Abstract
Background
The association between tobacco smoking and tuberculosis (TB) is increasingly coming to light and the literature is laden with the evidence of this association. However, only a few observational studies specifically investigated the association between smoking and TB treatment outcomes.
Aim and methods
The present study aims to determine the prevalence of smoking among TB patients in Penang and to compare the treatment outcomes between smoking and non-smoking TB patients. A retrospective estimate of smoking prevalence among patients with TB and a retrospective cohort study comparing smoking and non-smoking TB patients with regards to treatment outcomes was conducted. The data were extracted from medical records of newly diagnosed TB patients who registered at a chest clinic at a tertiary care hospital in the state of Penang, Malaysia from January 2006 to June 2008.
Results
The prevalence of ever-smoking among TB patients was 53.4%. Smoking was significantly associated with male gender, alcohol use, and intravenous drug use (IVDU). Ever smokers had increased likelihood of treatment failure (OR 7.48), default (OR 7.17) and were less likely to be cured (OR 0.34). After controlling for the effects of confounders using multivariate logistic regression, ever smokers were still less likely to be cured (aOR 0.31) and more likely to default treatment (aOR 3.24). Dying from TB did not reach statistical significance.
Conclusion
Prevalence of smoking was high among TB patients in Malaysia. This study further reaffirms that smoking is an independent predictor of poor TB treatment outcomes and prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, studies are confirming that smoking is an established risk factor not only for cancers, coronary heart disease, and stroke but also in the case of being infected with Mycobacterium tuberculosis, developing tuberculosis (TB) disease and dying from it (Hassmiller 2006; Bates et al. 2007). Most of these studies indicate that exposure to tobacco smoke substantially increases the risk of TB, independent of other risk factors such as intravenous drug use (IVDU), alcohol consumption and socioeconomic status (Altet-Gomez et al. 2005; Ariyothai et al. 2004; Leung et al. 2003, 2004). Tobacco is a leading cause of preventable premature death worldwide, whereas TB is a major cause of morbidity and mortality especially in the developing world. Globally, 9.4 million incident cases of TB and 1.3 million deaths occurred in 2008 (World Health Organization 2010). In Malaysia, in 2008, 17,506 new cases were registered with all forms of TB. Of these, 10,441 were infectious (Communicable Disease Section and Disease Control Division 2008).
Despite the increasing body of evidence which supports the strong association between tobacco smoking and TB, there are only a few observational studies that specifically investigated the association between smoking and TB treatment outcomes. Most of the published studies investigated potential risk factors for poor TB treatment outcomes in general (Chang et al. 2004; Santha et al. 2002; Thomas et al. 2005). In addition, the prevalence of smoking among TB patients is not widely documented. The present study was conducted to determine the prevalence of smoking among TB patients in Penang, Malaysia and to investigate the impact of active tobacco smoking on TB treatment outcomes.
Methods
Study design
The study comprised of: (1) an estimation of prevalence of smoking, by a retrospective review of patients’ medical records who registered for TB treatment in Penang, Malaysia between January 1 2006 and June 30 2008; (2) a retrospective cohort study comparing smoking and non-smoking TB patients with regard to treatment outcomes.
Study area and population
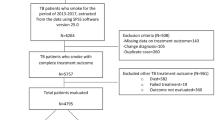
Newly diagnosed cases of TB of both sexes, above 15 years of age whose smoking status was documented and registered at the chest clinic of Penang General Hospital during the period 1 January 2006 to 30 June 2008 were included in the study. Category II (TB relapse, treatment failure and treatment default), category III (chronic cases) and HIV co-infected TB patients were excluded from the study. Furthermore, other category I cases comprised of pregnant women, patients with diabetes and those with incomplete data were also excluded. Figure 1 displays the procedure for selecting patients who fulfilled the eligibility criteria for the study. Owing to the main objective of the study, patients were grouped into either ever smokers (those who currently smoke cigarettes at the time of diagnosis of TB or who had previously quit smoking) or never smokers (those who never smoked or who have smoked less than 100 cigarettes during their lifetime). Standard definitions were used to evaluate TB treatment outcomes (Raviglione 2001). The outcomes of TB treatment were categorized as: cured, treatment completion, treatment default, treatment failure and death. A TB patient is defined as cured if he/she is smear-negative at/or 1 month prior to the completion of treatment and on at least one previous occasion. In addition, a patient who has completed treatment but without proof of cure is considered as a case of treatment completion. A patient whose treatment was interrupted for 2 months or more is referred to as a case of treatment default, whereas death was defined as a patient who dies for any reason during the course of treatment. Two hundred and seventy-four ever smokers were selected and considered to be the exposed group and 250 never smokers were selected and considered to be the non-exposed group.
Data collection
The eligible cases of TB patients were identified by reviewing the TB registration book at the chest clinic. Patients’ socio-demographic data, co-morbidities and clinical characteristics were collected by a retrospective review and assessment of the patients’ medical records for the period of TB treatment in order to evaluate treatment outcomes. A designed profoma was used to extract data from the time of diagnosis of TB until the end of treatment. This study was approved by the ethics committee of the Clinical Research Center, National Institutes of Health (NIH), Ministry of Health Malaysia.
Statistical analysis
Descriptive analyses were performed for quantitative (continuous) variables by calculating mean ± standard deviation (SD), while percentages and frequencies were determined for qualitative (categorical) variables. To compare the proportions between ever and never smokers, Chi-square (χ 2) test was applied, while the Mann-Whitney U test was used to evaluate statistical differences for quantitative variables that were not normally distributed. Ever-smoking TB patients were considered to be the exposed group, whereas never smokers were considered to be the non-exposed group. To examine whether tobacco smoking has contributed to poor treatment outcomes in TB patients, univariate analysis was performed using χ 2 test to estimate crude odds ratio (OR) and 95% confidence interval (CI). Furthermore, binary multiple logistic regression analysis was used to control for the effects of confounders (age, sex, alcohol use, IVDU and history of chronic diseases).
Results
Between 1 January 2006 to 30 June 2008, 1,270 patients comprised of all categories of TB were newly diagnosed and registered at the chest clinic of Penang General Hospital for TB treatment. Of these, 253 patients were excluded from the smoking prevalence analysis either because their smoking status was unknown or the diagnosis was changed during the period of treatment, giving rise to 1,017 TB patients whose smoking status was determined. The rate of ever smoking was higher than that of never smoking (53.4% vs. 46.6%, respectively). Figure 2 illustrates the prevalence of smoking among the patients diagnosed with TB.
Of the 1,017 TB patients whose smoking status was known, 493 were excluded from the retrospective cohort analysis, as they did not satisfy the eligibility criteria (Fig. 1). As a result, 524 TB patients were included in the analysis (274 ever smokers vs. 250 never smokers). Of this; 121 patients (23.1%) were cured, 291 (55.5%) completed treatment, 9 (1.7%) failed treatment, 40 (7.6%) defaulted and 63 (12.0%) died during the period of therapy. Ever-smoking TB patients compared with never smokers were significantly associated with older age and male gender. They also had higher proportion of other risk factors: alcohol consumption (22.3%; 61/274 vs. 1.2%; 3/250) and IVDU (17.5%; 48/274 vs. 0.8%; 2/250). Furthermore, the smoking status of TB patients was significantly associated with their race and initial Mantoux test. Table 1 shows the demographics and other clinical characteristics of ever- versus never-smoking TB patients.
In addition, ever-smoking TB patients were about seven times more likely to fail or default treatment (OR 7.489, CI 0.93–60.30 and OR 7.176, CI 2.76–18.62, respectively). They were also less likely to be cured from TB compared to never smokers (OR 0.342, CI 0.21–0.49) (Table 2). Using multivariate binary logistic regression to control for the effects of confounders (age, sex, alcohol consumption, IVDU and history of chronic diseases including cyanotic heart disease, liver disease, chronic renal failure, malabsorption syndrome, cancer, steroids treatment and others), ever smokers were still significantly less likely to be cured (aOR 0.312, CI 0.17–0.57) and more likely to default treatment (aOR 3.249, CI 1.01–10.45) compared to never smokers. The risk of a smoker to die from TB or to fail treatment was statistically not significant (Table 2). Unfortunately, in this retrospective study, smoking history was not recorded in sufficient detail to allow the number of cigarettes consumed per day among ever smokers to be calculated in a sizeable number; therefore no dose response relationship could be evaluated.
Discussion
In the present study, the prevalence of ever smoking was higher than that of never smoking among TB patients. Consistent with our results, a previous study conducted in Malaysia reported a higher prevalence rate of ever smokers compared with never smokers among TB patients (54.22% vs. 45.78%; Awaisu et al. 2010). These findings corroborate the results of other studies done elsewhere in the world, which supports the reported higher prevalence of smoking among patients with TB when compared with non-TB controls or the general population (Jee et al. 2009; Lin et al. 2009; Wang and Shen 2009 ). The finding that smoking was significantly associated with older age and male gender could account for the differences observed in the rate of TB between men and women. Previous studies have found that the differences in age and sex among TB patients were due to the smoking factor (Chan et al. 1995; Leung et al. 2002; Yu et al. 1988). A high proportion of IVDU were among the ever-smokers group when compared to never smokers (17.5%; 48/274 vs. 0.8%; 2/250, respectively). In China, ever-smoking TB patients were significantly associated with IVDU (P < 0.001) (Leung et al. 2003). Furthermore, excess alcohol use has clinical features associated with greater infectiousness of M. tuberculosis (Fiske et al. 2008; Lewis and Chamberlain 1963). Consistent with the previous literature, the current study found that alcohol use was significantly associated with ever-smoking TB patients.
The majority of TB patients in our cohort received 2EHRZ treatment for the intensive phase, followed by other modalities than those usually recommended, 2SHRZ and 2HRZ (53.6, 30.2, 8.0 and 7.6%, respectively). While for maintenance phase, the majority of TB patients received other modalities than those usually recommended, followed by 4H²R², 4HR and 4S²H²R² (45.4, 34.5, 5 and 0.4%, respectively).
In the cohort studied, the estimated cure rate was low. Low cure rates could actually increase the rate of transmission of the disease, and hence increased the number of cases (Dye et al. 1998 ). The rate of default was higher among ever-smoking TB patients compared to never smokers (12.8%; 35/274 vs. 2.0%; 5/250, respectively). After controlling for confounders including age, sex, alcohol use, IVDU and history of chronic disease, ever smokers were three times more likely to default from treatment compared to never smokers (aOR 3.249, CI 1.01–10.45). Previous studies have equally documented tobacco smoking as one of the predictors and risk factors significantly associated with higher default rate in TB care settings (Chang et al. 2004; Santha et al. 2002). Interestingly, a study from Hong Kong also showed that the risk of default from TB treatment under directly observed therapy could be accurately predicted by smoking (OR 3.00, 95% CI 1.41–6.39, P = 0.004; Chang et al. 2004). Patients who default treatment are at greatest risk for developing drug resistance and for spreading TB in the community (Shamaei et al. 2009). Treatment default was frequent among TB patients in Penang; therefore, interventions to improve treatment compliance in TB patients are necessary. Social support and incentive programs should be inclusively attainable to all patients considered at risk for treatment default. Improving compliance among smoking TB patients is a great challenge and should be addressed by looking for support from families and social organizations as well as providing smoking cessation interventions. Quit and win campaign in Italy is an effective approach to promote smoking cessation intervention and it could be used in our setting to encourage patients to quit smoking (Gianti et al. 2007).
Furthermore, we found that treatment failure was more common among current and ex-smokers (ever smokers) compared to non-smokers (2.9%; 8/274 vs. 0.4%; 1/250). Although, ever smokers were more likely to fail treatment (OR 7.489, CI 0.93–60.30), after controlling for covariates, the likelihood ratio failed to reach statistical significance (aOR 13.593, 95% CI 0.59–308.69). In contrast, a study from South India showed that smoking was significantly associated with treatment failure (Santha et al. 2002). The small number of patients who failed treatment decreases the power of statistical analysis and, probably was the reason why tobacco smoking was not found to be a risk factor for treatment failure after controlling for the effect of confounders in this study.
A study from India documented that the risk of dying from TB was about four times greater among smokers than non-smokers, and 61% of TB mortality has been attributed to smoking (P = 0.004; Gajalakshmi et al. 2003). Additionally, a study on mortality in relation to smoking showed that smoking TB patients were three times more likely to die from TB compared to non-smokers (Doll and Peto 1976). However, in the present study, we did not find a greater risk of mortality in ever-smoking TB patients; this may be partly explained by ease of access to healthcare facilities in Malaysia which is available to all patients registered for TB treatment and the subsidized health services provided by the government. Other studies also found that smoking was not associated with risk of dying from TB (Altet-Gomez et al. 2005; Santha et al. 2002). However, this divergence of observations calls for a more comprehensive investigation. It is hypothetical that, if smoking causes a rapid TB deterioration, then in communities where access to healthcare is constrained, the lives of smoking TB patients would possibly be at high risk. Our results further support the hypothesis of an increased susceptibility of smokers to poor treatment outcomes and prognosis. If smoking increases the risk of rapid disease deterioration, there is undoubtedly an immunopathological basis for the association, which calls for an integrated TB-tobacco intervention (Maurya et al. 2002; Pai et al. 2007; Yach 2000).
This study was subject to the potential limitations that are inherent to any retrospective study. First, there were potentials for information bias such as missing, unreported or incomplete clinical data in medical records; also it was unknown whether smoking status was recorded truthfully which may affect the differences observed between ever-smoking and never-smoking TB patients. Second, there was no evidence that the smoking status of the patients were biochemically verified. Third, it is difficult to completely exclude the effects of all confounders in a retrospective study, although the differences between ever smokers and never smokers in terms of treatment outcomes persisted after controlling for age, sex, history of chronic disease, alcohol use and IVDU. Finally, the results of the current study may not be generalizable to all TB patients who are smokers in Malaysia since the study patients were selected from a single tertiary care referral hospital.
Conclusions
The prevalence of smoking was high among TB patients in Malaysia. The results of the present study demonstrate an association between smoking and outcomes of TB. The study further reaffirms that tobacco smoking is a predictor of poor TB treatment outcomes. Therefore, smoking cessation interventions should be offered to all TB patients who are smokers when they are undergoing TB treatment. National TB programs should vigorously integrate tobacco dependence treatment into the management of TB and should provide capacity building for healthcare providers caring for TB patients.
References
Altet-Gomez M, Alcaide J, Godoy P, Romero M, Hernandez Del Rey I (2005) Clinical and epidemiological aspects of smoking and tuberculosis: a study of 13038 cases. Int J Tuberc Lung Dis 9(4):430–436
Ariyothai N, Podhipak A, Akarasewi P, Tornee S, Smithtikarn S, Thongprathum P (2004) Cigarette smoking and its relation to pulmonary tuberculosis in adults. Southeast Asian J Trop Med Public Health 35(1):219–227
Awaisu A, Nik Mohamed MH, Abd Aziz N, Syed Sulaiman SA, Mohamad Noordin N, Muttalif AR et al (2010) Tobacco use prevalence, knowledge, and attitudes among newly diagnosed tuberculosis patients in Penang State and Wilayah Persekutuan Kuala Lumpur, Malaysia. Tob Induc Dis 8(1):3
Bates M, Khalakdina A, Pai M, Chang L, Lessa F, Smith K (2007) Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med 167(4):335–342
Chan C, Woo J, Or K, Chan R, Cheung W (1995) The effect of age on the presentation of patients with tuberculosis. Tuber Lung Dis 76(4):290–294
Chang K, Leung C, Tam C (2004) Risk factors for defaulting from anti-tuberculosis treatment under directly observed treatment in Hong Kong. Int J Tuberc Lung Dis 8(12):1492–1498
Communicable Disease Section, Disease Control Division (2008) Annual report of tuberculosis. Ministry of Health Malaysia, Kuala Lumpur
Doll R, Peto R (1976) Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J 2(6051):1525–1536
Dye C, Garnett GP, Sleeman K, Williams BG (1998) Prospects for worldwide tuberculosis control under the WHO DOTS strategy: directly observed short-course therapy. Lancet 352(9144):1886–1891
Fiske CT, Hamilton CD, Stout JE (2008) Alcohol use and clinical manifestations of tuberculosis. J Infect 57(5):385–391
Gajalakshmi V, Peto R, Kanaka T, Jha P (2003) Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43 000 adult male deaths and 35 000 controls. Lancet 362(9383):507–515
Gianti A, Vianello S, Casinghini C, Roncarolo F, Ramella F, Maccagni M et al (2007) The" Quit and Win" campaign to promote smoking cessation in Italy: results and one year follow-up across three Italian editions (2000–2004). Ital J Public Health 5:59–64
Hassmiller K (2006) The association between smoking and tuberculosis. Salud Pública de México 48:201–216
Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM (2009) Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol 170(12):1478–1485
Leung C, Yew W, Chan C, Chau C, Tam C, Lam C et al (2002) Tuberculosis in older people: a retrospective and comparative study from Hong Kong. Geriatrics 50(7):1219–1226
Leung C, Yew W, Chan C, Tam C, Lam C, Chang K et al (2003) Smoking and tuberculosis in Hong Kong. Int J Tuberc Lung Dis 7(10):980–986
Leung C, Li T, Lam T, Yew W, Law W, Tam C et al (2004) Smoking and tuberculosis among the elderly in Hong Kong. Am J Resp Crit Care 170(9):1027–1033
Lewis J, Chamberlain D (1963) Alcohol consumption and smoking habits in male patients with pulmonary tuberculosis. Brit Med J 17(3):149–152
Lin H-H, Ezzati M, Chang H-Y, Murray M (2009) Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Resp Crit Care 180(5):475–480
Maurya V, Vijayan V, Shah A (2002) Smoking and tuberculosis: an association overlooked. Int J Tuberc Lung Dis 6(11):942–951
Pai M, Mohan A, Dheda K, Leung CC, Yew WW, Christopher DJ et al (2007) Lethal interaction: the colliding epidemics of tobacco and tuberculosis. Expert Rev Anti Infect Ther 5(3):385–391
Raviglione M (2001) Revised international definitions in tuberculosis control. Int J Tuberc Lung Dis 5(3):213–215
Santha T, Garg R, Frieden T, Chandrasekaran V, Subramani R, Gopi P et al (2002) Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis 6(9):780–788
Shamaei M, Marjani M, Chitsaz E, Kazempour M, Esmaeili M, Farnia P et al (2009) First-line anti-tuberculosis drug resistance patterns and trends at the national TB referral center in Iran: eight years of surveillance. Int J Infect Dis 13(5):e236–e240
Thomas A, Gopi P, Santha T, Chandrasekaran V, Subramani R, Selvakumar N et al (2005) Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis 9(5):556–561
Wang J, Shen H (2009) Review of cigarette smoking and tuberculosis in China: intervention is needed for smoking cessation among tuberculosis patients. BMC Public Health 9(1):292
World Health Organization (2010) Global Tuberculosis Control: a short update to the 2009 report. WHO, Geneva
Yach D (2000) Partnering for better lung health: improving tobacco and tuberculosis control. Int J Tuberc Lung Dis 4(8):693–697
Yu G, Hsieh C, Peng J (1988) Risk factors associated with the prevalence of pulmonary tuberculosis among sanitary workers in Shanghai. Tubercle 69(2):105–112
Acknowledgements
The authors are grateful to the staff of the chest clinic at Penang General Hospital for their support in supplying the data of this study.
Conflict of interest
The authors have no competing interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dujaili, J.A., Syed Sulaiman, S.A., Awaisu, A. et al. Outcomes of tuberculosis treatment: a retrospective cohort analysis of smoking versus non-smoking patients in Penang, Malaysia. J Public Health 19, 183–189 (2011). https://doi.org/10.1007/s10389-010-0365-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-010-0365-3