Abstract
Purpose
To investigate the clinical characteristics of eyes with cuticular drusen in Japanese individuals, while paying special attention to large colloid drusen (LCD).
Study design
Retrospective case series.
Methods
Eyes with cuticular drusen, from patients of 4 medical institutes in Japan, were investigated. Multimodal imaging findings were used to diagnose cuticular drusen. LCD was defined as cuticular drusen > 200 µm.
Results
Twenty-four eyes from 12 patients (8 women, 4 men) were diagnosed with cuticular drusen. The mean age of all patients (n = 12) was 60.8 years. The mean age of patients without additional macular pathology (n = 5) was 55.4 years. Of the 7 patients with additional macular pathology, 6 (85.7%) exhibited age-related macular degeneration-associated macular pathology, including drusenoid pigment epithelial detachment (PED) (8 eyes from 4 patients), geographic atrophy (2 eyes from 1 patient), and occult choroidal neovascularization (1 eye). LCD were found in 6 eyes of 3 patients (25%), those with LCD were on average 53.7 ± 8.7 years old and those without 69.9 ± 14.1 years of age (P = 0.064, Mann–Whitney U test).
Conclusions
Cuticular drusen were predominantly seen in females, and drusenoid PED was most frequently seen in eyes with additional macular pathology. LCD were seen in 25% of eyes with cuticular drusen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drusen are yellowish round deposits seen in the posterior pole in the aged retina. Histologically, they are accumulations of extracellular material located between the basal lamina of the retinal pigment epithelium (RPE) and inner collagenous layer of Bruch’s membrane [1]. They contain various components associated with inflammation and immune-associated processes [2], and can enlarge and aggregate over time. Though macular drusen have been considered to be a hallmark feature of early age-related macular degeneration (AMD), the risk of developing advanced AMD varies depending on number, distribution pattern, and size of drusen, presence or absence of pigment changes in RPE, and the fellow-eye condition [3, 4].
Among various subtypes of drusen clinically associated with AMD, cuticular drusen were first described by Gass in 1977 as an atypical form of drusen characterized by innumerable yellowish, small (25–75 µm in diameter), discretely round dots in the central macula or mid-periphery [5]. Drusen of this type are clearly visualized on fluorescein angiography showing “stars-in-the-sky” appearance in the early arteriovenous phase. Histopathologic studies have revealed that cuticular drusen appear as nodular elevations of the inner collagenous layer of Bruch’s membrane [6]. Cuticular drusen are associated with advanced AMD, including neovascular AMD, geographic atrophy (GA), and adult onset vitelliform lesions [7], as well as with several loci of genetic polymorphism associated with AMD [8].
In 2012, Guigui et al. [9] first reported the angiographic features of early onset drusen > 200 µm and termed them large colloid drusen (LCD). They demonstrate that LCD showed hyperfluorescence in fluorescein angiography. Multimodal imaging in subsequent case reports revealed further characteristics of LCD [10, 11]. Furthermore, a recent study proposes LCD as a variant of cuticular drusen [12].
To date, reports on cuticular drusen are more prevalent in patients of European descent than in Asian descent [13]. Therefore, we performed the present multicenter study in Japan to clarify the clinical characteristics of Japanese patients with cuticular drusen.
Methods
This retrospective study was conducted by the collaboration between 4 medical institutes: Yamanashi University Hospital, Nihon University Hospital, Kobe University Hospital, and Gunma University Hospital. Cuticular drusen phenotype in these institutes, seen between January 2018 and October 2018, were included in this study. Medical records of eyes with cuticular drusen were retrospectively reviewed at each institute. This study was approved by the Institutional Review Board of the University of Yamanashi and conducted in accordance with tenets of the Declaration of Helsinki.
Inclusion criteria consisted of eyes receiving multimodal imaging examination including color fundus photography, spectral-domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg Engineering) or swept-source optical coherence tomography (SS-OCT; DRI-OCT1: Topcon), fundus autofluorescence (FAF) using either Topcon fundus camera or Spectralis HRA, and near infrared reflectance, fluorescein angiography (FA), and indocyanine green angiography (ICGA) using Spectralis HRA/OCT. Exclusion criteria consisted of eyes which did not receive the aforementioned multimodal imaging. Since this study was retrospective, OCT scan patterns were not determined. However, horizontal and vertical scans through the fovea were performed for all study eyes.
All images of cuticular drusen suspects at each institute were collected, and presence or absence of cuticular drusen was evaluated by 2 independent graders (Y.S and K.T) on the basis of multimodal imaging. Discordant diagnosis was resolved through open arbitration. Diagnosis of cuticular drusen was made by the early-mid phase hyperfluorescence on FA, OCT patterns (dome-shaped, saw-toothed, or pointed) as previously reported, and/or punctate hypoautofluorescence with a ring of hyperautofluorescence on Topcon FAF [14,15,16]. Representative cases without macular pathology are shown in Figs. 1 and 2.
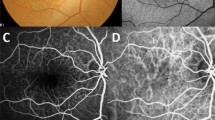
A 61-year-old woman with cuticular drusen (Case 5). a Color photographs show the numerous yellowish small deposits mainly located around the macula. b Fundus autofluorescence exhibits numerous pinpoint hypoautofluorescence surrounded by hyperautofluorescence corresponding to the yellowish deposits in color fundus photograph (A). c Fluorescein angiography demonstrates innumerable hyperfluorescence of “stars-in-the-sky” appearance. d Spectral-domain optical coherence tomography shows “saw-tooth” pattern of retinal pigment epithelium corresponding to the drusen
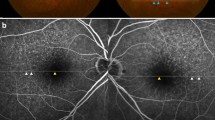
A 44-year-old man with cuticular drusen (Case 1). a Color photograph shows large drusen in the macula. b Wide-field fundus autofluorescence exhibits multiple pinpoint hypoautofluorescence corresponding to the drusen. c Fluorescein angiography demonstrates various sizes (greater or smaller than 200 µm) of hyperfluorescent spots. d Spectral-domain optical coherence tomography shows sharply peeked drusen of various heights
LCD were defined as cuticular drusen > 200 µm, as previously described [9].
Statistical analysis was performed using DR.SPSS (IBM). Continuous variables between the 2 groups were analyzed using Mann–Whitney U test, and P value < 0.05 was considered to be statistically significant.
Results
Nineteen patients were studied as cuticular drusen suspects and 12(63.2%) patients were diagnosed as having cuticular drusen. Discordant rate between the two graders at the first evaluation was 15.8% (3/19). Kappa coefficient was 0.63.
A total of 12 patients (8 women and 4 men), with a mean age of 60.8 years (range 44–93), were diagnosed with cuticular drusen (Table 1). Of 24 eyes, 12 (66.7%) demonstrated additional macular pathology, including drusenoid pigment epithelial detachment (PED) in 8 eyes of 4 patients, GA in 2 eyes of 1 patient, occult choroidal neovascularization in 1 eye, and branch retinal vein occlusion in 1 eye. Representative cases of cuticular drusen with GA and with drusenoid PED are shown in Figs. 3 and 4, respectively. LCD were found in 6 eyes of 3 patients (25%), those with LCD and those without were on average 53.7 ± 8.7 years and 69.9 ± 14.1 years of age respectively (P = 0.064, Mann–Whitney U test). Mean subfoveal choroidal thickness was 202 ± 80 µm among all 24 eyes.
A 61-year-woman with large colloid drusen (LCD) and geographic atrophy (GA) in both eyes (Case 6). a Color photographs show innumerable drusen with various sizes extending beyond the arcades, and larger drusen are considered as LCD. b Wide-field fluorescein angiography exhibits window defects corresponding to GA and innumerable hyperfluorescence with various sizes. c Fundus autofluorescence showed hypoautofluorescent corresponding to retinal pigment epithelial atrophy. d Spectral-domain optical coherence tomography shows steeply protruded retinal pigment epithelium. Enhanced choroidal transmission evidently corresponds to GA
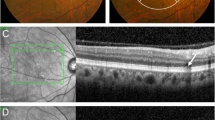
A 56-year-woman with cuticular drusen and drusenoid pigment epithelial detachment (PED) in both eyes (Case 3). a Color photographs show whitish confluent drusen covering the macula with surrounding area showing many large drusen. b Fluorescein angiography shows hypofluorescent macula with multiple large hyperfluorescence corresponding to large drusen. c Fundus autofluorescence shows several hypoautofluorescence dots inside the large drusen corresponding to hyperfluorescence on fluorescein angiography. d Spectral domain optical coherence tomography shows drusenoid PED in both eyes
Discussion
In this study, we describe Japanese patients with cuticular drusen, with or without additional macular pathology. Of 12 patients with cuticular drusen, additional macular pathology was present in 7 (83.3%) on initial presentation. Among those with additional macular pathology, drusenoid PED was most frequent.
Drusenoid PED comprises irregularly elevated RPE by multiple and confluent large drusen that can be easily distinguished from other PEDs, such as serous PED, hemorrhagic PED, and fibrovascular PED by fundus color photography and fluorescein angiography [17]. Drusenoid PED is known as a precursor lesion of GA because it generally grows slowly during long-term follow-up and eventually collapses resulting in GA [18]. Given that drusenoid PED is a manifestation of AMD, 11 eyes (45.8%) of 6 (50%) patients exhibited AMD on initial visit. A recent study demonstrates that over a 5-year follow-up of cuticular drusen eyes, acquired vitelliform lesions occurred at a frequency of 24.2%, GA at a rate of 12.5%, and neovascularization at a rate of 25% [7]; however, association of drusenoid PED is not described in this report. It is necessary to confirm independently whether drusenoid PED is an additional macular pathology specific to Asian eyes with cuticular drusen.
In the present study mean age of the 5 patients with cuticular drusen without additional macular pathology was 55.4 years (range 44–68). It is well known that cuticular drusen have an early onset in patients of European descent [15]. Although the exact onset of cuticular drusen is not evident in the present study, cuticular drusen seemed to manifest at a younger age among Japanese patients.
It is reported that cuticular drusen are predominantly seen in women of European descent [7]. The present results that 8(66.6%) out of 12 patients were women, is consistent with the report of a female predilection.
In our study, 6 eyes of 3 patients (Case 1, 3, and 6) presented with cuticular drusen > 200 µm and were classified as LCD. Early onset is a feature in patients with LCD. In this study, at initial presentation the mean age of patients with LCD was 53.7 ± 8.7 years, younger than those without LCD but the difference was not statistically significant, and is consistent with a previous report [9]. That study revealed that LCD occur in 25% of Japanese patients with cuticular drusen. Recently Terao et al. [19] presented three Japanese cases with cuticular drusen and 1(33%) out of 3 exhibited LCD. Further studies with larger cohorts are also likely reveal LCD in the Asians.
A major limitation of the present study is the small sample size of the cohorts. This was because cuticular drusen are rare in Asians though their exact prevalence has not been investigated. The other limitation is that there could have been a sampling bias. Since, in the present study fluorescein angiography was required for diagnosis of cuticular drusen and it is an invasive imaging modality, patients without additional macular pathology may have been fewer than those with additional macular pathology. Lastly, this was a cross-sectional study. A longitudinal study would be needed to determine whether cuticular drusen are associated with progression to AMD in the Japanese.
To summarize, cuticular drusen are predominantly seen in women and are associated with additional macular pathology, especially drusenoid PED. LCD are present in 25% of Japanese patients with cuticular drusen in the present study.
References
Khan KN, Mahroo OA, Khan RS, Mohamed MD, McKibbin M, Bird A, et al. Differentiating drusen: drusen and drusen-like appearances associated with ageing, age-related macular degeneration, inherited eye disease and other pathological processes. Prog Retin Eye Res. 2016;53:70–106.
Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–33.
Sakurada Y, Sugiyama A, Kikushima W, Yoneyama S, Tanabe N, Matsubara M, et al. Pseudodrusen pattern and development of late age-related macular degeneration in the fellow eye in the unilateral case. Jpn J Ophthalmol. 2019;63(5):374–81.
Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, et al. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol. 2005;123:1570–4.
Gass JD. Stereoscopic atlas of macular diseases; diagnosis and treatments. 2nd ed. St. Louis: Mosby; 1977. p. 46–50.
Russell SR, Mullins RF, Schneider BL, Hageman GS. Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. Am J Ophthalmol. 2000;129:205–14.
Balaratnasingam C, Cherepanoff S, Dolz-Marco R, Killingsworth M, Chen FK, Mendis R, et al. Cuticular drusen: clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology. 2018;125:100–18.
van de Ven JP, Smailhodzic D, Boon CJ, Fauser S, Groenewoud JM, Chong NV, et al. Association analysis of genetic and environmental risk factors in the cuticular drusen subtype of age-related macular degeneration. Mol Vis. 2012;18:2271–8.
Guigui B, Leveziel N, Martinet V, Massamba N, Sterkers M, Coscas G, et al. Angiography features of early onset drusen. Br J Ophthalmol. 2011;95:238–44.
Roberti NC, Dias JRO, Novais EA, Regatieri CS, Belfort R Jr. Large colloid drusen analyzed with structural en face optical coherence tomography. Arq Bras Oftalmol. 2017;80:122–4.
Veronese C, Maiolo C, Mora LD, Morara M, Armstrong GW, Ciardella AP. Bilateral large colloid drusen in a young adult. Retina. 2017;37:e132–4.
Sakurada Y, Parikh R, Gal-Or O, Balaratnasingam C, Leong BCS, Tanaka K, et al. Cuticular drusen: risk of geographic atrophy and macular neovascularization. Retina. 2018. https://doi.org/10.1097/IAE.0000000000002399.
Sato A, Senda N, Fukui E, Ohta K. Retinal angiomatous proliferation in an eye with cuticular drusen. Case Rep Ophthalmol. 2015;6:127–31.
Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–54.
Boon CJ, van de Ven JP, Hoyng CB, den Hollander AI, Klevering BJ. Cuticular drusen: stars in the sky. Prog Retin Eye Res. 2013;37:90–113.
van de Ven JP, Boon CJ, Smailhodzic D, Lechanteur YT, den Hollander AI, Hoyng CB, et al. Short-term changes of Basal laminar drusen on spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;154:560–7.
Cukras C, Agron E, Klein ML, Ferris FL 3rd, Chew EY, Gensler G, et al. Natural history of drusenoid pigment epithelial detachment in age-related macular degeneration: age-related eye disease study report no. 28. Ophthalmology. 2010;117:489–99.
Roquet W, Roudot-Thoraval F, Coscas G, Soubrane G. Clinical features of drusenoid pigment epithelial detachment in age related macular degeneration. Br J Ophthalmol. 2004;88:638–42.
Terao R, Matsuda A, Ogawa A, Shimizu K, Azuma K, Inoue T, et al. Optical coherence tomography angiography study of choroidal neovascularization associated with early-onset drusen. Retin Cases Brief Rep. 2019. https://doi.org/10.1097/icb.0000000000000856.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y. Sakurada, Lecture fee (Santen, Novartis, Bayer); K. Tanaka, None; A. Miki, None; H. Matsumoto, None; A. Kawamura, None; R. Mukai, None; H. Akiyama, None; S. Honda, None; R. Mori, None; H. Iijima, Lecture fee (Novartis), Speaker fee (Bayer, Novartis, Santen, Shionogi).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Yoichi Sakurada
About this article
Cite this article
Sakurada, Y., Tanaka, K., Miki, A. et al. Clinical characteristics of cuticular drusen in the Japanese population. Jpn J Ophthalmol 63, 448–456 (2019). https://doi.org/10.1007/s10384-019-00692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-019-00692-5