Abstract
Purpose
To investigate the 4-year outcome of aflibercept treatment using a treat-and-extend (TAE) regimen for recurrent neovascular age-related macular degeneration (AMD).
Study design
Retrospective observational study.
Methods
Data of eyes with recurrent AMD previously treated with anti-vascular endothelial growth factor agents or photodynamic therapy and had started aflibercept treatment using a TAE regimen for the first time were collected. Best-corrected visual acuity (BCVA), intervals of treatments, the presence of exudation, central foveal thickness (CFT), and central choroidal thickness (CCT) were analyzed.
Results
Of 47 consecutive eyes, 30 of the 47 eyes completed a 4-year follow-up. The mean BCVA (logMAR) was sustained over the 4 years (0.37 at baseline, 0.36 at 1 year, 0.36 at 2 years, 0.41 at 3 years, and 0.43 at 4 years, P = 0.21). Of the 30 eyes that completed the follow-up, BCVA of two eyes deteriorated by 0.3 logMAR or more at 4 years. At 4 years, 67% of eyes had extended treatment intervals to > 8 weeks, and 47% of eyes had extended intervals to > 12 weeks. Exudative changes in the macula, seen in all eyes at baseline, were only seen in 50% of the eyes at 4 years. The mean CFT and CCT decreased significantly at 4 years from 332 μm to 248 μm and from 218 μm to 183 μm, respectively.
Conclusion
In clinical settings, aflibercept treatment using a TAE regimen may successfully maintain visual acuity for up to 4 years even in recurrent cases of AMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neovascular age-related macular degeneration (AMD) is known to cause irreversible vision loss in middle-aged and older people due to repeated macular exudation. In the past, treatment for neovascular AMD was limited to highly invasive procedures such as retinal photocoagulation and surgical removal of choroidal neovascularization (CNV), which resulted in poor outcomes. Recently, the emergence of anti-vascular endothelial growth factor (VEGF) drugs such as ranibizumab and aflibercept has significantly changed the treatment of neovascular AMD and decreased the rate of blindness due to severe AMD [1]. The Minimally Classic/Occult Trial of theAnti-VEGF Antibody Ranibizumab in the Treatment ofNeovascular AMD (MARINA) and Anti-VEGF Antibodyfor the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR) studies, Phase 3 trials of ranibizumab for neovascular AMD, report that monthly doses of ranibizumab significantly improved visual acuity compared to baseline and maintained the improved visual acuity for 2 years [2,3,4]. Pro re nata (PRN) regimen, in which anti-VEGF drugs are administered “as needed”, i.e. when exudative changes are seen, was devised because of the burden imposed by frequent injections with fixed dosing. The Comparison Age-related macular degeneration Treatment Trials (CATT) study comparing monthly ranibizumab injections and ranibizumab injections following a PRN regimen shows non-inferiority of the PRN regimen at 1 year [5]. However, in the HORIZON study, which followed participants for an additional 2 years after the completion of the MARINA and ANCHOR phase 3 clinical trials, visual acuity regressed to the baseline visual acuity noted at the start of the MARINA and ANCHOR trials 2 years after release from both trials [6]. Furthermore, in the SEVEN-UP study, which followed participants for an additional 3 years after the HORIZON study, for a total of 7 years following the MARINA and ANCHOR trials, visual acuity decreased by 19.8 letters from the therapeutic peak upon completion of the MARINA and ANCHOR trials [7]. Moreover, the CATT trial also reveals an average visual acuity loss of 10.8 letters in the 3 years following the completion of the 2-year clinical trial [8]. These reports highlight the difficulty in maintaining visual acuity in clinical practice. Therefore, a treatment method called Treat-and-Extend (TAE), in which anti-VEGF drugs are administered continuously and aggressively at intervals adjusted according to the presence or absence of macular exudation, was introduced, and promising results are reported both in clinical trials and in clinical practice [9,10,11,12,13].
Besides the improvements in treatment protocols described above, an improved treatment, aflibercept, with a longer-lasting therapeutic effect than ranibizumab, has been developed. Phase 3 clinical trials VIEW 1 and VIEW 2 studies report that the bimonthly fixed dosing regimen with aflibercept is non-inferior to the monthly dosing regimen with ranibizumab [14]. Aflibercept has also begun to show favorable long-term results for treatment-naïve AMD with the TAE regimen in clinical trials and real-world settings [15,16,17]. To date, most reports investigating this regimen were in eyes with treatment-naïve AMD. Despite the considerable number of patients who experience recurrent AMD and were previously treated with anti-VEGF therapy or verteporfin photodynamic therapy (PDT), this population remains largely unstudied. Therefore, in this study, we investigated the long-term outcomes of aflibercept injections administered following the TAE regimen in eyes with recurrent neovascular AMD that had previously been treated with an anti-VEGF or PDT and report the 4-year outcomes of this treatment.
Subjects and methods
Study design
This was a single-center retrospective observational study. We collected data of consecutive eyes with previously treated neovascular AMD that had started aflibercept (2 mg/0.05 ml, Eylea, Bayer Yakuhin, Ltd.; Santen Pharmaceutical Co., Ltd.) administered according to a TAE regimen from January 2013 to April 2016 at Nagoya University Hospital. All eyes in the study exhibited recurrent cases of AMD and had previously undergone either anti-VEGF injections or PDT therapy, but no TAE regimen with aflibercept prior to this study. We conducted this study in accordance with the tenets of the Declaration of Helsinki and received the approval of the Institutional Review Board of the Nagoya University Graduate School of Medicine (18112) and Handa City Hospital (2019–015). This study was registered with the University Hospital Medical Information Network (UMIN000040311). The Institutional Review Board granted a waiver of informed consent for the present study because of its retrospective nature. We also published the study on the Nagoya University network to allow patients to decline participation in the present study. All patient data were anonymized before analysis.
Clinical measurements
Patients underwent comprehensive ophthalmologic examination including best-corrected visual acuity (BCVA) and spectral-domain optical coherence tomography (OCT, Spectralis, Heidelberg Engineering) at each visit. Color fundus photography, fluorescein angiography (FA), and indocyanine green angiography were performed for the diagnosis of AMD. The TAE regimen used in this study was as follows. Aflibercept was administered monthly until no macular exudation such as intraretinal fluid (IRF), subretinal fluid (SRF), and sub-retinal pigment epithelium (RPE) fluid was seen on OCT images. If no macular exudation was seen, the treatment interval was extended by 2 weeks, and if exudation was seen again, the treatment interval was shortened by 2 weeks. If massive exudation accompanied by subretinal hemorrhage was observed, the dosing interval was shortened to 4 weeks, and the TAE regimen was resumed when exudation was no longer present, with a minimum dosing interval of 4 weeks and a maximum dosing interval of 4 months (approximately 16 weeks). Whenever the intervals were extended and shortened two to three times in a row, the treatment interval was not extended further but was fixed at the doctors' discretion. Whether to give the first three initial treatments of aflibercept was up to the physicians. The time at which aflibercept treatments according to the TAE regimen started was considered as the baseline. The data of BCVA, treatment intervals of injections, presence of exudation on OCT at baseline and at 1, 2, 3 and 4 years and the number of aflibercept injections in the first, second, third and fourth years were collected from medical records. Central foveal thickness (CFT) and central choroidal thickness (CCT) were measured manually using the caliper function of the built-in software of the OCT device as the perpendicular length from the inner limiting membrane to Bruch's membrane and that from Bruch's membrane to the choroid-sclera boundary at the fovea. Whenever no RPE detachment was present at the fovea, the outer edge of the RPE was used as Bruch's membrane.
Statistical analysis
BCVA was recorded as decimal values and converted to logarithm of the minimal angle of resolution (logMAR) units for analysis, performed using SPSS version 26 (IBM Japan). For analysis of BCVA, data missing due to patient drop-out were supplemented by multiple imputations, and the data of all the cases were analyzed. We also analyzed data from the patients who completed the 4 years of follow-up. Only the data of patients who completed the 4 years of follow-up were used for the analysis of number of injections, intervals of injections, evaluation of macular fluid, CFT, and CCT. After testing for normal distribution with the Shapiro–Wilk test, comparisons between two groups were conducted using the Mann–Whitney t-test, comparisons in BCVA, change of BCVA, number of injections, CFT, and CCT using repeated measures ANOVA (Friedman's test), and comparisons between baseline data and at 4 years using the Wilcoxon signed-rank test; unless otherwise indicated. Data are presented as mean ± standard deviation and P-values smaller than 0.05 were considered significant.
Results
A total of forty-seven eyes met the inclusion criteria for this study; 30 of the 47 eyes completed a 4-year follow-up after aflibercept treatment with the TAE regimen. However, 10 of 47 eyes dropped out after 3 years, 5 between 2–3 years, and 2 between 1–2 years; there were no cases of drop-out within the first year. Of the 17 eyes that did not complete the 4-year follow-up, 6 continued to receive treatment from the local ophthalmologist, 7 had the treatment regimen changed from TAE to PRN due to patients’ preferences or doctors’ decision, and 4 exhibited self-interrupted scheduled visit for unknown reasons. The intervals for aflibercept injections at the time of dropout varied from 4–5 weeks for 2 cases, 8–12 weeks for 6 cases, and 13 weeks or more for 7 cases. The reasons for the switch from TAE to PRN in 7 cases were: selection of combined therapy with PDT followed by PRN because of the burden of monthly injections (n = 1), refusal to continue TAE treatment because of poor visual acuity in the affected eyes and good visual acuity in the fellow eyes (n = 2), development of neovascular AMD in the fellow eyes and prioritization of treatment for these eyes (n = 2); and expansion of geographic atrophy despite maintenance of 14- or 16-week treatment intervals (n = 2). For the 6 cases that continued treatment with their local ophthalmologists, the last treatment intervals at our hospital ranged from 8 to 12 weeks. The last treatment intervals for the four cases that self-interrupted their treatment ranged from between 13 to 16 weeks.
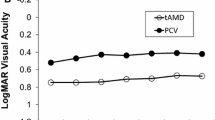
The baseline characteristics of patients are shown in Table 1. The group that completed a 4-year follow-up was significantly younger than the group that dropped out within 4 years. However, there were no significant differences in gender, subtypes of AMD, disease duration between the first diagnosis of AMD and initiation of aflibercept injections according to the TAE regimen, and BCVA at baseline. The mean BCVA and the changes in BCVA from baseline are shown in Fig. 1. The mean BCVA in the group that completed a 4-year follow-up (0.32 ± 0.32 at baseline, 0.31 ± 0.26 at 1 year, 0.32 ± 0.27 at 2 years, 0.32 ± 0.28 at 3 years, and 0.37 ± 0.30 at 4 years) were slightly better than those of total samples including dropout cases (0.37 at baseline, 0.36 at 1 year, 0.36 at 2 years, 0.41 at 3 years, and 0.43 at 4 years). Neither the group that completed follow-up (P = 0.23) nor the total samples’ group (P = 0.21) showed any significant changes in BCVA over 4 years. Furthermore, neither the group that completed follow-up (P = 0.38) nor the total samples’ group (P = 0.20) showed any significant changes in the change of BCVA over 4 years. The comparisons between BCVA at baseline and at 4 years showed no significant difference in either the group that completed follow-up (P = 0.34) nor the total samples’ group (P = 0.36). Of the total 30 eyes that completed the 4-year follow-up, two eyes experienced worsening in BCVA by 0.3 logMAR or more at 4 years. One of the two eyes had been showing residual serous retinal detachment over the follow-up period, but the patient did not agree to shorten the treatment interval, and the other eye developed cataract, which was surgically treated after the 4-year follow-up and the visual acuity was restored to 20/20. During the 4-year follow-up period, two out of the 30 eyes showed subretinal hemorrhages, the sizes of which were 2 and 4 disc-diameters; these eyes did not exhibit severe deterioration in visual acuity.
Mean best-corrected visual acuity (BCVA) and mean changes in BCVA. a Mean BCVA for the total sample (solid line) and eyes that completed the 4-year follow-up (dotted line) showed no significant changes over 4 years. b Mean changes in BCVA for the total sample (solid line) and eyes that completed the 4-year follow-up (dotted line) also showed no significant changes over 4 years
The eyes needed a significantly higher number of injections in the first year (7.0 ± 2.0 times, P < 0.001) compared to the second year (5.4 ± 2.1 times), third year (5.2 ± 2.3 times), and fourth year (5.4 ± 2.6 times) (Fig. 2a). The injection intervals at 1, 2, 3, and 4 years are shown in Fig. 2b. The percentage of eyes with intervals of 16 weeks increased each year, and a third of the eyes had extended intervals of 16 weeks by the end of the 4-year follow-up. The numbers of eyes with intervals of 4–8 weeks at 1, 2, 3, and 4 years were 10 (33%), 7 (23%), 9 (30%), and 11 (37%) respectively. At 4 years, 37% of eyes could not extend the intervals beyond 8 weeks; conversely, 63% of eyes had extended the intervals to over 8 weeks. To investigate the factors related to the injection intervals, we divided the eyes into two groups: those with intervals of 4–8 weeks and those with intervals of more than 8 weeks at 4 years. We compared the two groups for age, AMD subtypes, disease duration from the first diagnosis to the initiation of aflibercept injections according to the TAE regimen, baseline BCVA, change in BCVA at 4 years, presence of subretinal hyperreflective material (SHRM) on OCT images, baseline central choroidal thickness, and past history of PDT (Table 2). Eyes that needed treatment intervals of 4–8 weeks at 4 years showed better baseline BCVA and a lower prevalence of SHRM; however, they showed greater BCVA loss at 4 years than did eyes for which the intervals could be extended to more than 8 weeks.
At baseline, 30 out of 30 eyes had IRF, SRF, or sub-RPE fluid and were started on aflibercept treatments with the TAE regimen, and the percentage of eyes with IRF, SRF, or sub-RPE fluid gradually decreased, to 15 out of 30 eyes (50%) at 4 years (Fig. 3).
Figure 4 shows CFT and CCT during the follow-up period. CFT decreased significantly over the 4-year follow-up period from 332 ± 142 μm at baseline to 252 ± 68 μm at 1 year, 257 ± 81 μm at 2 year, 254 ± 85 μm at 3 year, and 248 ± 76 μm at 4 year (P < 0.001). CCT also gradually decreased from 218 ± 115 μm at baseline to 196 ± 107 μm at 1 year, 185 ± 102 μm at 2 year, 186 ± 105 μm at 3 year, and 183 ± 107 μm at 4 year (P < 0.001).
Discussion
In this study, we reported that aflibercept administration according to a TAE regimen maintained visual acuity over 4 years without any significant decline in visual acuity in a real world setting. To our knowledge, this is the first report of 4-year outcomes of TAE regimen for recurrent neovascular AMD.
In the VIEW 2 study, which included Asian population, a bimonthly fixed dosing of aflibercept for treatment-naïve AMD led to an improvement in visual acuity of 8.9 letters at 1 year [14]. In clinical practice, aflibercept treatment using the TAE regimen for treatment-naïve AMD was reported to lead to an improvement in 5.7–6.0 letters in visual acuity at 2 years [18, 19]. However, switching from ranibizumab to aflibercept is reported to improve macular anatomy, but the changes in visual acuity range from maintenance only in some reports to improvement in others [20,21,22,23,24,25]. The present study investigated recurrent cases of AMD previously treated with anti-VEGF drugs or PDT, or refractory cases that were not controlled with ranibizumab or aflibercept using a PRN regimen. There were, therefore, no additional improvements in visual acuity with the aflibercept treatment, as seen in some previous reports. It is also possible that irreversible changes in the macula were occurring because an average of 3.2 years had already passed since the first diagnosis of AMD. Nevertheless, it is worth noting that the visual acuity did not worsen during the 4-year follow-up period.
Traine et al. report a 4-year outcome of aflibercept for treatment-naïve AMD using the TAE regimen, in which 5.6% of the eyes exhibited worsened visual acuity by more than 3 lines [19]. Similarly, the present study shows that only 2 out of 30 eyes (6.7%) experienced deterioration in visual acuity by more than 0.3 logMAR. In addition, one of the two eyes that deteriorated had cataract surgery later, and their visual acuity improved, suggesting that aflibercept treatment using the TAE regimen is a treatment protocol is not likely to cause irreversible long-term deterioration in visual acuity.
Reducing patient burden and maintaining patient adherence to treatment are important issues in long-term management of AMD. A TAE regimen reportedly not only leads to better visual acuity, but also reduces the number of visits to hospitals and clinics compared to a PRN regimen, which require monthly monitoring [12, 26, 27]. The patients who dropped out during the 4-year follow-up in this study were significantly older than those who completed the 4-year follow-up highlights the difficulty of continuing treatments and making visits to hospital, especially by the elderly, particularly considering that some of the patients who dropped out continued their treatment at local hospitals or at clinics near their homes. Thus, it is important to establish a coordinated system of AMD treatment with local hospitals and clinics.
Whether anti-VEGF treatment for AMD should be stopped or not is still controversial. In this study, we did not set up a stopping protocol for the TAE regimen; however, 7 out of 17 cases which dropped out of the 4-year follow-up switched from the TAE to the PRN regimen due to patient preference or at the doctors’ discretion. Adrean et al. report that 29.4% of eyes experienced recurrence at an average of 14 months with the “treat-extend-stop protocol,” in which the dosing intervals were extended to 12 weeks, the eyes were confirmed 3 times to have dry macula, and then aflibercept treatment was discontinued [28]. Nguyen et al. report that when treatment was discontinued in eyes that had received at least five injections of aflibercept and then had no exudation for more than three months, 41% of eyes experienced recurrence within one year and 79% within five years. Although this study shows that visual acuity was maintained over 4 years in eyes which completed the 4-year follow-up, the long-term outcome after treatment discontinuation remains unknown [29]. Thus, caution should be exercised before suspending treatment, particularly in patients who wish to maintain a socially active lifestyle and continue to be able to drive.
Hosokawa et al. report that 62.7% of treatment-naïve AMD patients who received aflibercept injections had extended treatment intervals of TAE to more than 8 weeks at 1 year since starting treatment [30]. Similarly, Yamamoto et al. report that 62.7% of AMD patients had extended treatment intervals of TAE to more than 8 weeks at 1 year [31]. In the present study, 67% of eyes had extended treatment intervals to more than 8 weeks at 1 year and 63% at 4 years. Furthermore, 47% of eyes had extended intervals to more than 12 weeks at 4 years. Although it is still debatable whether the maximum intervals of treatments should be 12 or 16 weeks, we did not observe any significant exacerbation during the 4-year follow-up period, suggesting that aflibercept treatment using a TAE regimen can be performed safely even at a maximum treatment interval of 16 weeks.
We attempted to determine the characteristics of eyes with treatment intervals of 4–8 weeks at 4 years and found that these eyes showed better BCVA at baseline and a lower prevalence of SHRM, associated with poorer visual outcomes and more common in eyes with type 2 CNV that causes exudation directly in the subretinal space [32]. Furthermore, it is reported that type 1 CNV tends to be resistant to anti-VEGF therapy [33, 34]. Thus, most of the lesions in the eyes that required frequent injections at 4 years may have been type 1 CNV. It should be noted that eyes with intervals of 4–8 weeks at 4 years showed worsening of BCVA; this suggests that the frequent exudative changes did not allow extension of the treatment intervals and consequently led to the worsening of BCVA from baseline. Moreover, the number of eyes with intervals of 4–8 weeks was slightly higher at 4 years than at 2 years and 3 years, probably because eyes with frequent exudative changes may have had exacerbated CNV lesions, which resulted in the need for frequent treatments at 4 years. On the other hand, eyes with intervals of 16 weeks increased year by year. The eyes required frequent injections for the first year because the intervals were not adequately extended at that time. We think that there were cases where disease activity was reduced by continued proactive anti-VEGF therapy.
In the present study, dry macula was obtained in only 50% of cases at 4 years. This could be due to the fact that a TAE regimen extends or shortens the treatment intervals depending on the presence or absence of exudative changes; thus, in some cases, exudative changes were just present at 4 years. Furthermore, as in any real-world application of a therapy, patients’ enthusiasm may have influenced adherence to the treatment regimen. Nevertheless, the patients’ visual acuity was maintained for 4 years because even if the decision to extend or shorten treatment intervals was somewhat lax, anti-VEGF drugs were always administered at the time of visit, which may have reduced the risk for massive exudation and hemorrhage [35]. This is a benefit of proactive treatment in real-world settings.
Previous reports maintain that treatment-naïve eyes showed a decrease in CCT by 15%–18% at 2 years after aflibercept therapy using a TAE regimen [17, 36, 37]. The present study shows that CCT decreased by 15% at 2 years and 16% at 4 years, even though the eyes in this study were not treatment-naïve eyes.
The limitations of this study were its retrospective nature and the small number of cases. In addition, all subjects were of the Asian ethnicity, thus it is possible that other ethnic groups may respond differently to the treatment outlined here. A strength of our study is that it reports real-world and long-term outcomes of aflibercept using a TAE regimen.
In conclusion, this study showed that even in previously treated AMD cases, aflibercept treatment using a TAE regimen prevented worsening of visual acuity over the 4-year follow-up period. At 4 years, 63% of cases had extended the treatment intervals to more than 8 weeks, and 47% to more than 12 weeks, suggesting that a TAE-based aflibercept treatment may be an effective and feasible treatment option in clinical practice.
References
Morizane Y, Morimoto N, Fujiwara A, Kawasaki R, Yamashita H, Ogura Y, et al. Incidence and causes of visual impairment in Japan: the first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol. 2019;63:26–33.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65.
Group C, Martin DF, Maguire MG, Ying G-S, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
Singer MA, Awh CC, Sadda SV, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Group CoA-rMDTTCR, Maguire MG, Martin DF, Ying G-SS, Jaffe GJ, Daniel E, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–61.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration trex-amd 1-year results. Ophthalmology. 2015;122:2514–22.
Engelbert M, Zweifel SA, Freund KB. Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization using a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina. 2010;30:1368–75.
Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143:679–80.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117:2134–40.
Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration 2-year results of the trex-amd study. Ophthalmol Retina. 2017;1:314–21.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser P, Nguyen Q, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Eleftheriadou M, Gemenetzi M, Lukic M, Sivaprasad S, Hykin PG, Hamilton RD, et al. Three-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: evidence from a clinical setting. Ophthalmol Ther. 2018;7:361–8.
Morimoto M, Matsumoto H, Mimura K, Akiyama H. Two-year results of a treat-and-extend regimen with aflibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1891–7.
Matsumoto H, Hiroe T, Morimoto M, Mimura K, Ito A, Akiyama H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age-related macular degeneration. Jpn J Ophthalmol. 2018;62:144–50.
Barthelmes D, Nguyen V, Daien V, Campain A, Walton R, Guymer R, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38:20–8.
Traine PG, Pfister IB, Zandi S, Spindler J, Garweg JG. Long-term outcome of intravitreal aflibercept treatment for neovascular age-related macular degeneration using a “treat-and-extend” regimen. Ophthalmol Retina. 2019;3:393–9.
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156:29–35.
Singh RP, Srivastava S, Ehlers JP, Bedi R, Schachat AP, Kaiser PK. A single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysis. Br J Ophthalmol. 2014;98:i22–7.
Barthelmes D, Campain A, Nguyen P, Arnold JJ, McAllister IL, Simpson JM, et al. Effects of switching from ranibizumab to aflibercept in eyes with exudative age-related macular degeneration. Br J Ophthalmol. 2016;100:1640–5.
Kvannli L, Krohn J. Switching from pro re nata to treat-and-extend regimen improves visual acuity in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2017;95:678–82.
Giannakaki-Zimmermann H, Ebneter A, Munk MR, Wolf S, Zinkernagel MS. Outcomes when switching from a pro re nata regimen to a treat and extend regimen using aflibercept in neovascular age-related macular degeneration. Ophthalmologica. 2017;236:201–6.
Spooner K, Hong T, Wijeyakumar W, Chang AA. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017;11:161–77.
Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br J Ophthalmol. 2016;100:914–7.
Augsburger M, Sarra G-MM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2019;257:1889–95.
Adrean SD, Chaili S, Grant S, Pirouz A. Recurrence rate of choroidal neovascularization in neovascular age-related macular degeneration managed with a treat–extend–stop protocol. Ophthalmol Retina. 2018;2:225–30.
Nguyen V, Vaze A, Fraser-Bell S, Arnold J, Essex RW, Barthelmes D, et al. Outcomes of suspending vegf inhibitors for neovascular age-related macular degeneration when lesions have been inactive for 3 months. Ophthalmol Retina. 2019;3:623–8.
Hosokawa M, Morizane Y, Hirano M, Kimura S, Kumase F, Shiode Y, et al. One-year outcomes of a treat-and-extend regimen of intravitreal aflibercept for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2017;61:150–8.
Yamamoto A, Okada AA, Nakayama M, Yoshida Y, Kobayashi H. One-year outcomes of a treat-and-extend regimen of aflibercept for exudative age-related macular degeneration. Ophthalmologica. 2017;237:139–44.
Spaide RF, Jaffe GJ, Sarraf D, Freund BK, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data. Ophthalmology. 2019;127:616–36.
Suzuki M, Nagai N, Izumi-Nagai K, Shinoda H, Koto T, Uchida A, et al. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br J Ophthalmol. 2014;98:1186–91.
Nakano Y, Kataoka K, Takeuchi J, Fujita A, Kaneko H, Shimizu H, et al. Vascular maturity of type 1 and type 2 choroidal neovascularization evaluated by optical coherence tomography angiography. PLoS ONE. 2019;14:e0216304.
Okada M, Kandasamy R, Chong EW, McGuiness M, Guymer RH. The treat-and-extend injection regimen versus alternate dosing strategies in age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2018;192:184–97.
Ito A, Matsumoto H, Morimoto M, Mimura K, Akiyama H. Two-year outcomes of a treat-and-extend regimen using intravitreal aflibercept injections for typical age-related macular degeneration. Ophthalmologica. 2017;238:236–42.
Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, et al. Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration: a prospective study. Ophthalmol Retina. 2020;4:767–76.
Acknowledgements
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y. Tsunekawa, None; K. Kataoka, Honorarium for Lecturing (Novartis, Santen, Bayer, Senju); K. Asai, None; Y. Ito, Honorarium for Lecturing(Aichi Ophthalmologists Association, Bayer, Canon, ZEISS, Kowa, Novartis, Okazaki City Medical Association, Pfizer, Santen); H. Terasaki, Grant, Honorarium for Lecturing(Otsuka, Kowa, Santen, Senju, Alcon, Novartis, Wakamoto), Honorarium for Lecturing, Consultant fee, Travel fee(Bayer), Rohto Award Selection Committee(ROHTO), Honorarium for Lecturing (ZEISS), Grant (HOYA).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Keiko Kataoka
About this article
Cite this article
Tsunekawa, Y., Kataoka, K., Asai, K. et al. Four-year outcome of aflibercept administration using a treat-and-extend regimen in eyes with recurrent neovascular age-related macular degeneration. Jpn J Ophthalmol 65, 69–76 (2021). https://doi.org/10.1007/s10384-020-00783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00783-8