Abstract
Sylvatic plague, caused by the bacterium Yersinia pestis, is periodically responsible for large die-offs in rodent populations that can spillover and cause human mortalities. In the western US, prairie dog populations experience nearly 100% mortality during plague outbreaks, suggesting that multiple transmission pathways combine to amplify plague dynamics. Several alternate pathways in addition to flea vectors have been proposed, such as transmission via direct contact with bodily fluids or inhalation of infectious droplets, consumption of carcasses, and environmental sources of plague bacteria, such as contaminated soil. However, evidence supporting the ability of these proposed alternate pathways to trigger large-scale epizootics remains elusive. Here we present a short review of potential plague transmission pathways and use an ordinary differential equation model to assess the contribution of each pathway to resulting plague dynamics in black-tailed prairie dogs (Cynomys ludovicianus) and their fleas (Oropsylla hirsuta). Using our model, we found little evidence to suggest that soil contamination was capable of producing plague epizootics in prairie dogs. However, in the absence of flea transmission, direct transmission, i.e., contact with bodily fluids or inhalation of infectious droplets, could produce enzootic dynamics, and transmission via contact with or consumption of carcasses could produce epizootics. This suggests that these pathways warrant further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and Purpose
Recently, the World Health Organization (WHO) categorized plague as a re-emerging disease after human outbreaks occurred in countries that had been plague-free for greater than 30 years (Stenseth et al. 2008; Vogler et al. 2011; WHO http://www.who.int/csr/disease/en/, accessed 07/15/2015). Plague typically circulates in rodents and their fleas (sylvatic plague), with spillover to human populations associated with outbreaks in animals (epizootics). Indeed, mathematical models of plague dynamics in Kazakhstan have shown that when partially resistant gerbils and their fleas exceed population thresholds, plague epizootics resulting in human cases are more likely to occur (Davis et al. 2004, 2007, Davis et al. 2008; Samia et al. 2011; Reijniers et al. 2012). The Kazakhstan plague dynamic, however, may not be universal. Yersinia pestis has endemic foci on all continents but in Antarctica and Australia, it infects greater than 200 species of rodents and fleas (Serzhan and Ageyev 2000). Currently, considerable uncertainty exists in the drivers of plague epizootics in other endemic foci.
In the western US, many transmission pathways for sylvatic plague have been proposed (Fig. 1), yet the individual contributions of non-flea-vectored pathways to plague dynamics remain unclear. Y. pestis was introduced to the US in the early 1900s in San Francisco and is now considered endemic from Texas to southern Canada and west of the 100th meridian (Fig. 2; Matchett et al. 2010; Abbott and Rocke 2012; Antonation et al. 2014). In this endemic foci plague epizootics are primarily associated with ground squirrels and prairie dogs (family: Sciuridae) and their fleas (mainly Oropsylla spp.). Prairie dogs often suffer high mortality and colony extirpations (Cully and Williams 2001). Because diseases rarely cause the extirpation of their hosts without the presence of reservoirs (de Castro and Bolker 2005), many have suggested that a short-term reservoir or alternate host is needed to amplify plague dynamics (Gage and Kosoy 2005; Salkeld et al. 2010). With a focus on examining dynamics for plague in grassland ecosystems in the western US, our goals are to (1) review the current knowledge of plague transmission pathways (Fig. 1), (2) evaluate the contribution of each transmission pathway in black-tailed prairie dog populations (Cynomys ludovicianus) using a mathematical model, and (3) discuss parameter uncertainties and model sensitivities that warrant further investigation.
Conceptual diagram showing the hypothesized (dashed lines) and confirmed (solid lines) plague transmission pathways in the western US. Potential transmission pathways include carnivores (represented by the swift fox), small rodents (represented by grasshopper mice), prairie dogs, fleas, carcasses, and soil (represented by the image of a prairie dog burrow).
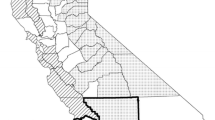
Plague occurrence in the western US, showing counties with positive animal or flea samples (green counties) and reported human cases (red dots) from 1970 to 2009. Reproduced with permission from Abbott and Rocke (2012), map based on information from Ken Gage, CDC.
Plague Transmission Pathways
Vector Transmission
For many years, the predominant paradigm in plague transmission was focused on flea-vectored transmission where “good” plague vectors develop midgut blockages increasing both their transmission efficiency and biting rate (Gage and Kosoy 2005). However, recent evidence suggests that many fleas efficiently transmit plague before developing blockages, 1–5 days after ingestion of an infectious blood meal (“early phase transmission”; Eisen et al. 2006). The main fleas (Oropsylla spp.) thought to be important in plague transmission in the US block infrequently and are capable of transmitting the bacteria prior to blocking (Eisen et al. 2009). Furthermore, the blocking process, which can take days to weeks, appears to be too slow and transient to drive western US plague dynamics (Webb et al. 2006). Thus, early phase transmission provides an alternate and more rapid transmission pathway (Eisen et al. 2009). Considerable uncertainty remains regarding vectorial capacity and transmission efficiencies for fleas on native hosts (studies to date have used standardized laboratory models; Eisen et al. 2008b; Eisen et al. 2009). However, even with low transmission rates for individual fleas, large flea population sizes that feed frequently on individual rodents magnify these rates, making efficient en masse transmission plausible (Burroughs 1947; Tripp et al. 2009).
Indeed, many investigators have noted an increase in flea abundance on prairie dogs, small rodents, and in burrows during plague epizootics (Salkeld and Stapp 2008; Stapp et al. 2009; Tripp et al. 2009; Friggens et al. 2010). Two mechanisms may drive the increase in average flea loads during epizootics: environmental conditions and host density favor exponential expansion of flea populations (Parmenter et al. 1999; Savage et al. 2011), or as host mortality increases, flea populations aggregate on fewer hosts (Tripp et al. 2009). Additional flea life history studies are needed to generate robust estimates of Oropsylla spp. population parameters in relation to host and climatic conditions to determine whether increasing flea loads are a trigger or sign of sylvatic plague epizootics in the western US. Either way, infected fleas most likely provide a short-term reservoir that contributes to rapidly spreading plague epizootics (Eisen and Gage 2009; Buhnerkempe et al. 2011).
Direct Transmission
Besides vector transmission, plague can also spread through direct transmission via three potential mechanisms, the inhalation of infectious droplets (usually blood/mucus) from sick hosts that have developed pneumonic plague (infection in the lungs), direct contact with the bodily fluids of a sick or dead host, or consumption of plague-killed carcasses. Preliminary evidence suggests that direct transmission may be currently underappreciated in sylvatic plague cycles. For example, pneumonia is a common histopathologic finding in prairie dogs that have died of plague in laboratory studies (Rocke, unpublished data; Bush and van Andel personal communication). Histopathologic evidence of pneumonia was consistent across inoculation doses in Gunnison’s prairie dogs that succumbed to plague (see methods in Busch et al. 2013, unpublished histopathological results in Table S1). Bloody noses or other pulmonary symptoms are common in plague-positive prairie dogs both in the field and under laboratory conditions (Williams et al. 1979; Quan et al. 1985; Rocke et al. unpublished), and isolation of Y. pestis from lungs of affected animals is common (Busch et al. 2013; Rocke et al. unpublished). Because prairie dogs are considered highly social and often perform “kiss” greetings (Hoogland 1995), direct contact transmission in prairie dogs may be underappreciated and currently understudied.
Similarly understudied, but potentially important, is direct contact with or consumption of plague-killed carcasses leading to increased infections in prairie dogs. Prairie dog carcasses are rarely encountered (Hoogland 1995), because carcasses are either below ground or actively scavenged by prairie dogs, other small rodents, and carnivores (Boone et al. 2009). Scavenging provides an opportunity for infected fleas to transfer to a live host and for direct transmission of the bacteria through the ingestion of infected tissues (Thomas et al. 1989; Arbaji et al. 2005) potentially leading to spillover of plague to other species. If Y. pestis survives and is infectious for any length of time within carcasses (early work suggests viable Y. pestis can be cultured from carcasses for at least 11 days; Ber et al. 2003), this direct transmission route may provide a potential short-term reservoir for Y. pestis during and between epizootics (Easterday et al. 2012). Prairie dogs are sometimes cannibalistic, highly social, and nest communally (Hoogland 1995) suggesting direct transmission pathways are certainly plausible.
Alternate Hosts
Very little evidence exists to support the notion that a separate enzootic cycle of plague occurs in coexisting but more resistant rodent or carnivore hosts in the western US foci (Salkeld and Stapp 2008; Stapp et al. 2008; Thiagarajan et al. 2008). Carnivores and small rodents, including deer mice (Peromyscus spp.) and northern grasshopper mice (Onychomys leucogaster), are rarely found infested with prairie dog fleas during inter-epizootic periods (Holmes et al. 2006; Salkeld and Stapp 2006; Salkeld and Stapp 2008; Stapp et al. 2008; Thiagarajan et al. 2008; Brinkerhoff et al. 2009; Stapp and Salkeld 2009). In addition, certain small rodent species (Dipomys spp.) and most carnivores are highly resistant to plague (Holdenried and Quan 1956; Salkeld and Stapp 2006), thus they most likely do not develop high enough bacteremia to transmit Y. pestis (Engelthaler et al. 2000). However, increased resistance to plague has been reported for grasshopper mice in enzootic plague areas compared to naive populations (Thomas et al. 1988). Findings from other endemic plague foci also suggest that individual variability in resistance within generally resistant rodent populations could contribute to maintenance and spread of plague epizootics (Zhang et al. 2012; Gascuel et al. 2013); this possibility needs additional study for co-occurring rodent species on prairie dog colonies. Still, though few short-lived rodent sampling studies during inter-epizootic periods are available (but see Holmes et al. 2006; Stapp and Salkeld 2009; Thiagarajan et al. 2008), more compelling evidence would have been expected if these rodents were the primary maintenance hosts of Y. pestis.
There is evidence, however, that small rodents may play a role in moving Y. pestis across prairie dog colonies during epizootics (Stapp et al. 2008; Stapp et al. 2009; Stapp and Salkeld 2009; Thiagarajan et al. 2008). For example, northern grasshopper mice have been observed to increase in abundance before and during epizootics in some locations. These mice can carry prairie dog fleas, have larger home ranges than prairie dogs, and enter multiple burrow systems per night, giving them the potential to facilitate movement of Y. pestis on a large spatial scale during plague outbreaks (Brinkerhoff et al. 2008; Salkeld et al. 2010; Kraft and Stapp 2013). However, northern grasshopper mice do not occur on all prairie dog colonies, and little is known about the movement of other small mammals in relation to plague dynamics. The evidence from northern grasshopper mice lends credence to the hypothesis that increased flea questing (due to host die-off) and movement of infected fleas by alternate hosts during outbreaks could potentially exacerbate ongoing plague epizootics.
Environmental Reservoirs
Another potential transmission pathway of plague is Y. pestis maintained in soil or other substrates. Y. pestis is closely related to Y. pseudotuberculosis, an enteropathogenic bacterium that can be maintained in contaminated soil (Skurnik et al. 2000; Bearden and Brubaker 2010) and has retained some genetic material that is not necessary for persistence in flea or mammal hosts, suggesting that carcass, invertebrate or soil existence may be possible (Easterday et al. 2012). Viable and infectious Y. pestis have been extracted from soil both in the laboratory and the field at least 3 weeks after contamination with septicemic blood (Ayyadurai et al. 2008; Eisen et al. 2008a). However, only ~1% of mice contracted plague through scarified skin exposure to highly contaminated soil in the laboratory (Boegler et al. 2012). Additional work is needed to clarify whether insects, other parasites, prairie dogs or their immature fleas, which consume debris as larvae, could become infected from contaminated soil, thus maintaining and spreading plague.
Simulating Plague Dynamics
Simulation Methods
To evaluate the contribution of the proposed transmission pathways, we modified the sylvatic plague ordinary differential equation (ODE) model described in Buhnerkempe et al. (2011; Tables 1, 2, Figure S1). This Susceptible-Exposed-Infectious-Resistant (SEIR) model includes two types of early phase flea transmission (EP1 and EP2) as well as transmission from infectious carcasses (M). Infected fleas are initially highly infectious (EP1) but transition to a less infectious state (EP2) unless they have additional infectious feeds (“booster feed cycle”; Buhnerkempe et al. 2011; Eisen et al. 2007). We added a compartment (D) and transmission parameters (β d , λ d ) for contaminated soil, as well as parameters for transmission from infected individuals (β i , ψ). To incorporate increased connectance of prairie dog towns via small rodents, we adjusted the spatial correction factor (i.e., the number of burrows used per day by prairie dogs, B) by the number of active prairie dog burrows used per day by grasshopper mice (B r , Kraft and Stapp 2013). Because adult fleas typically have reduced survival when starved (Rust and Dryden 1997; Krasnov et al. 2002; Wilder et al. 2008), we added a separate parameter for questing flea mortality (µ fq ). Lastly, we removed continuous host population growth because prairie dogs breed only once a year, and we were interested in the dynamics of a single epizootic within one above-ground season (April to October, 210 days). Because the parameter values and populations are constant, model dynamics will display an exponential decay over time, i.e., the classical epidemiological pattern with no opportunity for the disease to re-emerge. Parameter values were the same as those reported in Buhnerkempe et al. (2011) for black-tailed prairie dogs and O. hirsuta or extracted from the reviewed literature (Table 2). The resulting model is described with the following equations (Tables 1, 2; Fig. S1):
Hosts
Fleas
where flea infections from hosts were modeled as frequency dependent, while all other transmission pathways were modeled as density dependent, including infections from infected questing fleas to new hosts (a detailed and thorough description of the underlying model is included in Webb et al. (2006)).
We simulated 7 variations of the above model: the full model (all transmission pathways), rodent connectance, flea transmission, direct transmission (includes both direct contact and pneumonic transmission from live hosts), carcass transmission (includes both direct contact with and consumption of dead hosts), soil transmission (i.e., contact with contaminated soil), and no transmission (Table 3). We isolated transmission parameters for the above variations by setting the non-target transmission parameters to zero (except for increased connectance due to alternate rodent hosts, where we set the corresponding parameter, B r , to zero and compared to the full model). Stochasticity was incorporated into the parameter estimates by drawing random parameter values from a normal distribution with the standard deviation equal to 10% of the mean (value in Table 2). While our results are somewhat dependent on this assumption, we included stochasticity in the parameters to help identify potential thresholds where simulations consistently bifurcated between epizootics or disease die-out using realistic and potentially conservative estimates of variability in parameter estimates. We simulated dynamics for one above-ground season (210 days) 500 times, with initial host populations at carrying capacity (K = 200), initial flea populations equal to 8.4 fleas per host with 80% on host and 20% questing (Tripp et al. 2009), and 2% of hosts exposed to plague. We set a few external controls on model simulations; the spatial correction factor (B) for prairie dogs had to be greater than that for alternate rodent hosts (B r ), prairie dog or flea populations were set to zero if below 0.5, randomly drawn parameters that represented rates or proportions were constrained between 0 and 1, and on-host fleas became questing fleas when hosts went extinct (see Supplementary Material—R code sec 1.2).
We characterized the simulated plague dynamics by calculating six host and flea population metrics: the proportion of model simulations where prairie dogs went extinct (probability of extinction), the average number of prairie dogs remaining (survival), the average number of days prairie dogs persisted (host persistence), the average maximum number of infected questing fleas (infected fleas), the average number of days infected questing fleas persisted (flea persistence), and the average number of on-host fleas per prairie dog when prairie dog populations were greater than zero (flea intensity). The contribution of each pathway to total infections was assessed by calculating the average proportion of prairie dog infections contributed by each pathway in the full model. We then measured the one-at-a-time sensitivity of the full model to each parameter by calculating the resulting percentage change in flea and host metrics with each parameter ±25, 50, or 75%, maintaining stochasticity in parameter values and simulating each adjusted parameter 500 times. Using the same one-at-a-time approach, we also explored the sensitivity of each transmission pathway to its respective transmission parameters (β, λ). We considered the model to be ‘sensitive’ to a particular parameter if a 1% change in the parameter value had an average change in any of our model metrics greater than or equal to 0.5%. Our approach to sensitivity analyses assumes fairly consistent variability between parameters (at least in regards to orders of magnitude).
Because three parameters, p, λ m , and σ had rates supported in the literature that differed by more than one order of magnitude (e.g., they are potentially highly variable), we explored the effects of altering these parameters with a direct search over the potential parameter ranges. For p, which represents the proportion of hosts becoming resistant after exposure to Y. pestis, laboratory and field observations suggest natural resistance ranges from 0.01 to 0.6 in prairie dog populations (Cully and Williams 2001; Rocke et al. 2012; Busch et al. 2013). Similarly, the length of time infectious carcasses are available on the landscape, λ m (carcass decay rate), may be as long as 2 months in burrows (λ m = 0.017, Godbey 2006) or as short as 2.5 days on the surface (λ m = 0.4, Boone et al. 2009). The exposed period, i.e., the incubation period, also varies by individual, with reported exposed periods for prairie dogs in laboratory trials as short as 2 days and as long as 16 days (Rocke et al. 2012, 2015). We used the full model for evaluating the effects of resistance and the exposed period, p and σ, but used the carcass transmission variation to evaluate the effects of carcass decay rate, λ m , on plague dynamics (Table S2). All simulations were conducted using R 3.1.0 and the documented code can be found in the Supplementary Material.
Simulation Results
In the full model, large epizootics lasted for 33 days (4.7 weeks) and caused prairie dog extirpation (probability of population extinction = 1). This is consistent with field observations of plague outbreaks where black-tailed prairie dog populations were observed to decline more than 95% between 5 weeks and 4 months after the beginning of a plague epizootic (Cully et al. 2000; Pauli et al. 2006; Webb et al. 2006). In the full model, infectious questing fleas peaked at 19 days and persisted for 56 days (3 weeks after prairie dog populations go extinct, Table 3). This is also consistent with field patterns where infected fleas persisted for 1 to 4 months in burrows after the decline of prairie dog populations due to plague epizootics (St. Romain et al. 2013).
Three transmission pathways contributed to plague infections in the full model simulations; dynamics were not only dominated by the flea transmission pathway (72.11% ± 1.93 SD of total number of infections) but also included contributions from direct transmission (16.92% ± 0.97 SD) and carcass transmission (10.96% ± 0.90 SD). The carcass transmission, flea transmission, and full model led to large epizootics, extinction of prairie dogs, and large infectious flea reservoirs. In contrast, the no transmission and soil transmission variations led to the rapid termination of plague, large prairie dog populations, and small infectious questing flea reservoirs. The direct transmission variation had intermediate results, with extended durations of infections, reduced prairie dog populations that survived the epizootic, and moderate infectious flea reservoirs. Reducing the connectance of prairie dog colonies, by removing alternate rodent hosts from the model, had only a marginal impact on prairie dog and flea metrics (Table 3; Fig. 3). Flea intensities averaged 23.17 ± 1.09 SD fleas per host in the full model, which is within the range observed by Tripp et al. (2009) and estimated by Wilder et al. (2008) for epizootics (Table 3).
Simulation dynamics for isolated transmission pathways, showing the nonlinear dynamics for both the host (left) and flea (right) submodels. Darker lines represent the model average, while lighter lines of the same color group represent each individual model run. Parameter stochasticity was modeled by sampling from a random distribution with standard deviation equal to 10% of each parameter value and does not represent the underlying uncertainty in parameter estimates. Each transmission pathway was simulated 500 times.
The plague model was, in general, robust to variation in parameter values. The full model was sensitive to the length of the host exposed period (1/σ), which increased survival and reduced the probability of extinction, such that a 1% reduction in σ (longer exposed period) led to an average of 3.25% increase in host persistence, a 0.56% increase in host survival, and a 0.53% reduction in the probability of extinction. Flea intensity in the full model was sensitive to changes in the spatial correction factor (B), the transmission rate of hosts to vectors (γ), the disease induced host mortality rate (a), the flea growth rate (r f ), and the questing efficiency (a) (Fig. S2). When isolating transmission parameters in model variations, changes in direct and carcass transmission parameters were the most sensitive. In general, increasing direct transmission parameters (β i , β m , ψ) decreased the survival and persistence of hosts, while reducing the length of time carcasses were available (λ m ) increased the survival and persistence of hosts (Fig. S2).
The direct search over parameter values identified parameter thresholds where model dynamics bifurcated between epizootics and disease die-out. For values of p (proportion of hosts that gain resistance) above 0.05 (1 or more in 20 animals survives), the probability of extinction dropped to zero, hosts persisted, and survival of prairie dogs increased (Table S2, Fig. 4). Similarly, increasing the decay rate of carcasses (λ m ) reduced the impact of carcass transmission, with hosts persisting at values greater than 0.2 (carcasses infectious for 5 or fewer days; Table S2, Fig. 4). The direct search over the length of the exposed period, σ, highlighted similar threshold behavior around 8 days, with longer incubation periods leading to disease die-out and shorter incubation periods generating epizootics (Table S2, Fig. 4).
Results of the direct search over the proportion of hosts that gain resistance (p), decay rate of carcasses (λ m ), and exposed period (σ) illustrating the change in the average number of prairie dogs surviving for the various parameter values in the full model (p, σ) and the average size of the carcass reservoir for the various carcass decay rates (λ m ) in the carcass transmission variation of plague dynamics in the western US. The starred value represents the parameter value used in the full model simulation. Model metrics from the direct search of parameter values are summarized in Table S2.
Discussion
In our model simulations, transmission pathways that included short-term reservoirs, i.e., carcass or early phase flea transmission, resulted in extirpation of prairie dog populations both alone and in tandem with other pathways. This is similar to the results of Buhnerkempe et al. (2011) and agrees with the reviews of Gage and Kosoy (2005) and Eisen and Gage (2009). However, the ability of carcasses to drive an epizootic, combined with the 28% contribution of direct pathways to infection in the full model, suggests that the role of both of these pathways needs further investigation. For example, the current practice of dusting burrows with insecticides to prevent additional mortality from flea-borne transmission or spillover from sylvatic plague epizootics (Hoogland et al. 2004) may not be sufficient if large numbers of infected prairie dogs and carcasses are also present. We provide some preliminary and opportunistic evidence that secondary plague pneumonia may be common in prairie dogs and that plague-killed carcasses are potentially infectious for long periods (Godbey 2006; Busch et al. 2013, Table S1). However, a more systematic and thorough experimental approach is needed to develop robust estimates for the development and infectiousness of secondary pneumonia in rodent hosts and carcass availability and infectivity over time.
Inclusion of a soil transmission pathway for plague and increased connectance by small rodents did not affect the dynamics of plague in our model. This is not surprising, given the small rate of becoming infected via contaminated soil and the small impact the spatial correction factor (B) had on overall plague dynamics. However, prairie dogs have strong spatial structure within and among colonies (i.e., classic metapopulation dynamics) with discernable impacts on plague dynamics (Johnson et al. 2011; Collinge et al. 2005). For example, plague outbreaks are more likely in large, well-connected colonies (Stapp et al. 2004; Collinge et al. 2005; Johnson et al. 2011; George et al. 2013), leading to a reciprocal effect of increasing turnover and movement (extinction and colonization) of prairie dog colonies across the landscape (Augustine et al. 2008; George et al. 2013). Prairie dogs also have a strong spatial structure within colonies, created by the grouping of burrow systems and territoriality of coteries (family groups) that violate traditional “even mixing” assumptions in disease models. Yet, most plague models (this one included) have ignored explicit spatial dynamics within colonies (but see Salkeld et al. 2010 and Shoemaker et al. 2014 for lattice models), and we expect that incorporation of explicit space using realistic contact networks (Keeling 1999; Craft et al. 2011) may alter the contributions of alternate transmission pathways to plague dynamics.
The full model was sensitive to parameters that determined infection in hosts, such as the length of the exposed period (i.e., incubation period) and proportion of hosts gaining resistance after exposure to Y. pestis. The incubation period has high variation among individuals (Rocke et al. 2012, 2015), and given this parameter’s importance in model outcomes (i.e., plague epizootic vs disease die-out), may be a contributing factor to the regionally patchy distribution of plague epizootics. Similarly, the probability of gaining resistance, determined by laboratory studies, shows considerable variation (0.01–0.6) related to the history of plague exposure, host age, and host species (Busch et al. 2013; Rocke et al. 2012, 2015). As expected, increasing host resistance allowed for survival and persistence of prairie dogs. These results have implications for current efforts to develop an effective sylvatic plague vaccine strategy (Rocke et al. 2012, 2015), illustrating that even low vaccination rates may improve host survival and reduce plague dynamics.
Conclusion
Overall, we have confirmed that short-term reservoirs are necessary in order for large plague epizootics to develop in sylvatic plague foci in western US grasslands. However, we have also shown that either carcasses or infectious questing fleas can serve as this reservoir independently. We have highlighted several priorities for future research that would provide increased confidence in model parameters and better estimates of the impact of various transmission pathways on plague dynamics in prairie dogs and their fleas. These priorities include determining life history parameters for infected and uninfected fleas, transmission rates for direct contact, inhalation exposure, carcass consumption, and the incubation and infectious periods in prairie dogs. Increasing our confidence in these parameter estimates will lead to a stronger quantitative framework for predicting sylvatic plague epizootics and potential spillover risk to humans and other sensitive species in the western US.
References
Abbott RC, Rocke TE (2012) Plague: U.S. Geological Survey Circular 1372, 79 p., plus appendix. (Also available at http://pubs.usgs.gov/circ/1372.)
Antonation, K. S., T. K. Shury, T. K. Bollinger, A. Olson, P. Mabon, G. Van Domselaar, and C. R. Corbett. 2014. Sylvatic plague in a Canadian black-tailed prairie dog (Cynomys ludovicianus). Journal of Wildlife Diseases 50:699–702.
Arbaji, A., S. Kharabsheh, S. Al-Azab, M. Al-Kayed, Z. S. Amr, M. Abu Baker, and M. C. Chu. 2005. A 12-case outbreak of pharyngeal plague following the consumption of camel meat, in north-eastern Jordan. Annals of Tropical Medicine and Parasitology 99:789–793.
Augustine, D. J., M. R. Matchett, T. P. Toombs, J. F. Cully, T. L. Johnson, and J. G. Sidle. 2008. Spatiotemporal dynamics of black-tailed prairie dog colonies affected by plague. Landscape Ecology 23:255–267.
Ayyadurai S, Houhamdi L, Lepidi H, Nappez C, Raoult D, Drancourt M (2008) Long-term persistence of virulent Yersinia pestis in soil. Microbiology (Reading, England) 154:2865–2871
Bearden, S. W., and R. R. Brubaker. 2010. Recent findings regarding maintenance of enzootic variants of Yersinia pestis in sylvatic reservoirs and their significance in the evolution of epidemic plague. Vector-Borne and Zoonotic Diseases 10:85–92.
Ber, R., E. Mamroud, M. Aftalion, A. Tidhar, D. Gur, Y. Flashner, and S. Cohen. 2003. Development of an improved selective agar medium for isolation of Yersinia pestis. Applied and Environmental Microbiology 69:5787–5792.
Boegler, K. A., C. B. Graham, J. A. Montenieri, K. MacMillan, J. L. Holmes, J. M. Peterson, K. L. Gage, and R. J. Eisen. 2012. Evaluation of the infectiousness to mice of soil contaminated with Yersinia pestis-infected blood. Vector-Borne and Zoonotic Diseases 12:948–952.
Boone, A., J. P. Kraft, and P. Stapp. 2009. Scavenging by mammalian carnivores on prairie dog colonies: implications for the spread of plague. Vector-Borne and Zoonotic Diseases 9:185–190.
Brinkerhoff, J. R., C. Ray, B. Thiagarajan, S. K. Collinge, J. F. Cully, B. Holmes, and K. L. Gage. 2008. Prairie dog presence affects occurrence patterns of disease vectors on small mammals. Ecography 31:654–662.
Brinkerhoff, R. J., S. K. Collinge, Y. Bai, and C. Ray. 2009. Are carnivores universally good sentinels of plague? Vector-Borne and Zoonotic Diseases 9:491–7.
Buhnerkempe, M. G., R. J. Eisen, B. Goodell, K. L. Gage, M. F. Antolin, and C. T. Webb. 2011. Transmission shifts underlie variability in population responses to Yersinia pestis infection. PloS one 6:e22498.
Burroughs, A. 1947. Sylvatic plague studies. The vector efficiency of nine species of fleas compared with Xenopsylla cheopis. Journal of Hygiene 45:371–396.
Busch, J. D., R. Van Andel, N. E. Stone, K. R. Cobble, R. Nottingham, J. Lee, M. VerSteeg, J. Corcoran, J. Cordova, W. Van Pelt, M. M. Shuey, J. T. Foster, J. M. Schupp, S. Beckstrom-Sternberg, J. Beckstrom-Sternberg, P. Keim, S. Smith, J. Rodriguez-Ramos, J. L. Williamson, T. E. Rocke, and D. M. Wagner. 2013. The innate immune response may be important for surviving plague in wild Gunnison’s prairie dogs. Journal of Wildlife Diseases 49:920–31.
Collinge, S. K., W. C. Johnson, C. Ray, R. Matchett, J. Grensten, J. F. Cully Jr., K. L. Gage, M. Y. Kosoy, J. E. Loye, and A. P. Martin. 2005. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landscape Ecology 20:941–955.
Craft, M. E., E. Volz, C. Packer, and L. A. Meyers. 2011. Disease transmission in territorial populations: the small-world network of Serengeti lions. Journal of the Royal Society, Interface 8:776–86.
Cully, J. F., L. G. Carter, and K. L. Gage. 2000. New records of sylvatic plague in Kansas. Journal of Wildlife Diseases 36:389–392.
Cully Jr, J. F., and E. S. Williams. 2001. Interspecific comparisons of sylvatic plague in prairie dogs. Journal of mammalogy 82:894–905.
Davis, S., M. Begon, L. De Bruyn, V. S. Ageyev, N. L. Klassovskiy, S. B. Pole, H. Viljugrein, N. C. Stenseth, and H. Leirs. 2004. Predictive thresholds for plague in Kazakhstan. Science 304:736–8.
Davis, S., H. Leirs, H. Viljugrein, N. C. Stenseth, L. De Bruyn, N. Klassovskiy, V. Ageyev, and M. Begon. 2007. Empirical assessment of a threshold model for sylvatic plague. Journal of the Royal Society, Interface 4:649–57.
Davis, S., P. Trapman, H. Leirs, M. Begon, and J. A. P. Heesterbeek. 2008. The abundance threshold for plague as a critical percolation phenomenon. Nature 454:634–7.
de Castro, F. and B. Bolker. 2005. Mechanisms of disease-induced extinction. Ecology Letters 8:117-126.
Easterday, W. R., K. L. Kausrud, B. Star, L. Heier, B. J. Haley, V. Ageyev, R. R. Colwell, and N. C. Stenseth. 2012. An additional step in the transmission of Yersinia pestis? The ISME Journal 6:231–6.
Eisen, R. J., S. W. Bearden, A. P. Wilder, J. A. Montenieri, M. F. Antolin, and K. L. Gage. 2006. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proceedings of the National Academy of Sciences of the United States of America 103:15380–5.
Eisen, R. J., L. Eisen, and K. L. Gage. 2009. Studies of vector competency and efficiency of North American fleas for Yersinia pestis: state of the field and future research needs. Journal of Medical Entomology 46:737–744.
Eisen, R. J., and K. L. Gage. 2009. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Veterinary Research 40:1–14.
Eisen RJ, Lowell JL, Montenieri JA, Bearden SW, Gage KL (2007) Temporal dynamics of early-phase transmission of Yersinia pestis by unblocked fleas: secondary infectious feeds prolong efficient transmission by Oropsylla montana (Siphonaptera: Ceratophyllidae). Journal of Medical Entomology 44:672–677.
Eisen, R. J., J. M. Petersen, C. L. Higgins, D. Wong, C. E. Levy, P. S. Mead, M. E. Schriefer, K. S. Griffith, K. L. Gage, and B. C. Beard. 2008a. Persistence of Yersinia pestis in soil under natural conditions. Emerging Infectious Diseases 14:12–14.
Eisen, R. J., S. M. Vetter, J. L. Holmes, S. W. Bearden, J. A. Montenieri, and K. L. Gage. 2008b. Source of host blood affects prevalence of infection and bacterial loads of Yersinia pestis in fleas. Journal of Medical Entomology 45:933–938.
Engelthaler, D. M., B. J. Hinnebusch, C. M. Rittner, and K. L. Gage. 2000. Quantitative competitive PCR as a technique for exploring flea-Yersinia pestis dynamics. The American Journal of Tropical Medical Hygiene 62:552–560.
Eskey, C., V. Haas, and N. Good. 1940. Plague in the western part of the United States. Federal Security Agency, U.S. Public Health Service, Washington DC.
Evans, F., and R. Holdenried. 1943. A population study of the beechey ground squirrel in central California. Journal of Mammalogy 24:231–260.
Friggens, M. M., R. R. Parmenter, M. Boyden, P. L. Ford, K. Gage, and P. Keim. 2010. Flea abundance, diversity, and plague in Gunnison’s prairie dogs (Cynomys gunnisoni) and their burrows in montane grasslands in northern New Mexico. Journal of Wildlife Diseases 46:356–67.
Gage, K. L., and M. Y. Kosoy. 2005. Natural history of plague: perspectives from more than a century of research. Annual Review of Entomology 50:505–28.
Gani, R., and S. Leach. 2004. Epidemiologic determinants for modeling pneumonic plague outbreaks. Emerging Infectious Diseases 10:608–614.
Gascuel, F., M. Choisy, J.-M. Duplantier, F. Débarre, and C. Brouat. 2013. Host resistance, population structure and the long-term persistence of bubonic plague: contributions of a modelling approach in the Malagasy focus. PLoS Computational Biology 9:e1003039.
George, D. B., C. T. Webb, K. M. Pepin, L. T. Savage, and M. F. Antolin. 2013. Persistence of black-tailed prairie-dog populations affected by plague in northern Colorado, USA. Ecology 94:1572–1583.
Godbey BJL, Biggins DE, Garelle D (2006) Exposure of Captive Black-Footed Ferrets to Plague and Implications for Species Recovery. U.S. Geological Survey.
Hartwell, W. V, S. F. Quan, K. G. Scott, and L. Kartman. 1958. Observations on flea transfer between hosts; a mechanism in the spread of bubonic plague. Science 127:127–128.
Holdenried, R., and S. F. Quan. 1956. Susceptibility of New Mexico rodents to experimental plague. Public Health Reports 71:979–984.
Holmes, B. E., K. R. Foresman, and M. R. Matchett. 2006. No evidence of persistent Yersinia pestis infection at prairie dog colonies in north-central Montana. Journal of Wildlife Diseases 42:164–169.
Hoogland, J. L. 1995. The black-tailed prairie dog: Social life of a burrowing mammal. University of Chicago Press, Chicago, IL.
Hoogland, J. L., S. Davis, S. Benson-Amram, D. Labruna, B. Goossens, and M. A. Hoogland. 2004. Pyraperm kills fleas and halts plague among Utah prairie dogs. The Southwestern Naturalist 49:376-83.
Johnson, T. L., J. F. Cully, S. K. Collinge, C. Ray, C. M. Frey, and B. K. Sandercock. 2011. Spread of plague among black-tailed prairie dogs is associated with colony spatial characteristics. The Journal of Wildlife Management 75:357–368.
Keeling, M. J. 1999. The effects of local spatial structure on epidemiological invasions. Proceedings of the Royal Society B 266:859–867.
Kraft, J. P., and P. Stapp. 2013. Movements and burrow use by northern grasshopper mice as a possible mechanism of plague spread in prairie dog colonies. Journal of Mammalogy 94:1087–1093.
Krasnov, B. R., I. S. Khokhlova, L. J. Fielden, and N. I. Burdelova. 2002. Time of survival under starvation in two flea species (Siphonaptera: Pullicidae) at different air temperatures and relative humidities. Journal of Vector Ecology 27:70–81.
Lorange, E. A., B. L. Race, F. Sebbane, and B. J. Hinnebusch. 2005. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. Journal of Infectious Diseases 191:1907–1912.
Matchett, M. R., D. E. Biggins, V. Carlson, B. Powell, and T. Rocke. 2010. Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana. Vector-Borne and Zoonotic Diseases 10:27–35.
Parmenter, R. R., E. P. Yadav, C. A. Parmenter, P. Ettestad, and K. L. Gage. 1999. Incidence of plague associated with increased winter-spring precipitation in New Mexico. American Journal of Tropical Medicine and Hygiene 61:814–821.
Pauli, J. N., S. W. Buskirk, E. S. Williams, and W. H. Edwards. 2006. A plague epizootic in the black-tailed prairie dog (Cynomys ludovicianus). Journal of Wildlife Diseases 42:74–80.
Quan, T. J., A. M. Barnes, L. G. Carter, and K. R. Tsuchiya. 1985. Experimental plague in rock squirrels, Spermophilus variegatus (Erxleben). Journal of Wildlife Diseases 21:205–210.
Reijniers, J., S. Davis, M. Begon, J. A. P. Heesterbeek, V. S. Ageyev, and H. Leirs. 2012. A curve of thresholds governs plague epizootics in Central Asia. Ecology Letters 15:554–60.
Rocke TE, Tripp D, Lorenzsonn F, Falendysz E, Smith S, Williamson J, Abbott R (2015) Age at vaccination may influence response to sylvatic plague vaccine (SPV) in Gunnison’s prairie dogs (Cynomys gunnisoni). EcoHealth [ePub ahead of print].
Rocke, T. E., J. Williamson, K. R. Cobble, J. D. Busch, M. F. Antolin, and D. M. Wagner. 2012. Resistance to plague among black-tailed prairie dog populations. Vector-Borne and Zoonotic Diseases 12:111–6.
Rust, M. K., and M. W. Dryden. 1997. The biology, ecology, and management of the cat flea. Annual Review of Entomology 42:451–73.
Salkeld, D. J., M. Salathé, P. Stapp, and J. H. Jones. 2010. Plague outbreaks in prairie dog populations explained by percolation thresholds of alternate host abundance. Proceedings of the National Academy of Sciences the United States of America 107:14247–50.
Salkeld, D. J., and P. Stapp. 2008. No evidence of deer mouse involvement in plague (Yersinia pestis) epizootics in prairie dogs. Vector-Borne and Zoonotic Diseases 8:331–337.
Salkeld, D., and P. Stapp. 2006. Seroprevalence rates and transmission of plague (Yersinia pestis) in mammalian carnivores. Vector-Borne & Zoonotic Diseases 6:231–239.
Samia, N. I., K. L. Kausrud, H. Heesterbeek, V. Ageyev, M. Begon, K.-S. Chan, and N. C. Stenseth. 2011. Dynamics of the plague-wildlife-human system in Central Asia are controlled by two epidemiological thresholds. Proceedings of the National Academy of Sciences of the United States of America 108:14527–32.
Savage, L. T., R. M. Reich, L. M. Hartley, P. Stapp, and M. F. Antolin. 2011. Climate, soils, and connectivity predict plague epizootics in black-tailed prairie dogs (Cynomys ludovicianus). Ecological Applications 21:2933–2943.
Serzhan OS, Ageyev VS (2000) Geographical distribution and host complexes of plague-infected fleas in relation to some problems of paleogenesis of plague enzootics. In: Karantinye i Zoonoznye Infektsii v Kazakhstane, Atshabar BB, 2:183–92. Almaty: Kazakhskii Protivochumnyi Inst. Shoemaker KT, Lacy RC, Verant ML, Brook BW, Livieri TM, Miller PS, Fordham DA, Resit Akçakaya H (2014) Effects of prey metapopulation structure on the viability of black-footed ferrets in plague-impacted landscapes: a metamodelling approach. Journal of Applied Ecology 51:735–745.
Skurnik, M., A. Peippo, and E. Ervela. 2000. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Molecular Microbiology 37:316–330.
Stapp, P., M. F. Antolin, and M. Ball. 2004. Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Niño events. Frontiers in Ecology and the Environment 2:235–240.
Stapp, P., and R. J. Salkeld. 2009. Inferring host-parasite relationships using stable isotopes: implications for disease transmission and host specificity. Ecology 90:3268-73.
Stapp, P., D. J. Salkeld, R. J. Eisen, R. Pappert, J. Young, L. G. Carter, K. L. Gage, D. W. Tripp, and M. F. Antolin. 2008. Exposure of small rodents to plague during epizootics in black-tailed prairie dogs. Journal of Wildlife Diseases 44:724–730.
Stapp, P., D. J. Salkeld, H. A. Franklin, J. P. Kraft, D. W. Tripp, M. F. Antolin, and K. L. Gage. 2009. Evidence for the involvement of an alternate rodent host in the dynamics of introduced plague in prairie dogs. The Journal of Animal Ecology 78:807–17.
Stenseth, N. C., B. B. Atshabar, M. Begon, S. R. Belmain, E. Bertherat, E. Carniel, K. L. Gage, H. Leirs, and L. Rahalison. 2008. Plague: past, present, and future. PLoS Medicine 5:e3.
St. Romain K, Tripp DW, Salkeld DJ, Antolin MF (2013) Duration of plague (Yersinia pestis) outbreaks in black-tailed prairie dog (Cynomys ludovicianus) colonies of northern Colorado. EcoHealth 10:241–245.
Thiagarajan, B., Y. Bai, K. L. Gage, and J. F. Cully. 2008. Prevalence of Yersinia pestis in rodents and fleas associated with black-tailed prairie dogs (Cynomys ludovicianus) at Thunder Basin National Grassland, Wyoming. Journal of Wildlife Diseases 44:731–736.
Thomas, R. E., A. M. Barnes, T. J. Quan, M. L. Beard, L. G. Carter, and C. E. Hopla. 1988. Susceptibility to Yersinia pestis in the northern grasshopper mouse (Onychomys leucogaster). Journal of Wildlife Diseases 24:327–333.
Thomas, R. E., M. L. Beard, T. J. Quan, L. G. Carter, A. M. Barnes, and C. E. Hopla. 1989. Experimentally induced plague infection in the northern grasshopper mouse (Onychomys leucogaster) acquired by consumption of infected prey. Journal of Wildlife Diseases 25:477–480.
Tripp, D. W., K. L. Gage, J. A. Montenieri, and M. F. Antolin. 2009. Flea abundance on black-tailed prairie dogs (Cynomys ludovicianus) increases during plague epizootics. Vector-Borne and Zoonotic Diseases 9:313–321.
Vogler, A. J., F. Chan, D. M. Wagner, P. Roumagnac, J. Lee, R. Nera, M. Eppinger, J. Ravel, L. Rahalison, B. W. Rasoamanana, S. M. Beckstrom-Sternberg, M. Achtman, S. Chanteau, and P. Keim. 2011. Phylogeography and molecular epidemiology of Yersinia pestis in Madagascar. PLoS Neglected Tropical Diseases 5:e1319.
Webb, C. T., C. P. Brooks, K. L. Gage, and M. F. Antolin. 2006. Classic flea-borne transmission does not drive plague epizootics in prairie dogs. Proceedings of the National Academy of Sciences of the United States of America 103:6236–41.
Wilder, A. P., R. J. Eisen, S. W. Bearden, J. A. Montenieri, K. L. Gage, and M. F. Antolin. 2008. Oropsylla hirsuta (Siphonaptera: Ceratophyllidae) can support plague epizootics in black-tailed prairie dogs (Cynomys ludovicianus) by early-phase transmission of Yersinia pestis. Vector-Borne and Zoonotic Diseases 8:359–367.
Williams, J. E., M. A. Moussa, and D. C. Cavanaugh. 1979. Experimental plague in the California ground squirrel. Journal of Infectious Diseases 140:618-21.
Zhang, Y., X. Dai, X. Wang, A. Maituohuti, Y. Cui, A. Rehemu, Q. Wang, W. Meng, T. Luo, R. Guo, B. Li, A. Abudurexiti, Y. Song, R. Yang, and H. Cao. 2012. Dynamics of Yersinia pestis and its antibody response in great gerbils (Rhombomys opimus) by subcutaneous infection. PloS one 7:e46820.
Acknowledgments
This manuscript was greatly improved by comments from R. Abbott, E. Falendysz, M. Buhnerkempe, and two anonymous reviewers. RR and TR are supported by the U.S. Geological Survey’s National Wildlife Health Center. KR performed this work with the USGS while employed as a post-doctoral researcher at the University of Wisconsin. GB performed this work with the USGS while serving as a graduate student at the University of Wisconsin and is supported by a fellowship from the Morris Animal Foundation (D14ZO-412). The use of trade or product names does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interests to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richgels, K.L.D., Russell, R.E., Bron, G.M. et al. Evaluation of Yersinia pestis Transmission Pathways for Sylvatic Plague in Prairie Dog Populations in the Western U.S.. EcoHealth 13, 415–427 (2016). https://doi.org/10.1007/s10393-016-1133-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-016-1133-9