Abstract
We report a case of diffuse esophageal spasm (DES) successfully treated by esophageal long myotomy and Dor’s fundoplication. The patient was a 52-year-old man with a history of hepatitis C and severe chronic heart failure due to hypertensive cardiomyopathy. He had also undergone hemodialysis for chronic renal failure for 10 years. He had complained of dysphagia for 10 years. Diffuse esophageal spasm was diagnosed by fluoroscopy and esophageal manometry. We performed esophageal long myotomy through the opened hiatus and Dor’s fundoplication. The upper extent of the myotomy was confirmed by intraoperative endoscopic ultrasonography used to detect muscle thickening and low compliance of the esophageal wall. The procedure and postoperative recovery were uneventful, and the patient’s symptoms were relieved. This approach appears to be a potentially useful means of treating severe symptoms of DES resistant to conservative therapy, with ultrasound endoscopy being a helpful means of confirming the extent of esophageal myotomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse esophageal spasm (DES) is an idiopathic motility disorder that causes uncontrolled contractions of the esophagus. Reports on the surgical treatment of DES in Japan are rare. Here we present the case of a patient with DES successfully treated by esophageal long myotomy through the open hiatus and Dor’s fundoplication. The upper extent of the myotomy was confirmed by intraoperative endoscopic ultrasonography, used to detect thickening of the muscle and low compliance in the esophageal wall.
Case report
A 52-year-old man with 10-year history of solid food dysphagia and frequent regurgitation was referred to our hospital. Contrast-enhanced computed tomography (CT) demonstrated esophageal wall thickening, and a fluoroscopic esophagram showed esophageal dysmotility.
On admission, the patient was unable to take liquid orally. He had many comorbidities: hepatitis C, chronic heart failure secondary to hypertensive cardiomyopathy with an ejection fraction of 34 %, atrial fibrillation, and hemodialysis due to chronic renal failure. Laboratory data showed anemia as well as elevated creatinine and blood urea nitrogen as a likely consequence of chronic renal failure. Serum albumin and cholinesterase levels were decreased, probably secondary to liver cirrhosis caused by chronic hepatitis C infection.
The patient often complained of chest pain during dialysis, but no coronary artery disease could be detected on angiography and the cause remained unclear. Fluoroscopy revealed segmental contraction of the lower esophagus, narrowing of the abdominal esophagus into a spindle-like shape, and impaired flow of contrast medium into the stomach; however, normal peristalsis was evident in the upper esophagus (Fig. 1a). Thoracoabdominal CT showed thickening of the esophageal wall to the level of the carina (Fig. 1b). Although the abnormal mucosa could not be detected on endoscopy, a slight corkscrew-shaped change was found (Fig. 2a). Endoscopic ultrasonography (EUS) showed thickening of the muscle layer of the esophagus (Fig. 2b). High-resolution esophageal manometry (HRM) measurement was perfomed using a wet swallow technique; mean values are reported. It revealed no peristalsis in the esophageal body and fully synchronized waves of contraction with a marked increase in esophageal body pressure to 166 mmHg and a lower esophageal sphincter (LES) residual pressure of 10.6 mmHg (normal range: <8 mmHg). Lower esophageal sphincter pressure was normal at 31.3 mmHg (Fig. 3). High-resolution manometry also showed an 11-cm high-pressure band in the esophagus from 37 cm proximally to 48 cm distally. Preoperative endoscopic ultrasound (EUS) showed esophageal wall thickening from 15 cm proximally to the esophagogastric junction (EGJ). The extent of the esophageal wall thickening that was detected by the preoperative EUS extended cranially as far as the proximal portion of the middle thoracic esophagus [1]. The CT revealed that the esophageal wall was thickened from the EGJ cranially as far as the carina, which concurred with the preoperative EUS findings. The patient was diagnosed with DES and was administered a calcium channel antagonist for 3 months; however, as his symptoms did not improve, we performed esophageal long myotomy and Dor’s fundoplication. We determined that it would be feasible to perform a long myotomy at laparotomy.
Findings on preoperative examination. a Fluoroscopy revealed segmental contraction of the esophagus, narrowing of the abdominal esophagus into a spindle-like shape, and failure of discharge into the stomach from the esophagus. b Thoracoabdominal computed tomography showed thickening of the esophageal wall to the level of the carina
Esophageal manometry showed no peristaltic waves in the esophageal body and fully synchronized waves of contraction with a marked increases in esophageal body pressure to 166 mmHg and lower esophageal sphincter residual pressure to 10.6 mmHg (normal range: <8 mmHg). High-resolution manometry also showed an 11-cm high-pressure band in the esophagus from 37 cm proximally to 48 cm distally
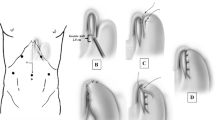
By means of an upper midline incision, the left lobe of the liver was freed by dividing the triangular ligament, and the vagus nerve and abdominal esophagus were identified. The diaphragm was opened from the hiatus to the sternum to expose the esophagus, then long myotomy was performed using simultaneous EUS with a water-filled balloon attached to the probe to confirm the areas of muscular thickening. The simultaneous EUS showed the thickening of the inner layer in the esophageal wall of the abdominal to thoracic esophagus between 35 and 42 cm from the incisor with low compliance. The EUS also showed a slightly thickened muscle layer proximally 35 cm from the incisor without low compliance. We undertook an 11-cm esophageal long myotomy proximally from the EGJ followed by a 2-cm gastric myotomy from the EGJ to the stomach. The muscle layer proximal to the upper end of the myotomy incision was slightly thickened, but as compliance was not reduced, we did not extend the incision further (Fig. 4). From the intra-abdominal view, the upper extent of the myotomy was 11 cm proximal to the EGJ. Myotomy was performed using an ultrasonic scalpel (Harmonic Scalpel II®, Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA).
Esophageal manometry was performed 14 days postoperatively and showed peristasis (Fig. 5); LES residual pressure had decreased from 10.6 to 3.1 mmHg and the esophageal body pressure from 166 to 56 mmHg. Inflow of barium to the remnant stomach had improved (Fig. 6). The patient’s symptoms of dysphagia and chest pain had resolved.
Discussion
This is the first report of the treatment of DES in Japan by transhiatal esophageal long myotomy guided by the use of intraoperative EUS to detect low compliance and thickening of the esophageal wall. This approach, combined with Dor’s fundoplication, proved to be a feasible option for the treatment of DES resistant to medication.
Diffuse esophageal spasm is defined a consistent clinical symptom such as chest pain or dysphagia in the presence of a manometric disorder [2]. The most common finding is the presence of simultaneous contractions in the distal esophagus, manifest as ‘synchronous’ pressure waves (propagating at >8 cm/s) with a minimum amplitude of 30 mmHg in 20–80 % of contractions, alternating with normal peristalsis [3]. In this case, no peristalsis was evident on esophageal manometry, but there were abnormal contractions in the distal esophagus on fluoroscopy; therefore, the diagnosis of DES was made.
Chest pain and dysphagia are typical symptoms of DES. The cause of the chest pain is not understood. The mechanism is presumed to be of esophageal origin [4]. It has been reported that the mechanisms responsible for functional chest pain include abnormal mechanophysical properties of the esophagus, central and peripheral hypersensitivity, and psychologic comorbidity. Our patient often complained of chest pain that proved not to be cardiac in origin, and the pain resolved after surgery. It is very likely that his chest pain was caused by DES.
There is no established conservative treatment for DES. Calcium channel blockers [5] and nitrites [6] can be administered to relax the smooth muscle of the esophagus, and oral psychoactive drugs or local injection of botulinum toxin may produce a beneficial effect [7]. Handa and colleagues [8] reported that antidepressants may be an effective treatment for DES. Surgical treatment is warranted when conservative management techniques fail to improve the symptoms.
In 1950, Lortat-Jacob [9] reported the first surgical treatment of DES. His procedure was a modified Heller myotomy, extending to the aortic arch through a left thoracotomy. In 1993, Anselmino and colleagues [10] performed a laparoscopic Heller cardiomyotomy and thoracoscopic esophageal long myotomy for a patient with DES, in which the myotomy was extended from the aortic arch, and a modified Dor’s fundoplication was also undertaken. In 2007, Leconte and colleagues [11] reported a series of open myotomies performed on 20 patients with DES in which the myotomy was extended up to 12–16 cm above the cardia to the level of the interior pulmonary veins, combined with Dor’s fundoplication. Their report did not reveal why the myotomies had been extended above the cardia. Symptoms of dysphagia improved in 18 of the 20 patients, and chest pain resolved in all those who had reported it. We chose to extend the myotomy to 11 cm on the basis of EUS findings.
There have been a small number of reports of surgical treatment of DES in Japan (Table 1). Katayama and colleagues [12] reported that they had performed a laparoscopic Heller-Dor procedure for DES resistant to conservative therapy. They undertook an esophageal myotomy approximately 6 cm from the EGJ and recommended intra-thoracic surgery should symptoms have persisted. Given our patient’s serious comorbidities, additional intra-thoracic surgery would have carried an even greater risk of complications, and a laparoscopic approach to an esophageal long myotomy can prove technically difficult in the mediastinum. Therefore, we opted for a transhiatal esophageal long myotomy. Long myotomy for DES was first reported in Japan by Sato and colleagues [13]. They performed a transphrenic esophageal myotomy via thoracotomy from the EGJ to the aortic arch together with Tal-Hatafuku oesophagogastroplasty [14] as fundoplication. A good postoperative outcome was reported that was still maintained after 5 years [13]. As laparotomy and thoracotomy may cause excessive surgical stress, we elected to perform an esophageal long myotomy via a transhiatal approach for our patient.
Compared with esophageal myotomy undertaken via thoracotomy, it may be difficult to obtain a satisfactory view of the lower esophagus through the esophageal hiatus in a closed-chest operation. In this case, we used endoscopic ultrasound to determine the site of the esophageal long myotomy and confirm esophageal muscle layer thickening. As a result, we were able to perform an adequate myotomy without the need to extend it to the level of the aortic arch, guided by the findings on EUS. We judged that the area of low compliance in the esophageal wall was likely to correspond to the high-pressure band detected by HRM. We suggest that it is not always necessary to perform the myotomy throughout the entirety of the thickened esophageal wall and that myotomy of the high pressure band is likely to be the most important part of achieving a good outcome in DES. Motoyama and colleagues [16] reported performing a laparoscopic and thoracoscopic esophageal long myotomy and Dor’s fundoplication for DES, which they described as a minimally invasive procedure; however, as our patient’s health was otherwise poor, we judged that the additional time required for laparoscopic and thoracoscopic surgery would have increased the risk of postoperative complications. Symptoms of dysphagia and chest pain resolved postoperatively, and at 1-year follow-up he also reported being able to eat. We judge that the improvement in chest pain symptoms was as result of the myotomy of the high-pressure band in the esophageal wall.
References
Daniel Pelot. Applied anatomy and anomalies of the esophagus. In: Edward J, editor. Berk Bockus Gastroenterology. Philadelphia: WB Saunders; 1985. pp. 666.
Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–24.
Salvador R, Costantini M, Rizzetto C, Zaninotto G. Diffuse esophageal spasm: the surgical approach. Dis Esophagus. 2012;25:311–8.
Fass R, Navarro-Rodriguez T. Noncardiac chest pain. J Clin Gastroenterol. 2008;42:636–46.
Nasrallah SM. Nifedipine in the treatment of diffuse oesophageal spasm. Lancet. 1982;2:1285.
Swamy N. Esophageal spasm: clinical and manometric response to nitroglycerine and long acting nitrites. Gastroenterology. 1977;72:23–7.
Miller LS, Parkman HP, Schiano TD, Cassidy MJ, Ter RB, Dabezies MA, Cohen S, Fisher RS. Treatment of symptomatic non achalasia esophageal motor disorders with botulinum toxin injection at the lower esophageal sphincter. Dig Dis Sci. 1996;41:2025–31.
Handa M, Mine K, Yamamoto H, Hayashi H, Tsuchida O, Kanazawa F, Kubo CJ. Antidepressant treatment of patients with diffuse esophageal spasm: a psychosomatic approach. Clin Gastroenterol. 1999;28(3):228–32.
Lortat-Jacob JL. Focal and diffuse myomatoses of the esophagus. Archives des maladies de l’appareil digestif et des maladies de la nutrition. 1950;39:519–24 [French].
Anselmino M, Hinder RA, Filipi CJ, Wilson P. Laparoscopic Heller cardiomyotomy and thoracoscopic esophageal long myotomy for the treatment of primary esophageal motor disorders. Surg Laparosc Endosc. 1993;3:437–41.
Leconte M, Douard R, Gaudric M, Dumontier I, Chaussade S, Dousset B. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg. 2007;94:1113–8.
Katayama K, Okitsu H, Tangoku A. A case of diffuse esophageal spasm treated by laparoscopic Heller-Dor operation. Shujutsu. 2009;63:1069–73 [Japanese].
Sato N, Watanabe M, Mastuno S, Nishinari N, Sasaki A, Saito K, Sasaki S, Sato K, Mori S. Long esophageal myotomy with a fundic patch procedure for treating diffuse esophageal spasm: report of a case. Surg Today. 1993;23:360–5.
Hatafuku T, Maki T, Thal AP. Fundic patch operation in the treatment of advanced achalasia of the esophagus. Surg Gynecol Obstet. 1972;134:617–24.
Maruyama K, Motoyama S, Okuyama M, Ohta H, Ogawa J. Successful surgical treatment for diffuse esophageal spasm. Nihon Kyobu Geka Gakkai Zasshi. 2005;53:169–72 [Japanese].
Motoyama S, Kitamura M, Ogawa J. Laparoscopic and thoracoscopic surgery for diffuse esophageal spasm. Shujutsu. 2004;58:2139–43 [Japanese].
Kato H, Kuwano H. Transthoracic esophageal long myotomy with fundic patch operation for diffuse esophageal spasm (DES). Shujutsu. 1999;53:2001–4 [Japanese].
Okuda T, Higashino M, Osugi H, Maekawa N, Tanimura S, Kinoshita H, Wakasa K. Case of diffuse esophageal spasm treated by long myotomy. Nihon Geka Gakkai Zasshi. 1993;94:1159–63 [Japanese].
Ethical Statement
This work, manuscript number ESOP-D-13-00031, is a case report. This article does not contain any studies with human or animal subjects performed by any authors.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murakami, H., Matsumoto, H., Kubota, H. et al. A case of long myotomy and fundoplication for diffuse esophageal spasm. Esophagus 10, 273–279 (2013). https://doi.org/10.1007/s10388-013-0398-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-013-0398-0