Abstract
The chytrid fungus Batrachochytrium dendrobatidis (Bd) has been implicated in amphibian declines on almost all continents. We report on prevalence and intensity of Bd in the United States amphibian populations across three longitudinally separated north-to-south transects conducted at 15 Department of Defense installations during two sampling periods (late-spring/early summer and mid to late summer). Such a standardized approach minimizes the effects of sampling and analytical bias, as well as human disturbance (by sampling restricted military bases), and therefore permits a cleaner interpretation of environmental variables known to affect chytrid dynamics such as season, temperature, rainfall, latitude, and longitude. Our prevalence of positive samples was 20.4% (137/670), and our mean intensity was 3.21 zoospore equivalents (SE = 1.03; range 0.001–103.59). Of the 28 amphibian species sampled, 15 tested positive. Three sites had no evidence of Bd infection; across the remaining 12 Bd-positive sites, neither infection prevalence nor intensity varied systematically. We found a more complicated pattern of Bd prevalence than anticipated. Early season samples showed no trend associated with increasing temperature and precipitation and decreasing (more southerly) latitudes; while in late season samples, the proportion of infected individuals decreased with increasing temperature and precipitation and decreasing latitudes. A similar pattern held for the east–west gradient, with the highest prevalence associated with more easterly/recently warmer sites in the early season then shifting to more westerly/recently cooler sites in the later season. Bd intensity across bases and sampling periods was comparatively low. Some of the trends in our data have been seen in previous studies, and our results offer further continental-level Bd sampling over which more concentrated local sampling efforts can be overlaid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, declines in amphibian populations are occurring at a rate several times faster than decreases in birds or mammals, with an estimated 42% of amphibian species in decline (Stuart et al. 2004; IUCN Red List 2014). In addition to global threats, such as habitat degradation and the impacts of invasive species that all organisms face, amphibians are also disproportionately affected by emerging infectious diseases. One of these, a chytrid fungus, Batrachochytrium dendrobatidis (Bd; Longcore et al. 1999), is considered a leading cause of amphibian declines (Berger et al. 1998; DiRosa et al. 2007; Skerratt et al. 2007; Briggs et al. 2010). Although the distribution of amphibians with Bd infections is nearly global, the distribution of lethal outbreaks of Bd-caused amphibian declines has to date been restricted to a few regions, notably Eastern Australia, Central America, and the western United States (Lips et al. 2006; Skerratt et al. 2007; Jones et al. 2008; Murray et al. 2009). As research on Bd continues, the complexity of this fungus and factors that affect it continue to be revealed. At the global scale, Bd detection is impacted by pressure factors such as trade and the introduction of alien host species (Liu et al. 2013) and associated with fundamental niche factors such as climate (Rohr and Raffel 2010; Rohr et al. 2011; Olson et al. 2013). Because of the preference of Bd for cool, moist environments, microhabitat characteristics such as temperature, moisture, and vegetation cover were empirically found to be important drivers of Bd infection (Raffel et al. 2010). Models also support these findings and indicate that mean diurnal temperature range and annual precipitation were important predictors of Bd occurrence (Murray et al. 2011), and natural vegetation and host species richness can be key factors associated with Bd occurrence (Becker and Zamudio 2011). Previous studies also suggest that Bd prevalence is predicted by host traits and host diversity (Venesky et al. 2013; Becker et al. 2014).
Other complexities of factors that affect Bd are known. For example, few die-offs connected to Bd have been reported in the eastern three-quarters of North America (east of the Rocky Mountains). This has led to the hypothesis that Bd is endemic in amphibian populations in this region (Rachowitz et al. 2006; Kinney et al. 2011). This scenario also suggests that in certain regions of the world, such as the majority of North America, much of the spread of Bd occurred decades ago (when it was epidemic) and that in these places it is now endemic (arising within the population). Lannoo et al. (2011) indicated that further testing of the endemic hypothesis involving surveys over broad geographic scales is warranted.
In regions where Bd is considered endemic, it can impact populations when environmental conditions are favorable (Retallick et al. 2004; Ouellet et al. 2005; Longo et al. 2010; Savage et al. 2011; Terrell et al. 2014). As a result, when sampling for Bd, it is important to distinguish between prevalence (percentage of individuals infected divided by number sampled) and intensity (strength of the infection, measured in zoospore equivalents). In populations where Bd is acting as an endemic disease, prevalence and intensity can be coupled (Briggs et al. 2010; Kinney et al. 2011); where Bd is acting in an epidemic fashion, prevalence and intensity can be uncoupled (Terrell et al. 2014). Both prevalence and intensity vary seasonally in endemic infections. Kinney et al. (2011) reported both Bd prevalence and intensity dropped from spring to summer in a population of crawfish frogs (Lithobates areolatus) located in southern Indiana—adults sampled in mid-summer showed no signs of infection. These same animals emerged from winter senescence and entered breeding wetlands with an infection prevalence of about 25%, and emerged from breeding wetlands with an infection rate of over 50%. Prevalence and intensity were linked, and chytrid-related deaths occurred following breeding. Post-breeding adults that returned to their burrows subsequently cleared the infection, presumably by basking.
When interested in natural rates of disease occurrence and transmission, it becomes useful to sample sites with a minimal disturbance history. Military installations are landscapes secured and protected in the interest of national security. These protections also extend to the natural resources contained on military bases—landscapes that have been shown to harbor the greatest density of threatened and endangered species and habitats of any federally owned lands in the United States (Stein et al. 2008).
The first objective of this study was to extend our previous work (Lannoo et al. 2011) by surveying for Bd prevalence (proportion of individuals infected) and intensity (strength of the infection, measured as zoospore equivalents) over three north-to-south transects at widely separated longitudes across the United States. To maintain comparability to the results of Lannoo et al. (2011), we used the same methodology and sampled United States Department of Defense (DOD) installations. Furthermore, for this study the same team of researchers following the same protocol collected field samples at all 15 study sites during the two sampling periods. This consistency reduces the confounding factors of sampling and analysis bias, as well as human disturbance on the dynamics of Bd infection transforming into the disease chytridiomycosis (Lannoo et al. 2011). Following the conclusions of Lannoo et al. (2011), our first hypothesis is that because hot, dry, sparsely vegetated habitats limit this fungus, Bd would be more often present and more intense at bases located in the north and east—that is, in the northern portion of the western transect, and throughout the midwestern and eastern transects (see below).
Our second objective was to assess the relationship of seasonality to the prevalence and intensity of Bd in the United States. Seasonality in Bd prevalence has been previously demonstrated (Berger et al. 2004; Gaertner et al. 2009; Kinney et al. 2011; Savage et al. 2011). As summer proceeds, the prevalence of Bd in amphibian populations decreases (Lannoo et al. 2011) as Bd-positive frogs clear their infection (Johnson and Speare 2005; Piotrowski et al. 2004; Woodhams et al. 2003, 2005; Kinney et al. 2011). This pattern is tied to the life history and physiological ecology of Bd, which thrives in cool, moist conditions—temperatures >28 °C are lethal (Fisher et al. 2009; Stevenson et al. 2013). We felt a study design consisting of three north-to-south transects would provide a wide range of both temporally and spatially mediated temperatures and precipitation levels, providing a further test of the relationship of Bd prevalence to temperature and precipitation. Our second hypothesis is that Bd would be more often present and more intense during our first sampling period (spring/early summer [March–June]), than our second (mid/late summer [July–September]). Our third hypothesis, building on the first, is that at any point in time, Bd prevalences and intensities would be lowest at our low-latitude sites, because temperatures are more likely to exceed the thermal maximum of Bd for longer portions of the year.
Materials and Methods
Ethics Statement
This research was conducted under Institutional Animal Care and Use Committee protocol number 11,217 issued by the University of Illinois at Urbana-Champaign, and state scientific collecting license permit numbers 2011-333 (Maine), 17496 (Minnesota), 0127112 (Mississippi), 11-SC00511 (North Carolina), SC2011062 (New Jersey), 080-11 (Oregon), 11-2011 (South Carolina), 3602 (Tennessee), 11-075 (Washington), and SCP-WCR-141-C-2011 (Wisconsin). No animals were harmed while collecting Bd samples.
Study Sites
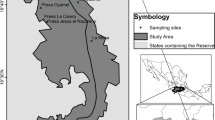
In 2011, we sampled for Bd at 15 DOD installations along three north–south transects spanning the length and breadth of the continental United States (Fig. 1). Transects were as follows.
-
West Coast Transect (Washington to California roughly along Interstate 5): Naval Radio Station Jim Creek (NRS Jim Creek) and Fort Lewis in Washington, Camp Rilea in Oregon, and Marine Corps Mountain Warfare Training Center Bridgeport (MWTC Bridgeport) and Fort Hunter Liggett in California.
-
Midwest Transect (Minnesota to Mississippi roughly along Interstates 94 and 55): Camp Ripley in Minnesota, Fort McCoy in Wisconsin, Scott Air Force Base in Illinois, Naval Support Activity Mid-South in Tennessee (NSA Mid-South), and Naval Air Station Meridian (NAS Meridian) in Mississippi.
-
East Coast Transect (Maine to Florida roughly along Interstate 95): Naval Computer and Telecommunications Area Master Station Cutler in Maine, Fort Dix in New Jersey, Marine Corps Base Camp Lejeune in North Carolina, Shaw Air Force Base in South Carolina, and Cape Canaveral Air Force Station in Florida.
We selected these military installations to maximize the range of variation in geography, habitat types, climate, and species diversity. Based on the installations selected and the distribution of United States amphibians, the potential existed to sample an estimated 50 species of amphibians—about one sixth of the total number of United States species (Lannoo 2005).
Field Sampling
We collected field samples during the northern hemisphere’s warm months of 2011. Each installation was sampled twice, once during a period encompassing spring/early summer (March–June), then again during a period encompassing mid/late summer (July–September). Samples were taken exclusively during the day and individual sites were not re-sampled during each field period. Between sites, we cleaned mud and other debris from gear, and disinfected gear with a dilute bleach solution.
Our goal was to sample at least 20 amphibians at each installation during each sampling period. To achieve this, three (range one to seven) wetland sites were generally sampled at each installation. We recorded sample sites using a Global Positioning System (GPS). In most cases, we sampled post-metamorphic animals (adults and juveniles), but we sampled tadpoles when no adults or juveniles were encountered.
We captured amphibians by hand or using a dip net. To prevent the spread of disease, we secured animals wearing nitrile gloves and placed animals individually in plastic bags for processing. We discarded gloves and bags after one use. We sampled all animals using sterile cotton, plastic-handled swabs (Medical Wire & Equipment Co., Corsham, England). For post-metamorphic animals, we rolled swabs over the body surface a total of 50 times as follows: five rubs each on the back, sides, belly, and head; between the thighs; and on the bottom of each foot. For tadpoles, we swabbed mouthparts and oral disks. Following swabbing, we broke the head of the swab into a 0.6 ml microcentrifuge tube (Fisherbrand 05-407-01; Pessier and Mendelson 2010). We stored samples at 4°C and shipped them on ice packs prior to analysis (described below). Following processing, we released animals at their site of capture.
Temperature and Precipitation Data
We obtained maximum daily temperature and precipitation data for a 30-day period prior to the sampling time from weather stations near or at each installation using National Oceanic and Atmospheric Administration (NOAA) databases (http://cdo.ncdc.noaa.gov/cgi-bin/climatenormals/climatenormals.pl). We then averaged all maximum daily temperatures and summed the daily precipitation for use as covariates in our analyses.
Laboratory Analyses
We used a real-time TaqMan PCR technique (Boyle et al. 2004; Hyatt et al. 2007) to analyze Bd swabs. Briefly, we prepared a DNA template with PrepMan Ultra (Applied Biosystems) and used an exogenous internal positive control labeled with TaqMan VIC (Applied Biosystems) for each sample to detect PCR inhibitors. For reactions, we used the TaqMan Environmental Mastermix 2.0 (Applied Biosystems). We ran assays in triplicate on an ABI/Applied Biosystems 7900HT thermocycler using 384 well plates. We considered samples that amplified at a Ct of <50 in 2 or more wells positive (Ct is the cycle number at which the fluorescent-labeled Bd probe crosses the threshold to indicate signal). We considered samples that amplified at a Ct of <50 in 1 well equivocal. For positive samples, we created quantification standards by growing Bd isolate JEL 197 on 1% Tryptone Agar and harvested zoospores by rinsing plates with 1x phosphate buffered saline. After collection, we counted zoospores three times on a hemocytometer to determine the range of zoospores ml -1. We generated standard curves with ten-fold serial dilutions (range 1 × 106 to 1 × 10−2 zoospores). In addition to positive controls (quantification standards), each plate included a negative control (TaqMan Mastermix and no sample DNA), as well as four positive and negative quality assurance controls consisting of swabs either inoculated with Bd zoospores or sham-inoculated.
We expressed the intensity of infection in the positive samples as the mean (averaged over the three reactions) number of zoospore equivalents (INT) per swab (Vredenburg et al. 2010). We calculated mean INT as the sum of all swab INT means divided by the number of positive swabs. We calculated Bd prevalence (PREV) as the number of positive swabs divided by the number of unequivocal swabs (equivocal swabs were eliminated).
Data Analysis
We excluded all individuals for which indeterminate results were obtained from all three PCR runs. Next we conducted a principal component analysis in R (R Core Team 2015) on the variables of latitude, longitude, average maximum 30-day temperature, and 30-day precipitation. We retained all principal components with an eigenvalue greater than one and conducted a Kaiser–Meyer–Olkin (KMO) test of sampling adequacy and Bartlett’s test of sphericity to determine if variable reduction was warranted and variances were equal. We then used a varimax rotation to determine which variables were associated with which components and retained the pc-scores as our new covariates.
To determine if the probability of Bd infection in amphibians varied by season, latitude, longitude, and with recent temperature, and rainfall, we conducted a series of mixed-effect binary logistic regressions using the R package lme4 (Bates et al. 2015). We used the species sampled as the random effect and season and principal component scores (see below) as the main effects. Next, we established a set of candidate models including the null (intercept only), global (all main effects and two-way interactions), all main effects models, all two-way main effects models, and all two-way main effects models with interaction. We assessed the candidate models using an information theoretic approach (Burnham and Anderson 2002) using the R package AICcmodavg (Mazerolle 2015). We then used the R package effects (Fox 2003) to determine how the presence/absence of Bd was affected by our main effects.
To analyze the intensity data, we first eliminated all Bd negative individuals from the dataset, then z-transformed our intensity estimates so they were centered and scaled by standard deviation units. We then followed the same methods for establishing and assessing candidate models as with the probability of infection analysis, except we used general linear mixed-effects models. For both analyses, if our candidate set of models (0.95 cumulative akaike weights) contained multiple models (i.e., low resolution), we performed model averaging of the parameters using AICcmodavg (Mazerole 2015). We then assessed the parameter effects by examining whether or not confidence intervals bounded zero.
Results
Across all bases and combining the two sampling periods, the 681 swabs collected (from 28 species) produced 670 unequivocal results. Of the 28 species sampled, 15 tested positive for Bd (Table 1). Among salamanders, only one individual, a Taricha granulosa sampled at NRS Jim Creek, tested positive. Bd-positive frog species included one bufonid (Anaxyrus americanus), four hylids (Acris crepitans, A. gryllus, Pseudacris regilla, and P. ornata), and nine ranids (L. catesbeianus, L. clamitans, L. palustris, L. pipiens, L. septentrionalis, L. sphenocephalus, L. sylvaticus, Rana aurora, and R. sierrae).
The number of animals sampled per site ranged from 21 (MWTC Bridgeport) to 64 (NCTAMS Cutler). The overall PREV was 20.4% (137 positives) and ranged from zero to 58.7% across installations (Table 2). Mean INT was 3.21 (SE = 1.03) and ranged from 0.001 to 103.59 across swabs (Table 3).
For the early season sampling period, the number of animals sampled per site ranged from 6 (NRS Jim Creek) to 39 (NCTAMS Cutler). Of the 341 early season swabs collected, 333 produced unequivocal results. The overall early season PREV was 21% (70 positives) and ranged from zero to 71.4% across installations (Table 2). Mean INT was 5.43 (SE = 1.95) and ranged from 0.001 to 103.59 across swabs (Table 3).
For the late season sampling period, the number of animals sampled at each site ranged from 0 (MWTC Bridgeport) to 30 (Fort Hunter Liggett). Of the 340 late season swabs we collected, 337 produced unequivocal results. The overall late season PREV was 19.9% (67 positives) and ranged from 0% to 68% across installations (Table 2). Mean INT was 0.90 (SE = 0.45) and ranged from 0.05 to 28.22 (Table 3) across swabs. We did not include MWTC Bridgeport in the analysis because no samples could be obtained in the late season period.
Variable Reduction
Our results for the KMO test of sampling adequacy (score = 0.574) and Bartlett’s tests of sphericity (χ 2 = 298.67, df = 6, p ≪ 0.001) suggested variable reduction was warranted among latitude, longitude, average maximum 30-day temperature, and 30-day precipitation. Two components were retained which explained 66.9% of the cumulative variance (Table 4). Latitude and 30-day precipitation were best explained in PC1 with a positive association for latitude and a negative for precipitation when rotated (Table 4). Longitude and average maximum 30-day temperature were best explained in PC2 with positive associations when rotated and because we designated longitudes west of the prime meridian as negatives, more easterly latitudes represented a higher PC score (Table 4).
Bd Infection Prevalence
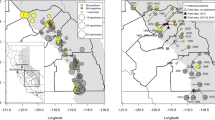
Of the eleven binary logistic mixed-effects models examined, the global model carried the lowest ΔAICc score and had an akaike weight of 1.00, suggesting it was the best model (Table 5). The strongest predictors in the global model were the parameters associated with the latitude/precipitation component and all interaction of effects and the intercept (Table 6). When examining the effects from the global model, we found season and the longitude/temperature components alone did not provide predictive power in discriminating the probability of Bd infection (Table 6; Fig. 2). Our latitude/precipitation component had strong resolution suggesting higher latitude with less recent precipitation had a greater probability of Bd infection (Table 6; Fig. 3). For the longitude/temperature component, we found more easterly sites with recent warmer temperatures had a higher probability of infection (Table 6; Fig. 3).
Predictive power greatly increased when accounting for the interactions among season, latitude/precipitation, and longitude/temperature (Table 6). There was a shift in the probability of Bd infection between seasons along a latitudinal/precipitation gradient (Table 6; Fig. 4). In the early season, a higher probability of infection was associated with higher latitudes with less recent precipitation (Table 6; Fig. 4). By the late season, a higher probability of infection was associated with lower latitudes and increased recent precipitation (Table 3; Fig. 4). A similar pattern held true for an east–west gradient with the highest probability of infection being associated with more easterly/recently warmer sites in the early season to more westerly/recently cooler sites in the later season (Table 6; Fig. 4).
The interactive effects of season and the latitude/climate component and longitude component on the probability of Bd infection in amphibians sampled from 14 military installations across the continental United States in 2011. The lighter shaded area represents the 95% confidence interval and the darker shaded area represents where confidence intervals overlap.
When examining the latitude/precipitation and longitude/temperature components, interaction was complex and shifted along both gradients (Table 6; Fig. 5). At low latitudes with less recent precipitation, we found that the probability of Bd infection decreased with more easterly sites with warmer recent temperatures (Table 6; Fig. 5). At higher latitudes with less recent precipitation, the pattern inverted where the highest probability of Bd infection increased with more westerly sites with cooler recent temperatures (Table 6; Fig. 5).
The interactive effects of the latitude/climate component and longitude component on the probability of Bd infection in amphibians sampled from 14 military installations across the continental United States in 2011. Lines represent the mean value of the latitudinal component evaluated at −2.16664, −1.24878, −0.33093, 0.586925, 1.50478, and 2.33085 (from top to bottom)
Bd Infection Intensity
Of the eleven mixed-effects general linear models, the global model performed the worst, although all models including an interaction term also performed poorly (Table 5). The null model performed the best. However, the resolution in discriminating between all other models that had combinations of season, latitude/climate, and longitude was low (Table 5). All of these models comprised the candidate set, and after model averaging of parameters, we found none of the main effects had any predictive power in determining the intensity of Bd infection (i.e., they all bounded zero; Table 6).
Discussion
Given the number of scientists studying Bd, as well as the individual and lab-based variations in swabbing techniques and sample transportation, storage, and analyses, it becomes useful to conduct frequent surveys by the same collaborators within a long-term monitoring framework and across large geographical areas to provide a template for comparison (Kriger et al. 2007). In this study, we surveyed for Bd prevalence and intensity on DoD installations over three north-to-south transects at widely separated longitudes across the United States using the same team of researchers and following the same protocol. Similar to the findings of Lannoo et al. (2011), we found Bd to be widespread spatially, with restricted military installations not naïve to Bd. Only three installations had no Bd-infected amphibians (NSA Mid-South, MWTC Bridgeport, Cape Canaveral); however, Bd is known from these regions in general (www.bd.maps.net; Olson et al. 2013).
One-fifth (20.4%) of the 670 amphibians we swabbed across the United States tested positive for the presence of Bd. Early season (21.0%) and late season (19.9%) prevalences were essentially identical. In contrast, overall infection intensities varied, averaging 3.21, with early season intensities being, on average, higher (5.93) than late season intensities (0.90). While these differences were not significant, they are consistent with our previous continental survey (Lannoo et al. 2011), and with the observation that animals exposed to warm and dry summer conditions can clear the infection (Woodhams et al. 2003; Kinney et al. 2011).
All of the species that tested positive for Bd in this investigation have tested positive in other studies (www.bd.maps.net; Lannoo et al. 2011). Similarly, other trends in our data have been seen in our previous research. For example, Bd prevalence rates increased from west to east on the North American continent (Lannoo et al. 2011). Interestingly, the relationship between the mean proportion of Bd infections differed depending on season. In the early season, there was no trend associated with increasing temperature and precipitation and decreasing (more southerly) latitudes; in contrast, during the late season, the mean proportion of infected individuals decreased with increasing temperature and precipitation and decreasing latitudes.
Our prevalence average of 20.4% aligns with other multispecies Bd surveys across the United States. For example, in the Pacific Northwest, Adams et al. (2007) reported a Bd prevalence of 21.5%. Working in the East, Tupper et al. (2011) found 18% prevalence in anurans on Cape Cod; Davidson and Chambers (2011) found an 18% Bd prevalence in Virginia; and Huang and Wilson (2013) found a 19% prevalence in the Piedmont and Blue Ridge ecoregions of northern Georgia. In the Midwest, Krynak et al. (2012) found a 20.2% Bd prevalence in Ohio, while Rodriguez et al. (2009) found a 22% prevalence working in northern Minnesota. Of course, other studies find higher or lower Bd prevalences depending on species sampled [e.g., Red-spotted Newts seem to have high prevalences (Groner and Relyea 2010; Bletz and Harris 2013)], region of the country (Chestnut et al. 2008; Saenz et al. 2010; Tatarian and Tatarian 2010; Gaertner et al. 2012), altitude (Hasken et al. 2009), life history stage (Kinney et al. 2011), season sampled (Kinney et al. 2011; Savage et al. 2011), and hydrologic regime (Terrell et al. 2014).
Our results suggest a pattern much more complicated than our hypotheses presupposed. Instead of prevalences and intensities being linked and varying in geographically and seasonally predictable ways, prevalences and intensities were uncoupled (probably because intensities were so low), and the predictive power of our prevalence models greatly increased when accounting for the interactions among season, latitude/precipitation, and longitude/temperature. In particular, we observed a shift in the probability of Bd infection between seasons along a latitudinal/precipitation gradient (Table 6; Fig. 4), as follows. In the early season, an infection prevalence was associated with higher latitudes with less recent precipitation (Table 6: Fig. 4), supporting hypothesis one. By the late season, however, prevalence was associated with lower latitudes and increased recent precipitation (Table 3; Fig. 4), refuting hypotheses one and three. A similar pattern held for the east–west gradient, with the highest prevalences associated with more easterly/recently warmer sites in the early season, supporting hypothesis one, then shifting to more westerly/recently cooler sites in the later season (Table 6; Fig. 4), refuting hypothesis one. Prevalences were equivalent between early (20.4%) and late (21%) season samples, refuting hypothesis two, while intensities increased from 3.21 to 5.43 (SE = 1.95 (Tables 1 and 2), also refuting hypothesis two.
We found Bd intensity to be comparatively low (Table 1). Further, we found that after model averaging, none of the main effects had predictive power (i.e., they all bounded zero; Table 6). The lack of effects on Bd intensity is likely the result of a combination of low observed intensity values and little variation among samples. Vredenburg et al. (2010) have suggested that at an intensity of about 10,000 zoospore equivalents, Bd shifts from being an infection to the disease, chytridiomycosis (see also Kinney et al. 2011). Therefore, our sampling did not reveal infection intensities high enough to trigger die-offs. However, with an average of one in five amphibians at these bases infected (the mean PREV was 20.4%), the potential exists for chytridiomycosis flare-ups should environmental conditions be conducive. Terrell et al. (2013) have shown that, in the face of varying climactic conditions, Bd prevalences remain relatively constant while intensities vary. Under the cool, wet conditions favored by the fungus, Bd infections can intensify and become fatal.
It has been suggested that habitat type may be an important factor potentially influencing Bd prevalence or intensity that certain aquatic habitats, especially cooler, shaded lentic ones, might be more conducive to Bd survival and therefore transmission among amphibians (Woodhams et al. 2003; Berger et al. 2004; Rohr and Raffel 2010; Lannoo et al. 2011). Although the scope of our project did not address this complicated, multifaceted environmental question, we do acknowledge that some of the variation in our results could be attributable to sampling amphibians in a variety of habitats.
This study provides important insight into the latitudinal and seasonal precipitation factors which affect Bd. Military installations in the United States have a tremendous density of native amphibians and have provided an important network of outdoor laboratories to sample for Bd across the North American continent. In the face of continuing environmental alterations brought about by habitat-independent factors such as climate change, disease dynamics, and invasive species, military installations may provide valuable controls to assess the effects of these factors on populations, communities, and ecosystems.
References
Adams MJ, Galvan S, Reinitz D, Cole RA, Payre S, Hahr M, Govindarajulu P (2007) Incidence of the fungus Batrachochytrium dendrobatidis in amphibian populations along the Northwest Coast of North America. Herpetological Review 38:430–431.
Bates D, Maechler M, Bolker BM, Walker S (2015) lme4: Linear mixed-effects models using Eigen and S4_. R package version 1.1-8. http://CRAN.R-project.org/package=lme4.
Becker CG, Zamudio KR (2011) Tropical amphibian populations experience higher disease risk in natural habitats. Proceedings of the National Academy of Science 108(24):9893–9898.
Becker CG, Rodriguez D, Toledo LF, Longo AV, Lambertini C, Corrêa DT, Leite DS, Haddad CF, Zamudio KR (2014) Partitioning the net effect of host diversity on an emerging amphibian pathogen. Proceedings of the Royal Society B: Biological Sciences 281(1795):20141796.
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (1998) Chytridiomycosis causes amphibian mortality associated with population decline in the Rain Forests of Australia and Central America. Proceedings of the National Academy of Science 95:9031–9036.
Berger L, Speare R, Hines HB, Marantelli G, Hyatt AD, McDonald KR, Skerratt LF, Olsen V, Clarke JM, Gillespie G, Mahony M, Sheppard N, Williams C, Tyler MJ (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Australian Veterinary Journal 82:434–439.
Bletz MC, Harris RN (2013) Occurrence of Batrachochytrium dendrobatidis in Notophthalmus viridescens in Northwestern Virginia, USA. Herpetological Review 44:257–259.
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian sample using real-time Taqman PCR assay. Disease of Aquatic Organisms 60:141–148.
Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungus pathogen of amphibians. Proceedings of the National Academy of Sciences 107:9695–9700.
Burnham KP, Anderson DR (2002) Model selection and multimodal inference: A practical information theoretic approach. 2nd ed. Springer, New York, New York.
Chestnut T, Johnson JE, Wagner RS (2008) Results of amphibian chytrid (Batrachochytrium dendrobatidis) sampling in Denali National Park, Alaska, USA. Herpetological Review 39:202–204.
Davidson SR, Chambers DL (2011) Occurrence of Batrachochytrium dendrodatidis in amphibians of Wise County, Virginia, USA. Herpetological Review 42:214–216.
DiRosa I, Simoncelli F, Fagotti A, Pascolini R (2007) The proximate cause of amphibian declines? Nature 447: E4–E5.
Fisher MC, Garner TWJ, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Ann Rev Microbiol 63: 291–310.
Fox J (2003) Effect displays in R for generalized linear models. Journal of Statistical Software. 8:1–27.
Gaertner JP, Brown DJ, Mendoza JA, Forstner MRJ, Bonner T, Hahn D (2012) Geographic variation in Batrachochytrium dendrobatidis occurrence among populations of Acris crepitans blanchardi in Texas, USA. Herpetological Review 43:274–278.
Gaertner JP, Forstner MRJ, O’Donnell L, Hahn D (2009) Detection of Batrachochytrium dendrobatidis in endemic salamander species from Central Texas. EcoHealth 6:20–26.
Groner ML, Relyea RA (2010) Batrachochytrium dendrobatidis is present in northwest Pennsylvania, USA, with high prevalence in Notophthalmus viridescens. Herpetological Review 41:462–465.
Hasken J, Newby JL, Grelle AM, Boling J, Estes J, Garey LK, Wilmes T, McKee R, Gomez D, Jackson T, Gibson N, Davinroy E, Montgomery DE, Kelrick MJ (2009) Evaluation of chytrid infection level in a newly discovered population of Anaxyrus boreas in the Rio Grande National Forest, Colorado, USA. Herpetological Review 40(4):426–428.
Huang R, Wilson LA (2013) Batrachochytrium dendrobatidis in amphibians of the Piedmont and Blue Ridge provinces in northern Georgia, USA. Herpetological Review 44:95–98.
Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Krieger K, Hero M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Colling A (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 73:175–192.
IUCN Red List 2014. http://www.iucnredlist.org/initiatives/amphibians/analysis. Accessed 24 March 2014
Johnson ML, Speare R (2005) Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Diseases of Aquatic Organisms 65:181–186.
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–993.
Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ (2011) Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg’s “10,000 zoospore rule.” PLoS ONE 6(3): e16708. doi:10.1371/journal.pone.0016708.
Kriger KM, Pereoglou F, Hero J-M. 2007. Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conservation Biology 21:1280–1290.
Krynak TJ, Robison TL, Scott JJ (2012) Detection of Batrachochytrium dendrobatidis in amphibian populations of northeast Ohio. Herpetological Review 43:87–89.
Lannoo, MJ (ed.). 2005. Amphibian declines: The conservation status of United States species. University of California Press, Berkeley.
Lannoo MJ, Petersen C, Lovich RE, Nanjappa P, Phillips C, Mitchell, J, McAllister, I (2011) Do frogs get their kicks on Route 66? Continental U.S. transect reveals spatial and temporal patterns of Batrachochytrium dendrobatidis infection. PLoS ONE 6(7):e22211. doi:10.1371/journal.pone.0022211.
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Ecology 103:3165–3170.
Liu X, Rohr JR, Li Y (2013) Climate, vegetation, introduced hosts and trade shape a global wildlife pandemic. Proceedings of the Royal Society B: Biological Sciences 280(1753):20122506.
Longcore J, Pessier A, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227.
Longo AV, Burrowes PA, Joglar RL (2010) Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Diseases of Aquatic Organisms 92:253–260.
Mazerolle MJ (2015) AICcmodavg: Model selection and multimodal inference based on (Q)AIC(c). R package version 2.0-3. http://CRAN.R-project.org/package=AICcmodavg
Murray KA, Skerratt LF, Speare R, McCallum H (2009) Impact and dynamics of disease in species threatened by the amphibian chytrid fungus, Batrachochytrium dendrobatidis. Conservation Biology 32:1242–1252.
Murray KA, Retallick RWR, Puschendorf R, Skerratt LF, Rosauer D, Mccallum HI, Berger L, Speare R, Vanderwal J (2011) Assessing spatial patterns of disease risk to biodiversity: implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. Journal of Applied Ecology 48(1):163–173.
Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, The Bd Mapping Group, Fisher MC (2013) Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PloS One 8(2):e56802.
Ouellet M, Mikaelian I, Pauli BD, Rodrigues J, Green DM 2005. Historical evidence of widespread chytrid infection in North American amphibian populations. Conservation Biology 19:1431–1440.
Pessier AP, Mendelson III JR (2010) A manual for control of infectious diseases in amphibian survival assurance colonies and reintroduction programs. Proceedings from a Workshop 16–18 February 2009, San Diego Zoo, San Diego, California, USA
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15.
Rachowicz LJ, Knapp RA, Morgan JAT, Stice MJ, Vredenburg VT, Parker JM, Briggs CJ (2006) Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87:1671–1683.
Raffel TR, Michel PJ, Sites EW, Rohr JR (2010) What drives chytrid infections in newt populations? Associations with substrate, temperature, and shade. EcoHealth 7(4):526–536.
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Retallick RWR, McCallum H, Speare R (2004) Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biology 2:1965–1971.
Rodriguez EM, Gamble T, Hirt MV, Cotner S (2009) Presence of Batrachochytrium dendrobatidis at the headwaters of the Mississippi River, Itasca State Park, Minnesota, USA. Herpetological Review 40:48–50.
Rohr JR, Raffel TR (2010) Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proceedings of the National Academy of Science USA 107(18):8269–8274.
Rohr JR, Halstead NT, Raffel TR (2011) Modeling the future distribution of the amphibian chytrid fungus: the influence of climate and human-associated factors. Journal of Applied Ecology 48(1):174–176.
Savage AE, Zamudio KR, Sredl MJ (2011) Disease dynamics vary spatially and temporally in a North American amphibian. Biological Conservation 144(6):1910–1915. doi:10.1016/j.biocon.2011.03.018.
Seanz D, Kavanagh BT, Kwiatkowski MA (2010) Batrachochytrium dendrobatidis detected in amphibians from national forests in eastern Texas, USA. Herpetological Review 41:47–49.
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134.
Stein BA, Scott C, Benton N (2008) Federal Lands and Endangered Species: The Role of Military and Other Federal Lands in Sustaining Biodiversity. BioScience 58:339–347.
Stevenson LA, Alford RA, Bell SC, Roznik EA, Berger L, et al. (2013) Variation in Thermal Performance of a Widespread Pathogen, the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis. PLoS ONE 8(9):e73830. doi:10.1371/journal.pone.0073830.
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW (2004) Status and trends of amphibians and extinctions worldwide. Science 306:1783–1786.
Tatarian P, Tatarian G (2010) Chytrid infection of Rana draytonii in the Sierra Nevada, California, USA. Herpetological Review 41:325–327.
Terrell VCK, Engbrecht NJ, Pessier AP, Lannoo MJ (2014) Drought reduces chytrid fungus (Batrachochytrium dendrobatidis) infection intensity and mortality but not prevalence in adult Crawfish Frogs (Lithobates areolatus). Journal of Wildlife Diseases 50:56–62.
Tupper TA, Streicher JW, Greenspan SE, Timm BC, Cook RP (2011) Detection of Batrachochytrium dendrobatidis in anurans of Cape Cod National Seashore, Barnstable County, Massachusetts, USA. Herpetological Review 42:62–65.
Venesky MD, Liu X, Sauer EL, Rohr JR (2013) Linking manipulative experiments to field data to test the dilution effect. Journal of Animal Ecology, 83(3):557–565.
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Science. 107(21):9689-94. doi:10.1073/pnas.0914111107.
Woodhams DC, Alford RA, Marantelli G (2003) Emerging disease of amphibians cured by elevated body temperature. Diseases of Aquatic Organisms 55:65–67.
Woodhams DC, Alford RA, Maraentelli G (2005) Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conservation Biology 19:1449–1459.
Acknowledgements
Support for this project (10-426) came from the Department of Defense Legacy Resource Management Program https://www.dodlegacy.org.legacy/index.aspx. The authors thank Chris Bucciantini, David Davis, Jackie Hancock, Ethan Kessler, John Maile, Jeff Phillips, and Dan Wylie for collecting specimens. For providing access to their installations and support in the field, we thank David Beckmann, Fort McCoy; Jay Brezinka, Camp Ripley; Chris Bucciantini, Naval Air Station Meridian; Angy Chambers, Cape Canaveral Air Force Station; Liz Clark, Fort Hunter Liggett; David Davis, Shaw Air Force Base; Chad Garber, Marine Corps Base, Camp Lejeune; Jackie Hancock, Fort Hunter Liggett; Jim Heide, Naval Support Activity Mid-South; Andrew Irvine, Marine Corps Mountain Warfare Training Center; Jim Lynch, Fort Lewis; Jeff Mach, Camp Rilea Armed Forces Training Facility; John Maile, Camp Ripley; John Miller, Jim Creek Naval Radio Station; Kari Moore, Naval Computer and Telecommunications Station, Cutler; Cindy Nolan, Scott Air Force Base; John Richardson, Fort Lewis; Bill Rogers, Marine Corps Base, Camp Lejeune; Kristen Sharp, Fort Dix; Roger Smith, Fort Dix; Linda Wagoner, Jim Creek Naval Radio Station; Rob Williamson, Naval Support Activity Mid-South.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersen, C.E., Lovich, R.E., Phillips, C.A. et al. Prevalence and Seasonality of the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis Along Widely Separated Longitudes Across the United States. EcoHealth 13, 368–382 (2016). https://doi.org/10.1007/s10393-016-1101-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-016-1101-4