fig. 9. The excitation voltage varies from 60 to 140 V. The result shows that the relationship between the maximum pump flow and the excitation voltage is linear. The explanation for this is, when the excitation frequency is very low compared with the natural frequency of the piezoelectric actuator (67,007 Hz), the nonlinear effect of the fluid-membrane coupling at zero pump pressure is negligible (Pan et al. 2001). The vibration response of the membrane to the excitation voltage is linear. Hence, the high excitation voltage at the constant excitation frequency is helpful to improve the flow rate. However, due to the limitation of the maximum allowable electric field strength for the piezoelectric material, the applied maximum excitation voltage must be selected in the actual application. When the excitation frequency is increased, the pump flow increases. It implies that when the excitation frequency is in the range from 200 to 500 Hz, the micropump at the high excitation frequency can generate large pump flow. In Fig. 10, the relationship between the displacement at the central of pump membrane and excitation frequency is shown for different excitation voltages. We found that the central displacement of pump membrane does not seem to be strongly affected by excitation frequency varied from 200 to 500 Hz. The excitation voltage is a more important factor effecting the central displacement compared with the excitation frequency.
The piezoelectric actuation is one of the important actuations for the valveless micropump. Piezoelectric actuator can provide moderate pressure at low power consumption as well as good reliability, compared with other actuators, such as electrostatic, electromagnetic, and thermopneumatic actuator. In order to predict and evaluate the effect of the piezoelectric actuator on the pump flow, the relationship between the maximum pump flow and the pump membrane thickness is shown in Fig. 11. The ratio of the pump membrane and the total thickness varies from 0.3 to 0.5. The excitation frequency is 400 Hz. We found that when the elastic pump membrane thickness increases, the pump flow becomes small. When the excitation voltage is 100 V, the cross-section deformation of the pump membrane for different pump membrane thicknesses is shown in Fig. 12. It can be seen that the thinner the pump membrane is, the larger the deformation magnitude and stroke volume are. Hence, the thickness of the pump membrane should be as thin as possible to improve the pump flow. However, due to the limitation of fabrication process and the membrane strength, there should be a minimum pump membrane thickness for the valveless micropump.
The relationship between the maximum pump flow and the piezoelectric membrane thickness for different pump membrane thicknesses h p is shown in Fig. 13. The maximum pump flow is given as a function of the piezoelectric membrane/total thickness ratio, varied from 0.5 to 0.9. Considering the maximum allowable electric field strength for the piezoelectric material, we applied a constant electric field strength of 500 V/mm in the finite element model. The frequency of excitation voltage is 400 Hz. The result shows that the pump flow for the valveless micropump with 0.1 mm pump membrane thickness reduces with the decrease of the piezoelectric membrane thickness, when the ratio of the piezoelectric membrane and the total thickness is less than 0.8. It is explained that the thin piezoelectric membrane can result in the small bending moment and reduce the stroke volume during the pumping cycle. If this ratio is more than 0.8, the pump flow decreases when the piezoelectric thickness increases. The explanation for this is that the flexural rigidity of the piezoelectric actuator becomes large when the piezoelectric membrane thickness increases. Hence, there is an optimal piezoelectric membrane thickness for the fixed thickness of the pump membrane. When the thickness of the pump membrane decreases, the optimal piezoelectric membrane/total thickness ratio becomes large. It implies that this optimal ratio is strongly affected by the pump membrane thickness. Figure 14 gives the relationship between the cross-section deformation of the pump membrane and the piezoelectric membrane thickness, when the thickness of the pump membrane is 0.1 mm. The result shows that when the thickness of the piezoelectric membrane is 0.4 mm, the central displacement of the pump membrane is the largest. The variation of the stroke volume with the thickness of the piezoelectric membrane is in direct proportion to the pump flow of the valveless micropump.
In order to select the suitable piezoelectric material, several commonly used materials, such as PZT-5A, PZT-4, and BaTiO3 are tested in the finite element model. The relationship between the maximum pump flow and the piezoelectric membrane/total thickness ratio for three piezoelectric materials is plotted in Fig. 15. It shows that the PZT-5A actuator can generate the largest pump flow, and the BaTiO3 actuator has the smallest pump flow. It can be seen from the curve that if this ratio varies from 0.5 to 0.8, the pump flow rapidly increases when the thickness of the piezoelectric membrane ranging from 0.1 to 0.4 mm increases. If this ratio varies from 0.8 to 0.9, the pump flow slightly decreases when the thickness of the piezoelectric membrane ranging from 0.4 to 0.9 mm increases. Hence, if the optimal thickness is not available, a thicker piezoelectric membrane is better than a thinner one to improve the micropump performance. However, the optimal thickness does not seem to be strongly affected by the piezoelectric material variation.
We investigate the dependency of the maximum pump flow on the piezoelectric membrane/total thickness ratio for three different membranes, namely, SiO2, Si, and Steel. Figure 16 shows that the pump flow for the SiO2 membrane is significantly larger than that for the Si membrane, and the pump flow for the steel membrane is slightly smaller than that for the Si membrane. It is believed that the deflection of the SiO2 membrane is the largest under the same excitation voltage, because the Young’s modulus for SiO2 is the largest among the three materials. In addition, the optimal piezoelectric membrane/total thickness ratio for the SiO2 membrane is the smallest. The result implies that the optimal piezoelectric thickness is also dependent on the pump membrane properties. Hence, the piezoelectric thickness should be optimized according to the practical pump membrane materials.
Furthermore, we investigate the sensitivity of the maximum pump flow to the material properties of pump membrane, including Young’s modulus, density, and Poisson ratio. The dependency of the normalized pump flow on these properties varied from 0.5 to 1.5 times of Si properties is plotted in Fig. 17. It is shown that the pump flow slightly increases with the increase of the pump membrane density, but dramatically decreases with the increase of the Young’s modulus. In particular, when the Young’s modulus ratio is less than one, the pump flow rapidly decreases with the increase of the Young’s modulus. The change of the pump flow resulted from the Poisson ratio variation is less than 1%. Hence, the result suggests that Young’s modulus of the elastic pump membrane is the dominant sensitive factor on the pump flow, and the density and Poisson ratio have very little effects on the pump performance.
Generally, the pump pressure of the valveless micropump is not sufficient to meet the maximum blood pressure for medical applications. It is desirable to investigate the influence of the actuator dimension on the pump pressure for improving the pumping performance. Figure 18 shows the relationship between the maximum pump pressure and the pump membrane/total thickness ratio ranging from 0.3 to 0.5. We found that the maximum pump pressure decreases as this ratio increases. This implies that it is desirable to have a thinner pump membrane to improve the pump pressure. When the excitation voltage becomes large, the pump pressure increases. The dependency of the maximum pump pressure on the thickness of the piezoelectric membrane is plotted in Fig. 19. A constant electric field strength of 500 V/mm is imposed in the finite element model. The piezoelectric membrane/total thickness ratio varies from 0.5 to 0.9. Thus, there is an optimal thickness ratio for a large pump pressure. For the valveless micropump with 0.1 mm thickness of the pump membrane, if this thickness ratio is less than 0.8, the pump pressure increases when the thickness of the piezoelectric membrane increases. However, when this ratio is beyond 0.8, the pump pressure decreases with the increase of the thickness of the piezoelectric membrane. If the thickness of the pump membrane is changed, the optimal thickness ratio differs. In addition, we also found that the influence of the dimension of the piezoelectric actuator on the maximum pump flow and pressure is similar.
7 Conclusion and future work
In this paper, we presented the design and simulation of an MEMS-based controlled drug delivery system potential for diabetics. This system is designed to achieve in vitro painless injections and automatically adjust the dosage of injected drugs according to the patient’s physiological information. If a wireless transportation device is integrated into the system, the new therapeutic mechanism of continuous communications between the physician and the patient can be built.
A valveless micropump is designed and simulated for the controlled drug delivery system. Based on the fluid–solid interaction solution algorithm, an electro-solid-fluid coupled simulation about the valveless micropump is carried out by using ANSYS software. From the simulation and analysis, we may draw the following important conclusions:
-
1.
The linear pressure-flow characteristics of the valveless micropump are obtained in good agreement with the experimental results (Olsson et al. 1995). The relationship between the maximum pump flow and the excitation voltage is linear. The larger the stroke volume of the pump membrane the better is the performance of the valveless micropump.
-
2.
When the excitation frequency is in the range from 200 to 500 Hz, the micropump at the high excitation frequency can generate a larger pump flow. The central displacement of the pump membrane does not seem to be strongly affected by the excitation frequency.
-
3.
The pump flow of the valveless micropump and the stroke volume of the pump membrane decrease when the thickness of the pump membrane increases, and if the thickness of the piezoelectric membrane is constant. The thickness of the pump membrane should be as thin as possible to improve the pump flow.
-
4.
There is an optimal thickness of the piezoelectric membrane for the fixed thickness of the pump membrane under a constant electric field. The dimension of the pump membrane can strongly affect the optimal ratio of the piezoelectric membrane and the total thickness.
-
5.
It is found that the PZT-5A actuator has the best performance among the three commonly used materials (SiO2, Si, and steel), and the optimal thicknesses for the three piezoelectric actuators do not seem to be significantly different. The variation of the pump membrane material may change the optimal thickness. The Young’s modulus of the pump membrane is the dominant sensitive factor on the pump flow, and the density and Poisson ratio have very little effects on the pump performance. As the Young’s modulus for SiO2 is the largest among the three materials, the micropump with the SiO2 membrane generates the largest pump flow.
-
6.
The pump pressure decreases when the thickness of the pump membrane increases and the excitation voltage decreases. Hence, a thin pump membrane is good for the increase of the pump pressure. When the thickness of the pump membrane is 0.1 mm, an optimal value for the piezoelectric membrane/total thickness ratio is about 0.8, which is affected by the thickness variations of the pump membrane.
Though the development of MEMS devices is advancing rapidly, the integration of different devices is still challenging. Our future work will focus on this aspect. Meanwhile, we will fabricate the optimized design of the valveless micropump and integrate it into the controlled drug delivery system for in vitro injection of diabetics and characterize them by experiments. In addition, we will also investigate how to solve the low backpressure resistance at the system-level.
Reference
Andersson H, Wijingaat Wvd, Nilsson P, Enoksson P, Stemme G (2001) A valve-less diffuser micropump for microfluidic analytical systems. Sens Actuators B 72:259–265
Bashir R (2004) BioMEMS: state-of-the-art in detection, opportunities and prospects. Adv Drug Deliv Rev 56:1565–1586
Cao L, Mantell S, Polla D (2001) Design and simulation of an implantable medical drug delivery system using microelectromechanical systems technology. Sens Actuators A 94:117–125
Fan X-J, Nhan P-T, Ng TY, Diao X (2002) Molecular dynamics simulation of a liquid in a complex nano channel flow. Phys Fluid 14:1146–1153
Fracais O, Dufour I (2002) Dynamic simulation of an electrostatic micropump with pull-in and hysteresis phenomena. Sens Actuators A 70:43–50
Kan J, Yang Z, Peng T, Cheng G, Wu B (2005) Design and test of a high-performance piezoelectric micropump for drug delivery. Sens Actuators A 121:156–161
Li S, Liu Yang, Cheng S (2001) Dynamic modeling and optimization of a valveless PZT micropump. In: Proceedings of SPIE, microfluidics and BioMEMS, pp 67–74
Li S, Chen S (2003) Analytical analysis of a circular PZT actuator for valveless micropumps. Sens Actuators A 104:151–161
Loy CT, Pradhan SC, Ng TY, Lam KY (1999) A series solution approach to an analytical load-deflection relation for the measurement of mechanical properties of thin films. J Micromech Microeng 9:341–344
Morris CJ, Foster FK (2000) Optimization of a circular piezoelectric bimorph for a micropump driver. J Micromech Microeng 10:459–465
Ng TY, Jiang TY, Li H, Lam KY, Reddy JN (2004) A coupled field study on the non-linear dynamic characteristics of an electrostatic micropump. J Sound Vib 273:989–1006
Olsson A, Stemme G, Stemme E (1995) A valve-less planar fluid pump with two pump chambers. Sens Actuators A 46–47:549–556
Olsson A, Stemme G, Stemme E (1996) Diffuser-element design investigation for valve-less pumps. Sens Actuators A 57:137–143
Olsson A, Enoksson P, Stemme G, Stemme E (1997) Micromachined flat-walled valveless diffuser pumps. J Microelectromech Syst 6:161–166
Olsson A, Stemme G, Stemme E (2000) Numerical and experimental studies of flat-walled diffuser elements for valve-less micropumps. Sens Actuators A 84:165–175
Pan LS, Ng TY, Liu GR, Lam KY, Jiang TY (2001) Analytical solutions for the dynamic analysis of a valveless micropump—a fluid-membrane coupling study. Sens Actuators A 93:173–181
Pan LS, Ng TY, Wu XH, Lee HP (2003) Analysis of valveless micropumps with inertial effects. J Micromech Microeng 13:390–399
Smits JG (1990) Piezoelectric micropump with three valves working peristaltically. Sens Actuators A 21:203–206
Stemme E, Stemme G (1993) A valveless diffuser/nozzle-based fluid pump. Sens Actuators A 39:159–167
Stoeber B, Liepmann D (2005) Arrays of hollow out-of-plane microneedles for drugs for drug delivery. J Microelectromech Syst 3:472–479
Singhal V, Garimella SV, Murthy JY (2004) Low Reynolds number flow through nozzle-diffuser elements in valveless micropumps. Sens Actuators A 113:226–235
Teymoori MM, Abbaspour-Sani E (2005) Design and simulation of a novel electrostatic peristaltic micromachined pump for drug delivery application. Sens Actuators A 117:222–229
White FM (1991) Viscous fluid flow, 2nd edn. McGraw-Hill, New York
Yamahata C, Lotto C, Al-Assaf E, Gijs MAM (2005) A PMMA valveless micropump using electromagnetic actuation. Microfluid Nanofluid 1:197–207
Yih TC, Wei C, Hammad B (2005) Modeling and characteration of a nanoliter drug-delivery MEMS micropump with circular bossed membrane. Nanomedicine 1:164–175
Zhang M, Tarn T, Xi N (2004) Micro-/Nano-devices for controlled drug delivery. In: international conference on robotics & automation, New Orleans, pp 2068–2073
Acknowledgments
The authors would like to express their gratitude to anonymous reviewers of this paper for their insightful comments and suggestions, which helped to improve the paper. No approval or endorsement by US National Institute of Standards and Technology is intended or implied. Supported by National Natural Science Foundation of China (grant no. 50575145).
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Cui, Q., Liu, C. & Zha, X.F. Study on a piezoelectric micropump for the controlled drug delivery system. Microfluid Nanofluid 3, 377–390 (2007). https://doi.org/10.1007/s10404-006-0137-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-006-0137-0