Abstract
In western Uganda, the landscape surrounding Kibale National Park (KNP) contains households, trading centers, roads, fields, and forest fragments. The mosaic arrangement of these landscape features is thought to enhance human–primate interaction, leading to primate population declines and increased bi-directional disease transmission. Using a social–ecological systems research framework that captures the complexity of interaction among people, wildlife, and environment, we studied five forest fragments near KNP and conducted intensive on-the-ground mapping to identify locations of human–primate spatial overlap. Primate locations and human activities were distributed within, on the edges, and far beyond fragment borders. Analysis of shared spaces indicated that 5.5% of human space overlapped with primate spaces, while 69.5% of primate spaces overlapped with human spaces. Nearest neighbor analysis indicated that human activities were significantly spatially clustered within and around individual fragments, as were primate locations. Getis–Ord statistics revealed statistically significant “hotspots” of human activity and primate activity, but only one location where spatial overlap between humans and primates was statistically significant. Human activities associated with collecting fuelwood and other forest products were the primary drivers of human–primate overlap; however, primates also spent time outside of forest fragments in agricultural spaces. These results demonstrate that fragmented landscapes are not uniform with respect to human–primate overlap, and that the implications of human–primate interaction, such as primate population declines and possible cross-species disease transmission, are spatially aggregated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social–ecological systems research aims to define research approaches that are robust, flexible, and dynamic enough to meet the challenges of coupled human and environmental interaction in times of rapid ecological change (Leslie et al. 2015). An evolution of classic systems theory, social–ecological systems theory is defined by its engagement with constructs such as complexity, resilience/vulnerability, emergence, and adaptability/transformability within an ecological challenge, such as declining fisheries or environmental degradation (Ostrom 2009). In this context, complexity refers to the phenomenon that multiple institutions and actors are unpredictably and co-constitutively entangled, across space and scale (Vogt et al. 2015); resilience and vulnerability refer to a system’s capacity to cope with undesirable change through principles and actions of local actors (Adger 2000; Leslie and McCabe 2013); emergence refers to the occurrence of events at higher levels of the system, which were set in motion by lower level components (Leslie et al. 2015); and adaptability/transformability refers to a system’s ability to preserve features within the system that need or should be preserved or to change where preservation is not desirable or possible (Walker et al. 2004).
Social–ecological systems research is characterized by field-based, microscale, interdisciplinary study design (Ostrom 2009; Poteete et al. 2010). This research project is framed by a social–ecological systems approach, focusing on three lower level components—conservation conflict, forest fragmentation, and subsistence livelihoods. These three components are interacting, complex, and tangible, with the potential to drive cross-species disease transmission through human–wildlife overlap.
Background
Wildlife conservation often conflicts with human subsistence livelihoods. These conflicts are especially pronounced in fragmented habitats (Murcia 1995; Wade et al. 2003; Fischer and Lindenmayer 2007; Ryan et al. 2015). Habitat fragmentation negatively impacts the wellbeing of wildlife (Bennett and Saunders 2010), particularly for species at higher trophic levels (Michalski and Peres 2005). Small sizes and isolation of habitat fragments constrain wildlife mobility and degrade community composition (Carlson and Hartman 2001; Michalski and Peres 2005; Panzacchi et al. 2009). Moreover, fragment edges are dynamic landscapes themselves. Indistinct boundary zones extend beyond the forest fragment itself (Didham 2010), and “edge effects” penetrate far into fragment interiors (Murcia 1995). For animals, life in fragmented habitats is associated with increased stress, immunocompromise, and exposure to infectious agents of people and domestic livestock (Salzer et al. 2007; Goldberg et al. 2008; Johnston et al. 2010; Chapman et al. 2013).
In the studies of human–wildlife interaction within a fragment matrix, one prevailing assumption is that a forest or habitat fragment is geographically homogeneous (Fahrig 2003; Didham 2010; Vaidyanathan et al. 2010). The ability to evaluate landscape change through the use of remotely sensed images over time has been a useful tool for measuring the spatial characteristics of habitat fragments (e.g., the FRAGSTATS computer program; (McGarical and Marks 1994). However, it is becoming increasingly clear that such approaches fail to account for the complexities of habitats within and outside of fragments (Vaidyanathan et al. 2010). Satellite images lack the refinement to identify, describe, or understand the role of the habitat fragment as a dynamic space in which human–wildlife interactions occur. Consequently, recent scholarship has been informed by data collected in the field to more fully investigate how, where, and ultimately why, people and wildlife interact (Chapman and Lambert 2000; Carter et al. 2012; Locatelli and Peeters 2012).
Fragmentation matters for disease transmission, as human-induced landscape change is a core driver for zoonoses, reverse zoonoses, and complex epidemiological processes of pathogen spread commonly referred to as “emergence” (Smolinski et al. 2003; Allan et al. 2003; Patz et al. 2004; Aguirre and Tabor 2008; McCord et al. 2014). Increased human accessibility to wildlife habitat appears to simultaneously drive human–wildlife contact and habitat fragmentation (Patz et al. 2008; Lambin et al. 2010; Murray and Daszak 2013; Gottdenker et al. 2014). Thus, landscape processes operate through human and animal behavior, modified by host factors (e.g., nutrition, stress, immunity), to precipitate changes in transmission dynamics.
Given these discrepancies between spatial approaches that consider habitat fragmentation to be a primary factor in cross-species disease transmission, and ecological approaches that emphasize human and animal behavior, we sought to understand the role of the ‘fragment’ in the context of infectious disease in a place highly relevant to the emergence of novel zoonotic diseases [e.g., filovirus outbreaks in bordering districts in 2008 (Mbonye et al. 2012)]. Specifically, we conducted an intensive, microscale spatial study of five forest fragments in Western Uganda, with the overall goal of characterizing human and animal fragment ecologies in the context of cross-species disease risk using an interdisciplinary research framework (Wood et al. 2012). We first sought to describe the spatial distribution of humans and non-human primates (hereafter “primates”) across a fragment matrix and to define specific areas of human–primate overlap. We then used this information to identify how, where, and why humans and primates exist in space, how that may impact cross-species disease exchange, and how that may be averted. The co-occurrence of forest fragmentation and emerging infectious diseases in this region provides an ideal scenario for which to examine these critical interactions.
Methods
Research was undertaken with approval from the University of Washington’s Human Subjects Division (#32300), the University of Illinois-Urbana Champaign’s Animal Use Protocol and Institutional Review Board, the Uganda Wildlife Authority, the Uganda National Council for Science and Technology (NS #221), and Local Leaders. Oral consent was obtained for all adults in the study at the time of enrollment and recorded using signed notation by field assistants approved to conduct human subjects research. Parents provided assent on behalf of children under 14 years old.
Study Sites
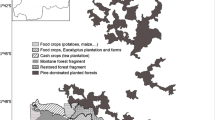
We targeted five forest fragments near the boundary of Kibale National Park (KNP), in western Uganda, that were also habitats for primates (Figure 1). Sites were defined as remnant forests (without the protected status that KNP holds) in use by both primates and humans (Table 1). In this region, forest fragments provide important human livelihood resources such as building materials, traditional medicine, water, and fuelwood (Naughton-Treves et al. 2007; Naughton-Treves et al. 2011; Goldberg et al. 2012; Ryan et al. 2015). However, for primates that reside within the KNP landscape matrix, fragmentation has deleterious effects including reduced health, enhanced cross-species microbial transmission, and local extirpations of primate populations (Goldberg et al. 2008).
Sample Selection
Primates included red colobus (Piliocolobus rufomitratus tephrosceles), black and white colobus (Colobus guereza), and red-tailed guenon (Cercopithecus ascanius). These species were selected due to their persistence in forest fragments, relevance to primate conservation, variable sensitivity to environmental change, and/or adaptability to modified habitats (Onderdonk and Chapman 2000; Chapman et al 2005; Goldberg et al. 2008; Goldberg, et al 2012). Vervet monkeys (Cercopithecus aethiops) were also present in these fragments but were not included due to their transient nature.
All households within 500 meters of each fragment were mapped and censused. Households were then randomly selected through spatial stratification by geographic quadrant for each fragment. For example, we assigned households into one of four locations according to their position relative to the forest fragment. The quadrants were northwest, northeast, southwest, and southeast. One household at a time was then selected from each quadrant by random draws of paper until we reached a total of 80 individuals. After consent was secured, all household members were enrolled in the study. This sampling scheme resulted in a random selection of spatially stratified households with a nested sample of participants.
Data Collection Through Mapping Human Activities and Primate Locations
From October 2008 through May 2009, local field assistants administered surveys to participants in the local language. The survey generated data on livelihood activities over the preceding four weeks for each household member. Livelihood activities included: tending livestock (goats, sheep, cows, pigs); working in fields; collecting firewood; collecting forest products; fetching water; guarding crops; making charcoal; brewing; maize daubing; and clearing the forest.
Survey data were then used to guide an activity space mapping exercise. Participants led field assistants on a guided tour indicating up to three locations where each livelihood activity previously mentioned had occurred. Field assistants recorded coordinates at each location and input codes that corresponded with each activity. Data were collected using a hand-held Global Positioning System unit (Magellan Explorist 500, Santa Clara, CA; accurate to 3 m).
Primate location data were collected using three methods. The first was a census of primates during which field assistants conducted exhaustive searches of each fragment and enumerated primate social groups and individuals, including their age, sex, and location. These census searches lasted at least 6 days. The field assistants began by searching independently for individuals or groups of primates and recording relevant demographic data. At the end of the day, the four field assistants compared data and resolved any inconsistencies. Subsequent interviews with local villagers (especially those self-identified as knowledgeable about local wildlife) were then used to determine whether primates could also be found outside of the fragment and where. These interviews informed a subsequent two days of searching for primates outside the forest fragment, which finalized the census.
The second method used to determine primate locations was collection of spatial data in support of on-going primate fecal sample collection (Salyer et al. 2012; McCord et al. 2014). Primates were followed and geographic coordinates were recorded when samples were collected. Finally, GPS waypoints were collected during primate behavioral observations, in which coordinates were recorded throughout the day, whenever primates were not moving. The resulting spatial data therefore do not represent primate home ranges, but rather the locations of primates during the day and when it was possible to observe them confidently.
Data for primates and humans were collected simultaneously. Two field assistants were assigned to follow primates, and three field assistants plus the project manager, collected data from people.
Statistical Analysis
Based on georeferenced data on households and forest boundaries, the geometry of each fragment study site was recreated and basic parameters (e.g., area, perimeter) were calculated (ArcGIS, Redlands, CA). Each human activity space was assigned a location descriptor to indicate its place in the landscape. The descriptors were ‘inside’, ‘outside’, or on the ‘edge’ of fragment. ‘Edge’ was defined as 5 m inside and 5 m outside of the boundary of the fragment, while ‘inside’ was the fragment itself less the 5 m included in ‘edge.’ The ‘outside’ area was calculated by creating a minimum convex polygon for each fragment containing all activity points for that fragment, excluding the ‘edge’ and ‘inside’ areas.
We conducted a G-test for goodness-of-fit to evaluate the statistical significance of human activities within, on the edge, or outside of the fragment using R 2.10 (Vienna, Austria) and the gstat package. The G-test, like a chi-square test, compares observed versus expected values, but specifies the distribution of expected values to be based on a factor that might affect raw counts (McDonald 2014). In this case, the factor considered was relative area of the different locations for each fragment (‘inside’, ‘edge’, and ‘outside’). The relative areas of inside/outside/edge were converted into percentages of the total area, which were then used to inform the expected distribution. We sought to examine whether certain activities occurred disproportionately inside, outside, or on the edge of the fragment.
Expected values for activities that occurred within fragment ‘edges’ were often quite small relative to ‘outside’ or ‘inside’ the fragment because the area of a fragment’s edge was proportionally much smaller than that inside or outside the fragment. As a consequence, all expected values for the edge area were less than five, which approaches the minimum recommended sample size for G-tests (Sokal and Rolf 2011; McDonald 2014). Therefore, we invalidated results if the total expected frequencies (inside and outside as well as edge) for a given activity in a given fragment were less than five.
Spatial Analysis (Visualization and Pattern Detection)
To investigate spatial clustering of humans and primates across each fragment, we conducted spatial autocorrelation and hotspot detection analyses. Using the Nearest Neighbor statistic, we calculated the distribution of clustering and dispersal (i.e., spatial autocorrelation) for human activity spaces and again for primate locations. Hotspots of human or primate activities were calculated through the Getis–Ord statistic. Both of the above calculations were run through the toolbox features in ArcGIS 10.1 (ESRI, Redlands, CA). Where human hotspots and primate hotspots overlapped, the human activity that occurred within the cluster was identified as a possible driver for overlap.
Results
Study Sites and Populations
Of the five study sites (Figure 1), four were situated along the northwest border of KNP (Bugembe, Rurama, Kyamazima, and Kitojo); the fifth (Byara) was on the northeastern side. The largest fragment was Bugembe (0.216 km2) and the smallest was Kyamazima (0.015 km2). The fragments were all in low-lying areas, on steep slopes, and/or in valley bottoms with persistent water (Table 1). These areas are non-arable, explaining the persistence of forest.
A total of 635 waypoints were collected to identify locations for approximately 124 primates across the five fragments. The average number of primates per fragment was 25 ± 3.25. Kitojo supported the largest number of primates with 32 red-tailed guenons. The fewest primates were found in Kyamazima (six red-tailed guenons, five black and white colobus, and three red colobus) (Table 1).
Across all five study sites, a total of 105 households were enumerated; of those, 61 households were enrolled. The largest number of participants lived in Rurama (26.5%, n = 97), while the smallest number lived in Kitojo (13.6%, n = 50). The average number of participants per fragment was 73 (Table 1). Across all sites, the majority of participants identified as members of the Mutooro cultural group (73.2%, n = 267) and as students (34.1% n = 124) or subsistence farmers (31.5%, n = 115). The median age was 15 years and the age range was 0.25–90 years. Participants were 50% (n = 184) female and 50% (n = 181) male. Data on a total of 2351 human activity spaces were collected for 367 participants.
Distribution of Human Activities Across the Fragments
The most frequent human activities (Table 2) were tending livestock (38.3%), working in fields (28.4%), collecting firewood (15.3%), and collecting water (13.8%). Results of the G-test showed that tending livestock, working in fields, collecting firewood, and fetching water were significantly spatially clustered either ‘outside’, ‘inside’, or along the ‘edge’ of the fragment (Table 3).
Visualizing the Spatial Configuration of Human Activities Across the Landscape
By mapping human activity spaces, we were able to infer spatial patterns of activity for people in the fragmented landscape. Our results indicate that activities occur in spatial configurations that differed among study sites. For example, human activity spaces were recorded inside, along the edge, and outside four fragments (Byara, Kitojo, Kyamazima, and Rurama). However, in Bugembe, no human activity spaces were recorded inside the fragment (Figures 2a–e).
Spatial Clusters of Human Activity Spaces and of Primate Locations
Nearest Neighbor Index showed that both human activity spaces and primate locations were significantly clustered for all fragments. Table 4 shows Bugembe displayed the highest amount of human activity space clustering. Kitojo displayed the highest amount of primate clustering and Kyamazima the least. All clustering was statistically significant (Table 4).
Figures 3a–e show hotspot maps for human activity spaces and primate locations, calculated using Getis–Ord statistic. Human activity space hotspots were located primarily outside of fragments. Byara was the only community with significant clustering of human livelihood activities inside of the fragment. In Kitojo, although clusters of human activity were observed, none were statistically significant.
Primate hotspots were found exclusively inside or on the edge of the fragment in Bugembe and Byara. In Kyamazima, eight primate location hotspots were identified, and half of those were located outside of the fragment. Primate hotspots were found exclusively outside of the forest fragments of Rurama and Kitojo, but with a z-score close to zero, implying lack of statistical power.
The only location where primate and human hotspot clusters definitively overlapped was on the edge of the Byara fragment. The human activity that most strongly contributed to this finding was firewood collection. The rarity of overlapping clusters may indicate avoidance of areas of high human use by primates.
Conversion of Points into Polygons to Estimate Spatial Overlap
Spatial locations of primates and humans for each fragment were converted into minimum convex polygons. Overlap of the human and primate polygons for each fragment were then quantified, and primate polygon area was expressed as a percentage of the total area of human usage and vice versa (Table 4). Kitojo had the highest proportion of human space overlapping with primates at 16.66%. Bugembe had the lowest proportion of human spaces overlapping with primates at 1.19%.
For all fragments, the proportion of primate spaces that overlapped with humans was much higher than the proportion of human spaces that overlapped with primate space. In fact, for Byara and Kyamazima, primate spaces overlapped 100% with human spaces.
Discussion
We conducted a fine-grained analysis of human–primate overlap in and around five forest fragments in Western Uganda. Our maps of human activity spaces revealed that livelihood activities are distributed across the landscape matrix. Our G-test and Nearest Neighbor analyses showed that human activity was spatially aggregated, and that subsistence livelihood activities, such as firewood collection and gardening, were the major determinants of human presence. Hotspot analysis using Getis–Ord statistic showed that both human activity spaces and primate locations were spatially clustered regardless of the absolute frequency with which people and primates were recorded. Thus, when certain locations were revisited frequently by multiple individuals, those locations were close together. In other parts of the landscape, few individuals and infrequent visits to those locations occurred, making them “not spots,” or areas of disproportionately low activity and overlap. Clearly, people and primates use their habitats deliberately, and this sometimes leads to use of shared space and sometimes to avoidance, depending on the particular characteristics of the landscape. This observation reinforces the notion that considering habitat fragments to be uniform spaces is an oversimplification (Anderson et al. 2007; Didham 2010).
Our analysis also revealed locations where people and primates overlapped in space. In most locations, hotspots for humans and primates were far apart. However, on the southern edge of Byara (Figure 3b), primate and human hotspots overlapped significantly. Collecting forest products, such as firewood and herbal medicines, is the driver for intensive and frequent use of this space by people. Thus, specific human activities can be considered hotspot drivers of overlap.
Through a social–ecological systems research framework, this study responds directly to calls for increased attention to the structures and interactions that underpin human–animal–ecological change in the context of zoonotic diseases. We have described scenarios where the risk of cross-species disease transmission is predicated upon the complexity of seemingly mundane, often invisible, social–ecological interfaces. Such approaches improve capacity to devise effective policy and practice recommendations that are informed by the realities of the lives that are targeted for intervention (King 2011; Leach and Scoones 2013; Brown and Kelly 2014). The multiple spatial methods used herein reveal spaces of potential risk for both human health and conservation.
Past research in this area has shown that fragmentation increases microbial transmission risk, as assessed using molecular epidemiological methods (Salzer et al. 2007; Johnston et al. 2010; Salyer et al. 2012; Ghai et al. 2014). Subsequent research has found that risk of human and primate contact is highest for people living in the fragment matrix compared to those who live alongside Kibale National Park itself, despite the fact that KNP is unsurpassed for its primate density and diversity (Paige et al. 2014).
Our study contributes to this growing literature by drawing upon social–ecological systems theory and concepts, which enables the consideration of phenomena on a finer scale. Our results show a remarkable degree of non-uniformity with respect to the distribution of people and primates within and among forest fragments. They also demonstrate that primates frequent areas beyond the fragment, as has been shown in other systems (Anderson et al. 2007). Given that forest fragments are increasingly common habitats for primates and other wildlife worldwide (Marsh 2003), understanding such fine-scale interactions will become increasingly important for both conservation and public health.
Conclusion
This study examines human–primate overlap in a mosaic landscape of forest fragments. People and primates overlap extensively in their use of space, but this varies considerably among forest fragments and as a result of different human behaviors. To the extent that habitat overlap informs disease transmission risk, interventions that target risky human–wildlife context may not be generalizable among locations. This conclusion emphasizes that decisions about outbreak mitigation must be place-specific and informed by local data (Holmes 2008; Lloyd-Smith et al. 2009; Rabinowitz et al. 2013; Rock and Degeling 2015). Grounded, micro-scale investigations, as represented by social–ecological systems research approaches, should continue to inform studies of social and ecological context to describe, and possibly, predict the context of human–primate spatial overlap, interaction, and thus the possibility of cross-species disease transmission.
References
Adger WN (2000) Social and ecological resilience: are they related? Prog Hum Geogr 24:347–364. doi: 10.1191/030913200701540465
Aguirre AA, Tabor GM (2008) Global factors driving emerging infectious diseases. Ann N Y Acad Sci 1149:1–3. doi: 10.1196/annals.1428.052
Allan BF, Keesing F, Ostfeld RS (2003) Effect of Forest Fragmentation on Lyme Disease Risk. Conserv Biol 17:267–272. doi:10.1046/j.1523-1739.2003.01260.x
Anderson J, Rowcliffe JM, Cowlishaw G (2007) Does the matrix matter? A forest primate in a complex agricultural landscape. Biol Conserv 135:212–222. doi:10.1016/j.biocon.2006.10.022
Bennett AF, Saunders DA (2010) Habitat fragmentation and landscape change. In: Sodhi N, Ehrlich P (eds) Conservation Biology for All. Oxford University Press, Oxford, pp 88–106
Brown H, Kelly AH (2014) Material proximities and hotspots: toward an anthropology of viral hemorrhagic fevers. Med Anthropol Q 28:280–303. doi:10.1111/maq.12092
Carlson A, Hartman G (2001) Tropical forest fragmentation and nest predation – an experimental study in an Eastern Arc montane forest, Tanzania. Biodivers Conserv 10:1077–1085.
Carter NH, Shrestha BK, Karki JB, Man N, Pradhan B, Liu J (2012) Coexistence between wildlife and humans at fine spatial scales. Proc Natl Acad Sci 109:15360–15365. doi:10.1073/pnas.1210490109/-/DCSupplemental.www.pnas.org/c
Chapman CA, Ghai R, Jacob A, Koojo SM, Reyna-hurtado R, Rothman JM, Twinomugisha D, Wasserman MD, Goldberg TL (2013) Going, Going, Gone: A 15-Year History of the Decline of Primates in Forest Fragments near Kibale National Park, Uganda. In: Marsh LK, Chapman CA (eds) Primates in fragments: complexity and resilience. Springer New York, New York, NY, pp 89–100
Chapman CA, Lambert JE (2000) Habitat alteration and the conservation of African primates: case study of Kibale National Park, Uganda. Am J Primatol 50:169–85. doi:10.1002/(SICI)1098-2345(200003)50:3<169::AID-AJP1>3.0.CO;2-P
Chapman CA, Struhsaker TT, Lambert, JE (2005) Thirty years of research in Kibale National Park, Uganda, reveals a complex picture for conservation. International Journal of Primatology 26(3): 539-555.
Didham RK (2010) Ecological Consequences of Habitat Fragmentation. doi:10.1002/9780470015902.a0021904
Fahrig L (2003) Effects of Habitat Fragmentation on Biodiversity. Annu Rev Ecol Evol Syst 34:487–515.
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 16:265–280.
Ghai RR, Simons ND, Chapman CA, Omeja PA, Davies TJ, Ting N, Goldberg T. (2014) Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Neglected Tropical Disease. doi:10.1371/journal.pntd.0003256
Goldberg TL, Gillespie TR, Rwego IB, Estoff EL, Chapman CA (2008) Forest Fragmentation as Cause of Bacterial Transmission among Nonhuman Primates, Humans, and Livestock, Uganda. Emerg Infect Dis 14:1375–1382. doi:10.3201/eid14.9.071196
Goldberg TL, Paige SB, Chapman CA (2012) The Kibale EcoHealth Project: Exploring connections among human health, animal health, and landscape dynamics in western Uganda. In: Aguirre A, Ostfeld RS, Daszak P (eds) New Directions in Conservation Medicine: Applied Cases of Ecological Health. Oxford Universtiy Press, New York, pp 452–465
Gottdenker NL, Streicker DG, Faust CL, Carroll CR (2014) Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. Ecohealth. doi:10.1007/s10393-014-0941-z
Holmes BJ (2008) Communicating about emerging infectious disease: The importance of research. Heal Risk Soc 10:349–360. doi:10.1080/13698570802166431
Johnston AR, Gillespie TR, Rwego IB, McLachlan TLT, Kent AD, Goldberg TL (2010) Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Negl Trop Dis 4:e683. doi:10.1371/journal.pntd.0000683
King B (2011) Spatialising livelihoods: Resource access and livelihood spaces in South Africa. Trans Inst Br Geogr 36:297–313. doi:10.1111/j.1475-5661.2010.00423.x
Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V (2010) Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr 9:54. doi:10.1186/1476-072X-9-54
Leach M, Scoones I (2013) The social and political lives of zoonotic disease models: Narratives, science and policy. Soc Sci Med 88:10–17. doi:10.1016/j.socscimed.2013.03.017
Leslie HM, Basurto X, Nenadovic M, Sievanen L, Cavanaugh KC, Cota-Nieto JJ, Erisman BE, Finkbeiner E, Hinojosa-Arango G, Moreno-Baez M, Nagavarapu S, Reddy SMW, Sanchez-Rodriguez A, Siegel K, Ulibarria-Valenzuela JJ, Weaver AH, Aburto-Oropeza O (2015) Operationalizing the social-ecological systems framework to assess sustainability. Proc Natl Acad Sci 112:5979–5984. doi:10.1073/pnas.1414640112
Leslie P, McCabe JT (2013) Response Diversity and Resilience in Social-Ecological Systems. Curr Anthropol 54:114–143. doi:10.1086/669563
Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT (2009) Epidemic dynamics at the human-animal interface. Science 326:1362–7. doi:10.1126/science.1177345
Locatelli S, Peeters M (2012) Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS 26:659–73. doi:10.1097/QAD.0b013e328350fb68
Marsh LK (2003) Primates in Fragments: Ecology and Conservation. Kluwer Academic/Plenum Publishers, New York
Mbonye A, Wamala J, Tugumizemo V, Aceng J, Makumbi I (2012) Repeated outbreaks of Viral hemorrhagic fevers in Uganda. Afr Health Sci 12:579–583. doi:10.4314/ahs.v12i4.31
McCord A, Chapman CA, Weny G, Tumukunde A, Hyeroba D, Klotz K, Koblings AS, Mbora DNM, Cregger M, White BA, Leigh SR, Goldberg TL (2014) Fecal microbiomes of non-human primates in Western Uganda reveal species-specific communities largely resistant to habitat perturbation. Am J Primatol 76:347–54. doi:10.1002/ajp.22238
McDonald J. (2014) Handbook of Biological Statistics, 3rd edn. Sparky House Publishing, Baltimore, MD
McGarical K, Marks BJ (1994) FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure. Gen Tech Rep PNW-GTR-351 US … 97331:134.
Michalski F, Peres CA (2005) Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol Conserv 124:383–396. doi:10.1016/j.biocon.2005.01.045
Murcia C (1995) Edge effects in fragmented forests: Implications for conservation. Trends Ecol Evol 10:58–62.
Murray KA, Daszak P (2013) Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Curr Opin Virol 3:79–83. doi:10.1016/j.coviro.2013.01.006
Naughton-Treves L, Alix-Garcia J, Chapman CA (2011) Lessons about parks and poverty from a decade of forest loss and economic growth around Kibale National Park, Uganda. Proc Natl Acad Sci U S A 108:13919–13924. doi:10.1073/pnas.1013332108
Naughton-Treves L, Kammen DM, Chapman CA (2007) Burning biodiversity: Woody biomass use by commercial and subsistence groups in western Uganda’s forests. Biol Conserv 134:232–241.
Onderdonk D A,Chapman CA (2000) Coping with forest fragmentation: The primates of Kibale National Park, Uganda. International Journal of Primatology 21: 587-611.
Ostrom E (2009) A general framework for analyzing sustainability of social-ecological systems. Science (80)324: 419–422. doi:10.1126/science.1172133
Paige SB, Frost SDW, Gibson MA, Jones JH, Shankar A, Switzer WM, Ting N, Goldberg TL (2014) Beyond Bushmeat: Animal Contact, Injury, and Zoonotic Disease Risk in Western Uganda. Ecohealth. doi:10.1007/s10393-014-0942-y
Panzacchi M, Linnell JDC, Odden M, Odden J, Andersen R (2009) Habitat and roe deer fawn vulnerability to red fox predation. J Anim Ecol 78:1124–1133. doi:10.1111/j.1365-2656.2009.01584.x
Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ (2004) Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environ Health Perspect 112:1092–8.
Patz JA, Olson SH, Uejio CK, Gibbs HK (2008) Disease emergence from global climate and land use change. Medical Clinics of North America 92:1473–91, xii. doi:10.1016/j.mcna.2008.07.007
Poteete AR, Janssen MA, Ostrom E (2010) Working Together: Collective Action, the Commons, and Multiple Methods in Practice. Princeton University Press, Princeton, NJ
Rabinowitz PM, Kock R, Kachani M, Kunkel R, Thomas J, Gilbert J, Wallace R, Blackmore C, Wong D, Karesh W, Natterson B, Dugas R, Rubin C (2013) Toward proof of concept of a one health approach to disease prediction and control. Emerg Infect Dis. doi:10.3201/eid1912.130265
Rock MJ, Degeling C (2015) Public health ethics and more-than-human solidarity. Soc Sci Med 129:61–7. doi:10.1016/j.socscimed.2014.05.050
Ryan SJ, Southworth J, Hartter J, Dowhaniuk N, Fuda RK, Diem JE (2015) Household level influences on fragmentation in an African park landscape. Appl Geogr 58:18–31. doi:10.1016/j.apgeog.2015.01.005
Salyer SJ, Gillespie TR, Rwego IB, Chapman CA, Goldberg TL (2012) Epidemiology and molecular relationships of Cryptosporidium spp. in people, primates, and livestock from Western Uganda. PLoS Negl Trop Dis 6:e1597. doi:10.1371/journal.pntd.0001597
Salzer JS, Rwego IB, Goldberg TL, Kuhlenschmidt MS, Gillespie TR (2007) Giardia sp. and Cryptosporidium sp. infections in primates in fragmented and undisturbed forest in western Uganda. J Parasitol 93:439–440. doi:10.1645/GE-970R1.1
Smolinski M, Hamburg M, Lederberg J (2003) Microbial Threats to Health: Emergence, Detection, and Response. The National Academies Press, Washington, DC
Sokal R, Rolf F (2011) Biometry: The Principles and Practices of Statistics in Biological Research, 4th edn. W. H. Freeman, New York, NY
Vaidyanathan S, Krishnaswamy J, Samba Kumar N, Dhanwatey H, Dhanwatey P, Ullas Karanth K (2010) Patterns of tropical forest dynamics and human impacts: Views from above and below the canopy. Biol Conserv 143:2881–2890. doi: 10.1016/j.biocon.2010.04.027
Vogt JM, Epstein GB, Mincey SK, Fischer BC, McCord P (2015) Putting the “E” in SES: Unpacking the ecology in the Ostrom social-ecological system framework. Ecology and Society. doi:10.5751/ES-07239-200155
Wade TG, Riitters KH, Wickham JD, Jones KB (2003) Distribution and causes of global forest fragmentation. Conservation Ecology 7(2): 7. http://www.consecol.org/vol7/iss2/art7
Walker B, Holling CS, Carpenter SR, Kinzing A (2004) Resilience, Adaptability and Transformability in Social-Ecological Systems. Ecology and Society 9(2): 5. http://www.ecologyandsociety.org/vol9/iss2/art5
Wood JLN, Leach M, Waldman L, Macgregor H, Fooks AR, Jones KE, Restif O, Dechmann D, Hayman DTS, Baker KS, Peel AJ, Kamins AO, Fahr J, Ntiamoa-Baidu Y, Suu-Ire R, Breiman RF, Epstein JH, Field HE, Cunningham AA (2012) A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos Trans R Soc Lond B Biol Sci 367:2881–92. doi:10.1098/rstb.2012.0228
Acknowledgements
We are grateful to Geoffrey Weny, Annet Nyamuwija, Patrick Katuramu, John Rusoke, Joseph Baruhanga, Edith Mbabazi, and Alice Mbabazi for their assistance in the field. We are grateful for institutional capacity and support of Makerere University Biological Field Station, the Uganda Wildlife Authority, and the District Health Office of Kabarole. Finally, we thank the Uganda National Council of Science and Technology for granting permission to conduct this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paige, S.B., Bleecker, J., Mayer, J. et al. Spatial Overlap Between People and Non-human Primates in a Fragmented Landscape. EcoHealth 14, 88–99 (2017). https://doi.org/10.1007/s10393-016-1194-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-016-1194-9