Abstract
The Natal multimammate mouse (Mastomys natalensis) is the reservoir host of Lassa arenavirus, the etiological agent of Lassa fever in humans. Because there exists no vaccine for human use, rodent control and adjusting human behavior are currently considered to be the only options for Lassa fever control. In order to develop efficient rodent control programs, more information about the host’s ecology is needed. In this study, we investigated the spatial behavior of M. natalensis and other small rodents in two capture-mark-recapture and four dyed bait (Rhodamine B) experiments in Lassa fever-endemic villages in Upper Guinea. During the capture-mark-recapture studies, 23% of the recaptured M. natalensis moved between the houses and proximate fields. While M. natalensis was found over the entire study grid (2 ha), other rodent species (Praomys daltoni, Praomys rostratus, Lemniscomys striatus, Mus spp.) were mostly trapped in the surrounding fields. Distances between recapture occasions never exceeded 100 m for all rodent species. During the dyed bait experiments, 11% of M. natalensis and 41% of P. daltoni moved from the fields to houses. We conclude that commensal M. natalensis easily moves between houses and proximate fields in Guinea. We therefore consider occasional domestic rodent elimination to be an unsustainable approach to reduce Lassa virus transmission risk to humans, as M. natalensis is likely to reinvade houses quickly from fields in which rodents are not controlled. A combination of permanent rodent elimination with other control strategies (e.g., make houses rodent proof or attract predators) could be more effective for Lassa fever control, but must be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Natal multimammate mouse, Mastomys natalensis (Smith, 1834), is the natural host of Lassa virus (LASV), an arenavirus that causes Lassa hemorrhagic fever (LF) in humans (Monath 1987). In West Africa, these rodents thrive in houses and surrounding fields of rural villages where they shed the virus through feces, urine and saliva (Mccormick et al. 1987; Walker et al. 1975). Humans can become infected by ingestion of contaminated food or water, inhalation of virus particles, touching contaminated objects or by direct consumption of the rodents (Stephenson et al. 1984; McCormick 1999; Bonwitt et al. 2016). Secondary human-to-human transmission is also possible and occurs typically within households or health care facilities (Carey et al. 1972; Fisher-Hoch et al. 1995; Lo Iacono et al. 2015). Annually around 200,000 people are affected, with a fatality rate of 1–2% (Monath 1987; McCormick 1999; World Health Organization 2016). Since no human vaccine exists and therapeutic options are limited to the broad-spectrum antiviral ribavirin, rodent control and adjusting human behavior are currently considered to be the only options for LASV prevention (Bausch et al. 2010; Fisher-Hoch et al. 2000; Hastie et al. 2017).
In order to develop sustainable rodent control programs, basic knowledge of the rodent’s spatial behavior is essential to determine when and where to control and to anticipate recolonization events (Singleton et al. 1999, 2010). Since M. natalensis is the most important rodent pest species in sub-Saharan Africa, plenty of information can be found in the literature and outbreak forecast models have been developed (Leirs 1994; Leirs et al. 1997; Monadjem and Perrin 1998; Massawe et al. 2011; Borremans et al. 2015). While most of these studies investigated M. natalensis living in large agricultural fields or natural savannah habitat in East or Southern Africa, little attention has been paid to its ecology in LF-endemic areas in West Africa (Swanepoel et al. 2017). Here, the rodent flourishes in houses and surrounding cultivations of rural villages, where it reaches between 95 and 98% of all indoor captures (Demby et al. 2001; Fichet-calvet et al. 2009). The reason why M. natalensis is found in houses in remote areas in West Africa could be the absence of competing Rattus species (Demby et al. 2001), which are suggested to scare away M. natalensis from buildings in East and South Africa (Monadjem et al. 2011). Because several environmental factors (e.g., resource availability, predation risk or mating system) can affect rodent behavior considerably, additional research is needed to better understand M. natalensis’ ecology in these commensal environments (Mulungu et al. 2015; Fichet-Calvet et al. 2007).
To our knowledge, only one longitudinal survey investigated the spatial behavior of M. natalensis in LF-endemic areas. Fichet-Calvet et al. 2007 trapped rodents inside houses and surrounding cultivations in three villages in Upper Guinea. They found clear seasonal fluctuations in abundance according to habitat type. While M. natalensis was as numerous inside as outside houses during the rainy season, its abundance increased significantly inside but decreased outside houses during the dry season. This pattern suggests a change in habitat preference driven by fluctuations in food availability. When the dry season starts, the harvest is cut and the crops are stored inside the houses. At the same time, fields are left fallow or burned for the cultivation of crops the following year. These environmental conditions might attract M. natalensis from the surrounding fields to the houses where they find food and shelter. Because houses in these villages typically consist of one room in which the harvest is stored close to the bed, the living space of humans and M. natalensis seems to overlap almost completely during the dry season (Bonwitt et al. 2017). An increased transmission risk of LASV to humans can therefore be expected and is also noted by clinicians who observed more LF cases in local hospitals during this period (Mccormick et al. 1987; Bausch et al. 2001; Asogun et al. 2012).
In this study, we examined the spatial behavior of M. natalensis in LF-endemic villages during the dry season at three levels. First, we investigated if M. natalensis moves from the surrounding fields to the houses, as hypothesized by Fichet-Calvet et al. (2007). Secondly, we reported the mean and maximum distances that this rodent moved between captures, which we used as an indicator of its home-range size. Finally, we investigated the rodent’s microhabitat choice within and close to the village. Although we were mainly interested in the spatial behavior of M. natalensis as the reservoir host of LASV, we included other captured rodent species in our analysis as well. These rodents are not reservoirs of LASV, but spillover infections to these animals (Praomys daltoni, Praomys rostratus, Lemniscomys striatus, Mus matthei/minutoides) have been observed (Fichet-Calvet et al. 2014). We performed two types of experiments: capture-mark-recapture (CMR) experiments in which traps were set in both houses and surrounding fields, and Rhodamine B (RB) experiments in which colored bait was set in the fields and rodents were trapped inside houses.
Methods
Selection of Study Sites
Because we wanted to build on the results of Fichet-Calvet et al. 2007, we chose the same area used in that study for our experiments, which is the prefecture of Faranah in Upper Guinea. This area was chosen for its high mean human LASV seroprevalence (approx. 35%) and the abundance of M. natalensis in the houses (Demby et al. 2001; Lukashevich et al. 1993). In Faranah, the rainy season starts in April and continues until October with an annual mean rainfall of 1458 mm. Four rural villages were selected: Tambaya (10°18′25″N; 10°51′45″W), Silimi (09°58′32″N, 10°39′07″W), Brissa (10°13′00″N, 10°41′20″W) and Yarawalia (9°57′18″N, 10°43′56″W). They were chosen because of their remote location from a paved road, a size not exceeding 1000 inhabitants (Tambaya 1000, Silimi 960, Brissa 655, and Yarawalia 570 inhabitants), and less than 45 min driving time from Faranah. Experiments were performed during the beginning of the dry season in the months October and November of the years 2015 and 2016. This period was chosen because the harvest is recently stored inside the houses and the fields are left fallow, which we hypothesized to be the main drivers for M. natalensis to enter the houses.
Capture-Mark-Recapture Studies
Two capture-mark-recapture (CMR) experiments were performed: one in Tambaya (14/10/2015–6/11/2015) and a second in Silimi (17/10/2016–8/11/2016). The two villages were chosen for biosafety reasons, as the LASV prevalence in the rodent population was zero in Tambaya and low in Silimi (1/42) in comparison with other villages in this area (Fichet-Calvet et al. 2016).
During 1 month, rodents were live-trapped every week for three consecutive nights using Sherman live traps (Sherman Live Trap Co., Tallahassee, FL, USA). Trapping was done in 2-ha rectangular grids of 20 × 10 traps spaced 10 m apart. The grids were located on the edge of the villages in a way that both houses and surrounding fields were included. The habitat type was noted for each location where a trap was set: inside a house, or outside in bush, grass, cultivations or open-space (public square) habitat (Fig. 1). The traps were baited (with a mixture of peanuts, dry fish and wheat flour) in the evening and checked in the morning. Captured rodents were taken to a small building, where species, sex, weight and reproductive status were recorded (Leirs 1994; Sluydts et al. 2007). Identification of rodent species was based on external morphology following Kingdon et al. 2013. Mice were considered to be adults if signs of sexual activity were observed (scrotal testes in males, perforated vagina, lactating nipples or pregnancy in females). All animals were individually marked by toe clipping or tattooing (Leirs 1994; Hess 2009; Borremans et al. 2015) and released at the same location as where they were previously captured (even if they were captured inside a house).
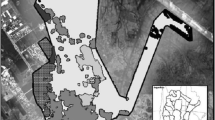
Movements of recaptured M. natalensis during the capture-mark-recapture experiments in Tambaya (left) and Silimi (right). Traps were spaced 10 m apart. Arrows represent the direction and minimum distance that an individual moved between two successive captures. Points (two times) and triangles (three times) represent recaptures on the same location. Colors of the arrows indicate sex and reproductive status of the animals (red = male and adult, black = female and adult, blue = juvenile). The colors of the different coordinates represent the habitat type where the trap was placed (white = house, pink = open space, orange = cultivations, yellow = grass, green = bush).
The mean distance between successive captures (MDSC) and maximum distance between all captures (MDAC) were calculated for each recaptured individual based on the Euclidian distance between trapping locations. The MDSC was used as an indicator of home-range size which could not be calculated directly due to the low number of recaptures per animal (Monadjem and Perrin 1998; Slade and Russell 1998). The R-packages ‘colorRamps’ and ‘fields’ were used for the plotting of habitat types, rodent movements and capture locations in the village (R Core Team 2016).
Microhabitat choice was analyzed by relating habitat type per trapping station to trapping success per station (i.e., total number of rodents captured on that station/number of trapping occasions). The habitat type around each station was determined and divided into five categories: house (indoor), cultivations (outdoor, surrounded by agricultural plants), bush (outdoor, surrounded by trees or shrubs > 1 m), grass (outdoor, surrounded by Poaceae plants) or open space (outdoor, no vegetation). We did not include the habitat ‘open space’ in the analyses because there were only a few stations placed in this habitat. The relation was analyzed with a generalized linear model (GLM) with negative binomial distribution (glm.nb function in R), as there was considerable evidence of overdispersion when we used a Poisson distribution. Multiple comparisons (glht function in R) between the different vegetation types were analyzed by a Tukey’s test using the Rpackage ‘Multcomp’(R Core Team 2016). Separate analyses were performed for the two villages, as rodent diversity and abundance differed considerably between them. We only retained rodent species of which there were at least 25 captures (i.e., M. natalensis, P. daltoni, P. rostratus, Mus spp. and L. striatus).
Rhodamine B Experiments
Rhodamine B (RB) is a dye that, once ingested, becomes incorporated into keratinous structures of animals where it is detectable for up to several weeks under fluorescent light (Jacob et al. 2002). The dye is often used to examine dispersal of mammals and was previously shown to be effective to study rodent movements (Mohr et al. 2007; Monadjem et al. 2011; Rahelinirina et al. 2009). Four RB experiments were performed: two in Brissa (first 23/10/2015-11/11/2015; second 26/10/2016-14/11/2016) and two in Yarawalia (first 1/11/2015-18/11/2015; second 4/11/2016-23/11/2016). These villages were chosen for practical reasons, as they are part of ongoing studies in which LASV viral prevalence was followed in the rodent population for several years.
For the preparation of the bait, 2 g RB powder (Sigma-Aldrich) was mixed in 1 kg of conventional bait (Monadjem et al. 2011). Bait stations were filled with 50 g RB bait and placed in (one to three) fields close to the village border in a grid formation spaced 5 m apart (Fig. 3). The number of bait stations differed between the 2 years: While 100 bait stations were placed in both villages in the year 2015, 200 stations were placed in both villages in 2016. The stations stayed in the field for eight consecutive days, where they were rebaited each 2 days and checked for signs of consumption. Eight days after removal of the stations, 120 traps were placed in 60 houses that were randomly chosen along a transect from the RB fields to the village center. In order to be sure that rodents were willing to eat the bait, 50 additional traps were placed in the RB fields. All traps were baited during three consecutive evenings and checked every morning. Captured rodents were humanely killed, measured morphometrically (as described in Fichet-Calvet et al. 2007) and checked for external signs of RB. The whiskers (including the follicle) and a piece of the fur were removed, put in small ziploc bags and stored in a − 20°C freezer until further analysis followed. Coordinates of the houses where rodents were trapped were recorded with a GPS.
At least six whiskers and a piece of the fur were eventually placed on a microscope slide in a drop of water and covered with a coverslip. Slides were examined for signs of RB under a fluorescence microscope (UV light at 530–585 nm) at low magnification and always compared with a positive and negative control sample. Distances between the houses and the fields were estimated based on the GPS coordinates and calculated with the ‘Vincenty (ellipsoid)’ method from the Rpackage ‘geosphere’. Locations of the RB fields and houses were plotted in Google Maps (with the Rpackage ‘ggmap’) (R Core Team 2016).
Ethical Statement
During all manipulations, standard procedures for BSL3 work in the field were followed (Mills et al. 1995). The investigation and permission to conduct research on wild animals were approved by the National Ethics Committee of Guinea (permit n° 12/CNERS/12 and 129/CNERS/16) and carried out in accordance with the approved guidelines. The experiments were performed in collaboration with the local health authorities (Prefecture de Faranah) and in agreement with the village chiefs. Trapping and releasing of rodents in houses was only performed if permission was obtained from the individual house owners.
Results
Capture-Mark-Recapture Study
During 4800 trap nights, we captured 378 animals of which 109 individuals were recaptured a total of 203 times (Table 1). The exact number of times that individuals were recaptured is presented per species in the additional information table 1.
The species richness and evenness differed considerably between the two villages (Table 1). Almost all animals trapped in Tambaya were M. natalensis (94%), and only one other rodent (P. daltoni) and Crocidura species were found. M. natalensis was also the most abundant rodent in Silimi (37%), but other rodents [P. daltoni (5%), P. rostratus (20%), L. striatus (11%) and Mus spp. (14%)] were captured frequently as well. Other trapped species in Silimi were Grammomys butingi, Uranomys ruddi, Lophuromys sikapusi, Gerbilliscus guineae, Rattus rattus and Crocidura spp. Although we could not identify all individuals to the species level, we know from previous studies that three sibling species of Mus (M. mattheyi, M. minutoides and M. baoulei) and Crocidura (C. theresae, C. buettikoferi and C. lamottei) can occur in this area (Fichet-calvet et al. 2009).
Direct movements into or out of houses or recaptures in the same house were mainly found for M. natalensis (Fig. 1 and additional information table 2). We captured three M. natalensis (two in Tambaya and one in Silimi) first inside and afterward outside a house, eight M. natalensis (five in Tambaya and three in Silimi) first outside and afterward inside a house, one M. natalensis (in Silimi) in one house and later in another house, and six M. natalensis (four in Tambaya and two in Silimi) always in the same house. In addition, we captured two P. daltoni, one P. rostratus, one L. striatus and two M. musculus (all in Silimi) first inside and later outside a house, two P. daltoni and one L. striatus first outside and later inside a house, and one P. daltoni two times in the same house. Outdoor movements between different vegetation types were observed for fifteen M. natalensis, seven P. daltoni, twelve P. rostratus, eleven L. striatus, three Crocidura spp and seven Mus spp. captures (additional information: table 2).
Distances between captures were small for all rodent and shrews: the mean distance between successive captures (MDSR) lay between 10.0 and 40.6 m and maximum distance between all captures (MDAR) between 10.0 and 60.4 m depending on the species (Table 1). Some individuals moved larger distances, but the MDAR never exceeded 100 m for all species (Fig. 2).
Differences in microhabitat choice were observed for all rodent species, but significant differences were only found for M. natalensis and P. rostratus (Table 2; additional information table 3 for the p values). In both villages, we trapped significantly more M. natalensis inside houses than in bush vegetation patches (z value = 0.35, p = <0.01). We also trapped more M. natalensis in houses compared to cultivations in Tambaya (z value: 3.22, p = <0.01), and in houses compared to grass in Silimi (z value = 0.38, p < 0.01). We trapped almost no other rodent species inside houses, but only P. rostratus was captured significantly more frequently in grass patches compared to houses (z value = −2.59, p = <0.01). Significant differences between vegetation types were only found for M. natalensis, which we trapped more often in cultivations compared to bush vegetation in Silimi (z value = 0.31, p = <0.01).
Rhodamine B Experiment
During 2040 trap nights, we captured 223 animals of which 174 individuals were trapped inside a house and 49 in a RB field (Table 2). The most dominant species in both villages was M. natalensis (86%) followed by P. daltoni (13%). Other trapped species were P. rostratus, L. striatus, Mus spp., R. rattus and Crocidura spp.
We found 65 animals with signs of RB (Table 3). The majority of trapped rodents in the RB fields were RB positive, indicating that these animals were willing to eat the dyed bait. In the houses, 18 M. natalensis and 7 P. daltoni were found to be RB positive, which means that these individuals had moved from the fields to the houses during our study (thus within maximally 16 days) (Fig. 3). Most of these RB-positive individuals were found in houses that were close (< 25 m) to a RB field (Fig. 4). The number of RB-positive animals decreased with distance from the RB fields, and no positive individuals were found in houses that were more than 100 m from the closest RB field.
Overview of the villages (Brissa and Yarawalia) where Rhodamine B experiments were performed per year (Google Maps image). Red polygons represent Rhodamine B fields. Points and squares represent houses where rodents were captures (Color: black = Rhodamine B-negative rodents, red = Rhodamine B-positive rodents; symbol: points = M. natalensis; squares = P. daltoni).
Minimal distances between Rhodamine B fields and houses where rodents (left = M. natalensis and right = P. daltoni) were trapped. Bars represent the number of trapped individuals per distance interval away from the closest Rhodamine B field (black = rodents without Rhodamine B signs, red = rodents with RB signs).
Discussion
We hypothesized that M. natalensis moves from the surrounding fields to the houses during the dry season to search for food and shelter, which is abundant inside but scarce outside houses at that time (Fichet-Calvet et al. 2007). While we observed direct movements from the fields to the houses in all experiments, we did not find overall migration in this direction only. In fact, more individuals moved from the houses to the fields during the CMR experiments. The low number of recaptured animals in the CMR experiments and the fact that we only checked for movements from the fields to the houses in the RB experiments made it impossible to statistically investigate the overall direction in which M. natalensis actually migrated.
Although we could not statistically confirm the migration hypothesis, our study shows that M. natalensis can easily enter and leave houses, stay for a long time inside the same house or move from one house to another. These results suggest that an individual home range of M. natalensis can include both houses and outdoor fields in rural villages. Therefore, it remains likely that M. natalensis stays for longer periods inside houses when food availability is limited outdoor, which might explain the increased indoor rodent abundance and LASV transmission risk to humans during the dry season (Bausch et al. 2001; Fichet-Calvet et al. 2007). Similar results were found in other field studies performed in Tanzania and Swaziland, where M. natalensis visited houses after which it retreated up to 100 m into the surrounding fields (Monadjem et al. 2011). Our results are also consistent with a telemetry study performed in Namibia in which radio-tracked M. natalensis was found to enter houses regularly during the post-harvest season, but not during the pre-harvest or pre-planting seasons (Monadjem et al. 2011). Half of the radio-tracked individuals remained inside the same house after entering, while the other half returned to the fields. In contrast, radio-tracked M. natalensis stayed predominantly in the fields in rural villages in Swaziland, probably due to the abundant presence of competing Rattus species in the houses (Monadjem et al. 2011).
Most M. natalensis moved little between captures and distances presented here are similar to those observed in other CMR studies performed in agricultural fields in Africa. Since the MDSC was suggested to be a good indicator of M. natalensis’ home-range size (Monadjem and Perrin 1998; Slade and Russell 1998), we can assume that sizes in Guinea are similar to those observed in South Africa (± 680 m2) and Tanzania (± 650 m2) (Borremans et al. 2013; Leirs et al. 1997; Monadjem and Perrin 1998). These sizes could, however, be underestimations as home ranges of radio-tracked M. natalensis were considerably larger in agricultural fields in Tanzania (± 1200 m2), rural villages in Swaziland and Namibia (± 4300 m2) and natural vegetation in Uganda (± 8400 m2) (Hoffmann and Klingel 2001; Leirs et al. 1997; Monadjem et al. 2011). Animals with home ranges of this magnitude would indeed be able to move easily between houses and surrounding fields.
The patchy vegetation structure of the fields allowed us to assess microhabitat choice of M. natalensis within and close to the villages. Overall, M. natalensis seems to move easily among vegetation types without showing clear preferences between grass, bush or cultivations. There were, however, some differences between the two villages. M. natalensis was trapped more frequently in cultivations than in bush and grass in Silimi, although the opposite was true in Tambaya. More vegetables were still present on the cultivations in Silimi compared to Tambaya, which might explain this result. In Tambaya, M. natalensis was most often trapped in grass, which is also assumed to be its natural habitat (Leirs 1994). In East Africa, abundances are generally lower in grasslands compared to agricultural fields, except during the early breeding season (Leirs 1994). This suggests that M. natalensis migrates from agricultural fields to grasslands for reproduction, probably because juveniles are better protected against predators in the denser grass vegetation. Since M. natalensis breeds throughout the year in Guinea (Fichet-Calvet et al. 2008), abundances in grass vegetation might be generally higher here.
We also captured several other rodent and shrew species in this study besides M. natalensis. These species were predominantly captured outdoor and assumed not to be reservoirs of LASV. Nevertheless, we included these animals in this study for two reasons. First, antibodies against LASV, or LASV-like arenaviruses, were found previously in some of these species, indicating that spillover infections could occur (Fichet-Calvet et al. 2014). Secondly, according to our knowledge, no other studies investigated the spatial behavior of these animals in rural villages. Although observed habitat choices and distances between captures were not exceptional for most species (Happold 1977), a high proportion of P. daltoni trapped in houses close to the RB field (< 25 m) showed signs of RB consumption. This proportion was considerably higher compared to M. natalensis (63 vs 30%, respectively), suggesting that P. daltoni visits houses for short periods after which it returns to the fields, where it is trapped most often. In other countries such as Ghana, Senegal or Nigeria, P. daltoni can represent a larger proportion of the commensal rodent populations (Bâ et al. 2012; Kronmann et al. 2013; Olayemi et al. 2017). Praomys daltoni seems to become the main commensal species when M. natalensis is rare or the other way around.
Overall results from this study are rather disappointing for the development of simple rodent control programs. Occasional rodent elimination seems pointless, as M. natalensis is likely to reinvade houses or cultivations rapidly from fields (e.g., bush or grass patches) where rodents are not controlled. In support of this reasoning, local villagers in Sierra Leone (a neighboring country) had the impression that rodents returned quickly after they set poison or traps, especially in houses close to the village border (Bonwitt et al. 2017). We therefore recommend that, in Guinea, rodents must be controlled on a permanent basis in order to be effective, at least during the dry season when M. natalensis abundances are high in the houses. Similar suggestions were made during experimental studies performed in rural villages in Namibia where contamination and loss of grain were clearly lower in year-round controlled than in non-controlled villages (Taylor et al. 2012). We also recommend combining rodent elimination with other control strategies. Rodent proofing of houses and storing food in airtight containers were found to be highly effective in discouraging rodents from entering houses, while keeping cats and dogs or attracting wildlife predators (e.g., owls, civets) might scare them away (Hopkins et al. 2002; Labuschagne et al. 2016; Mahlaba et al. 2017; Mdangi et al. 2013). Future research is necessary to see if these ecologically based control strategies can indeed decrease LASV transmission risk to humans in Guinea.
References
Asogun DA, Adomeh DI, Ehimuan J, Odia I, Hass M, Gabriel M, Olschlager S, Becker-Ziaja B, Folarin O, Phelan E, Ehiane PE, Ifeh VE, Uyigue EA, Oladapo YT, Muoebonam EB, Osunde O, Dongo A, Okokhere PO, Okogbenin SA, Momoh M, Alikah SO, Akhuemokhan OC, Imomeh P, Odike MAC, Gire S, Andersen K, Sabeti PC, Happi CT, Akpede GO, Günther S (2012) Molecular diagnostics for Lassa fever at irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0001839
Bâ K, Kane M, Gauthier P, Granjon L (2012) Ecology of a typical West African Sudanian savannah rodent community. Afr J Ecol 51:447–455.
Bausch DG, Demby AH, Coulibaly M, Kanu J, Goba A, Bah A, Condé N, Wurtzel HL, Cavallaro KF, Lloyd E, Baldet FB, Cissé SD, Fofona D, Savané IK, Tolno RT, Mahy B, Wagoner KD, Ksiazek TG, Peters CJ, Rollin PE (2001) Lassa Fever in Guinea: I. Epidemiology of Human Disease and Clinical Observations DANIEL. Vector borne zoonotic Dis 1:269–281.
Bausch DG, Hadi CM, Khan SH, Lertora JJL (2010) Review of the Literature and Proposed Guidelines for the Use of Oral Ribavirin as Postexposure Prophylaxis for Lassa Fever. Clin Infect Dis 51:1435–1441. https://doi.org/10.1086/657315
Bonwitt J, Kelly AH, Ansumana R, Agbla S, Sahr F, Saez AM, Borchert M, Kock R, Fichet-Calvet E (2016) Rat-atouille: A Mixed Method Study to Characterize Rodent Hunting and Consumption in the Context of Lassa Fever. Ecohealth 13:234–247. https://doi.org/10.1007/s10393-016-1098-8
Bonwitt J, Sáez AM, Lamin J, Ansumana R, Dawson M, Buanie J, Lamin J, Sondufu D, Borchert M, Sahr F, Fichet-Calvet E, Brown H (2017) At home with mastomys and rattus: Human-rodent interactions and potential for primary transmission of lassa virus in domestic spaces. Am J Trop Med Hyg 96:935–943. https://doi.org/10.4269/ajtmh.16-0675
Borremans B, Hughes NK, Reijniers J, Sluydts V, Katakweba AAS, Mulungu LS, Sabuni CA, Makundi RH, Leirs H (2013) Happily together forever: temporal variation in spatial patterns and complete lack of territoriality in a promiscuous rodent. Popul Ecol 56:109–118. https://doi.org/10.1007/s10144-013-0393-2
Borremans B, Sluydts V, Makundi RH, Leirs H (2015) Evaluation of short-, mid- and long-term effects of toe clipping on a wild rodent. Wildl Res 42:143–148.
Carey DE, Kemp GE, Pinneo HAW, Addy RF, Fom ALMD, Stroh G, Henderson BE (1972) Lassa fever epidemiological aspects of the 1970 epidemic, Jos, Nigeria. Trans R Soc Trop Med Hyg 66:402–408.
Demby AH, Inapogui A, Kargbo K, Koninga J, Kourouma K, Kanu J, Coulibaly M, Wagoner KD, Ksiazek TG, Peters CJ, Rollin PE, Bausch DG (2001) Lassa Fever in Guinea: II. Distribution and prevalence of Lassa virus infection in small mammals. Vector borne zoonotic Dis 1:283–299.
Fichet-calvet E, Audenaert L, Barrie P, Verheyen E (2009) Diversity, dynamics and reproduction in a community of small mammals in Upper Guinea, with emphasis on pygmy mice ecology. Afr J Ecol 48:600–614.
Fichet-Calvet E, Becker-Ziaja B, Koivogui L, Günther S (2014) Lassa serology in natural populations of rodents and horizontal transmission. Vector Borne Zoonotic Dis 14:665–74. https://doi.org/10.1089/vbz.2013.1484
Fichet-Calvet E, Lecompte E, Koivogui L, Daffis S, ter Meulen J (2008) Reproductive characteristics of Mastomys natalensis and Lassa virus prevalence in Guinea, West Africa. Vector Borne Zoonotic Dis 8:41–8. https://doi.org/10.1089/vbz.2007.0118
Fichet-Calvet E, Lecompte E, Koivogui L, Soropogui B, Doré A, Kourouma F, Sylla O, Daffis S, Koulémou K, Ter Meulen J (2007) Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in Guinea, West Africa. Vector Borne Zoonotic Dis 7:119–28. https://doi.org/10.1089/vbz.2006.0520
Fichet-Calvet E, Ölschläger S, Strecker T, Koivogui L, Becker-Ziaja B, Camara AB, Soropogui B, Magassouba N, Günther S (2016) Spatial and temporal evolution of Lassa virus in the natural host population in Upper Guinea. Sci Rep 1–6. https://doi.org/10.1038/srep21977
Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB (2000) Effective Vaccine for Lassa Fever. J Virol 74:6777–6783. https://doi.org/10.1128/jvi.74.15.6777-6783.2000
Fisher-Hoch SP, Tomori O, Nasidi A, Perez-Oronoz GI, Fakile Y, Hutwagner L, McCormick JB (1995) Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. BMJ 311:857–859. https://doi.org/10.1136/bmj.311.7009.857
Happold DCD (1977) A population study on small rodents in the tropical rain forest of Nigeria. LaTerre la Vie 31:385–458.
Hastie KM, Zandonatti MA, Kleinfelter LM, Heinrich ML, Rowland MM, Chandran K, Branco LM, Robinson JE, Garry RF, Saphire EO (2017) Structural basis for antibody-mediated neutralization of Lassa virus. Science 356:923–928.
Hess BH (2009) Mouse Ear Tattoo: A Quick and Easy Alternative to Ear Punches and Tags. J Am Assoc Lab Anim Sci 48:587–588.
Hoffmann A, Klingel H (2001) Spatial and temporal patterns in Mastomys cf. natalensis by radiotracking. In: African small mammals, Collection Colloques et seminaires, IRD Edition, Paris. Proceedings of the 8th International African Small Mammal Symposium, Paris 1999.
Hopkins AS, Whitetail-eagle JOE, Corneli AMYL, Person B, Ettestad PJ, Dimenna M, Norstog JON, Creswell J, Khan ALIS, Olson JG, Cavallaro KF, Bryan RT, Cheek JE, Begay B, Hoddenbach GA, Ksiazek TG, Mills JN (2002) Experimental Evaluation of Rodent Exclusion Methods Native American Community in New Mexico. Vector borne zoonotic Dis 2:61–68.
Jacob J, Jones D, Singleton G (2002) Retention of the bait marker Rhodamine B in wild house mice. Wildl Res 29:159–164.
Kingdon J, Happold D, Butynski T, Hoffmann M, Happold M, Kalina J (2013) The mammals of, hares and rabbits. Bloomsbury Publishing, London
Kronmann KC, Nimo-paintsil S, Obiri-danso K, Ampofo W, Fichet-calvet E (2013) Arenaviruses Detected in Pygmy Mice, Ghana. Emerg Infect Dis 19:1832–1835.
Labuschagne L, Swanepoel LH, Taylor PJ, Belmain SR, Keith M (2016) Are avian predators effective biological control agents for rodent pest management in agricultural systems? Biol Control 101:94–102. https://doi.org/10.1016/j.biocontrol.2016.07.003
Leirs H (1994) Population ecology of Mastomys natalensis (Smith, 1834). Implications for rodent control in Africa. Brussels
Leirs H, Verhagen R, Sabuni C, Mwanjambe P, Verheyen W (1997) Spatial patterns in Mastomys natalensis in Tanzania (Rodentia, Muridae). Belgian J Zool 127:29–38.
Lo Iacono G, Cunningham AA, Fichet-Calvet E, Garry RF, Grant DS, Khan SH, Leach M, Moses LM, Schieffelin JS, Shaffer JG, Webb CT, Wood JLN (2015) Using Modelling to Disentangle the Relative Contributions of Zoonotic and Anthroponotic Transmission: The Case of Lassa Fever. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0003398
Lukashevich IS, Clegg JCS, Sidibe K (1993) Lassa virus activity in Guinea: Distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J Med Virol 40:210–217. https://doi.org/10.1002/jmv.1890400308
Mahlaba TAM, Monadjem A, McCleery R, Belmain SR (2017) Domestic cats and dogs create a landscape of fear for pest rodents around rural homesteads. PLoS One 12:1–9. https://doi.org/10.1371/journal.pone.0171593
Massawe AW, Mulungu LS, Makundi HR, Eiseb SJ, Kirsten F, Mahlaba T, Malebane P, Maltitz E Von, Monadjem A, Taylor P, Tutjavi V, Belmain SR (2011) Spatial and temporal population dynamics of rodents in three geographically different regions in Africa : Implication for ecologically-based. African Zool 46:393–405
Mccormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES, Africa W (1987) A Prospective Study of the Epidemiology and Ecology of Lassa Fever carried out primarily in the eastern province of Sierra. J Infect Dis 155:437–444.
McCormick JB (1999) Lassa Fever, Emergence. Elsevier, Berlin Heidelberg
Mdangi M, Mulungu LS, Massawe AW, Eiseb SJ, Tutjavi V, Kirsten F, Mahlaba T, Malebane P, von Maltitz E, Monadjem A, Dlamini N, Makundi RH, Belmain SR (2013) Assessment of rodent damage to stored maize (Zea mays L.) on smallholder farms in Tanzania. Int J Pest Manag 59:55–62. https://doi.org/10.1080/09670874.2012.744495
Mills JN, Childs JE, Ksiazek TG, Peters CJ, Velleca WM (1995) Methods for trapping and sampling small mammals for virologic testing. Atlanta: Centers for disease control and prevention,
Mohr K, Leirs H, Katakweba A, Machang R (2007) Monitoring rodents movements with a biomarker around introduction and feeding foci in an urban environment in Tanzania. African Zool 42:294–298.
Monadjem A, Mahlaba T, Dlamnini N, Eiseb S, Belmain S, Mulungu L, Massawe AW, Makundi RH, Mohr K, Taylor PJ (2011) Impact of crop cycle on movement patterns of pest rodent species between fi elds and houses in Africa. Wildl Res 38:603–609.
Monadjem A, Perrin M (1998) The effect of supplementary food on the home range of the multimammate mouse Mastomys natalensis. South African J Wildl Res 28:1–3.
Monath TP (1987) Lassa Fever: New Issues Raised by Field Studies in West Africa. J Infect Dis 155:433–436. https://doi.org/10.1093/infdis/155.3.433
Mulungu LS, Borremans B, Ngowo V, Mdangi ME, Katakweba AS, Tesha P, Mrosso FP, Mchomvu M, Kilonzo BS (2015) Comparative study of movement patterns of Mastomys natalensis in irrigated rice and fallow fields in eastern Tanzania. Afr J Ecol 53:1–7. https://doi.org/10.1111/aje.12233
Olayemi A, Obadare A, Oyeyiola A, Fasogbon S, Igbokwe J, Igbahenah F, Ortsega D, Günther S, Verheyen E, Fichet-Calvet E (2017) Small mammal diversity and dynamics within Nigeria, with emphasis on reservoirs of the lassa virus. Syst Biodivers 2000:1–10. https://doi.org/10.1080/14772000.2017.1358220
R Core Team (2016) R: a language and environment for statistical 688 computing. R Foundation, Vienna
Rahelinirina S, Duplantier J, Ratsimba M, Ratovonjato J, Ramilijaona O, Papillon Y, Rahalison L (2009) Assessment of Rhodamine B for labelling the plague reservoir Rattus rattus in Madagascar. Afr J Ecol 48:662–666.
Singleton G, Belmain S, Brown P, Hardy B (2010) Rodent Outbreaks : Ecology and Impacts. Los Baños (Philippines): Inter- national Rice Research Institute.
Singleton GR, Hinds LA, Leirs H, Zhang Z (1999) Ecologically-based management of rodent pests-re-evaluating our approach to an old problem. Canberra, Australia: ACIAR.
Slade NA, Russell LA (1998) Distances as Indices to Movements and Home-Range Size From Trapping Records of Small Mammals. J Mammal 79:346–351. https://doi.org/10.2307/1382871
Sluydts V, Crespin L, Davis S, Lima M, Leirs H (2007) Survival and maturation rates of the African rodent, Mastomys natalensis: density-dependence and rainfall. Integr Zool 2:220–32. https://doi.org/10.1111/j.1749-4877.2007.00065.x
Stephenson E, Larson E, JW (1984) Effect of Environmental Factors on Aerosol-Induced Lassa Virus Infection. J Med Virol 14:295–303.
Swanepoel LH, Swanepoel CM, Brown PR, Eiseb SJ, Goodman SM, Keith M, Kirsten F, Leirs H, Mahlaba TAM, Makundi RH, Malebane P, Von Maltitz EF, Massawe AW, Monadjem A, Mulungu LS, Singleton GR, Taylor PJ, Soarimalala V, Belmain SR (2017) A systematic review of rodent pest research in Afro-Malagasy small-holder farming systems: Are we asking the right questions? PLoS One. https://doi.org/10.1371/journal.pone.0174554
Taylor PJ, Downs S, Monadjem A, Eiseb SJ, Mulungu LS, Massawe AW, Mahlaba TA, Kirsten F, Von Maltitz E, Malebane P, Makundi RH, Lamb J, Belmain SR (2012) Experimental treatment-control studies of ecologically based rodent management in Africa: Balancing conservation and pest management. Wildl Res 39:51–61. https://doi.org/10.1071/wr11111
Walker DH, Wulff H, Lange J V, Murphy FA (1975) Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull World Health Organ 52:523–34.
World Health Organization (2016) Lassa fever. [online]. In: Available www.who.int/csr/disease/lassafever/en/ [Accessed 13 Jun. 2016].
Acknowledgements
Joachim Mariën is a research fellow of the Flemish Interuniversity Council (VLIR-UOS). We are particularly grateful to the field assistants of the Projet des Fièvres Hémorragiques en Guinée, who participated to the rodent sampling: Amara Camara, Mamadou Condé, Moussa Fofana, and Morlaye Sylla. We thanks Dr Mory Cherif, who facilitated the field work by his constant medical support toward the communities. We thank Dr Mami Cécé Gowara, Directeur Régional de la Santé, Dr N’Faly Bangoura, Directeur Préfectoral and Sayon Oulare who facilitated our work in the Faranah prefecture. The research was funded by the German Research Foundation (DFG FI 1781/1&2-1 and LE SPP 1596) and by the University of Antwerp and the Antwerp study centre for disease (ASCID) Grant Number GOA BOF FFB3567.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mariën, J., Kourouma, F., Magassouba, N. et al. Movement Patterns of Small Rodents in Lassa Fever-Endemic Villages in Guinea. EcoHealth 15, 348–359 (2018). https://doi.org/10.1007/s10393-018-1331-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-018-1331-8