Abstract
Purpose
To compare the efficacy of 577- and 810-nm subthreshold micropulse laser photocoagulation (SMLP) combined with direct photocoagulation to microaneurysms in diabetic macular edema (DME).
Methods
A prospective nonrandomized interventional case series. Forty-nine consecutive patients (53 eyes) with DME were recruited. In 20/24 (83.3 %) eyes, 810-nm SMLP (810-nm MP) to achieve a confluent grid pattern was followed by direct photocoagulation to microaneurysms via a continuous 561-nm wavelength laser. In 21/29 (72.5 %) eyes, 577-nm SMLP (577-nm MP) was combined with direct photocoagulation to microaneurysms via the same instrument. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were examined 1, 2, 3, 6 and 12 months after treatment.
Results
The mean power required for SMLP was lower in the 577-nm than in the 810-nm MP group (204.1 vs. 954.1 mW) (p < 0.0001). Significant reductions in CMT persisted from 3 to 12 months after treatment in all patients (p < 0.01). There were no significant intergroup differences in CMT until 12 months. In both groups, mean BCVA remained stable until 12 months after treatment. Additional treatment for persistent macular edema was performed within 12 months in 4/24 eyes (16.7 %) in the 810-nm MP group and 1/29 eyes (3.4 %) in the 577-nm MP group.
Conclusion
Either 577-nm MP or 810-nm MP combined with direct photocoagulation for microaneurysm closure reduced DME, maintained visual acuity and reduced the additional treatment rate within 12 months. The 577-nm MP apparatus required less energy for SMLP than the 810-nm MP instrument and was suitable for direct photocoagulation of microaneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) is the most common cause of visual acuity loss in patients with diabetes. In 1985, the Early Treatment Diabetic Retinopathy Study (ETDRS) showed that focal photocoagulation in “clinically significant” DME substantially reduces the risk of visual loss [1]. However, the progressive enlargements of laser scars [2], subretinal fibrosis [3] and subretinal neovascular membranes [4, 5] are reported as complications from laser tissue damage, resulting in the occurrence of scotomas or color vision loss. Less invasive treatment strategies have been advocated in order to reduce the amount of laser energy applied and thus avoid tissue damage.
Advances in laser technology have led to the development of selective photocoagulation for treatment of the retinal pigment epithelium (RPE) using the subthreshold micropulse laser photocoagulation (SMLP) method [6–15]. This modality is designed to target the RPE, with minimal effects on the sensory retina and choroid [6].
In 1997, Friberg et al. [7] reported on the clinical application of 810-nm SMLP (810-nm MP) in the treatment of DME. Several clinical studies have since confirmed the efficacy of this approach [6, 8–15]. Recent clinical studies demonstrated that SMLP achieves effects similar or superior to those of modified ETDRS photocoagulation [9, 15]. We previously reported that 810-nm MP was effective in reducing DME with or without microaneurysm closure [13, 16]. However, modified ETDRS photocoagulation with microaneurysm closure significantly reduced macular edema compared to mild macular grid photocoagulation without microaneurysm closure [17].
The combination of SMLP and direct photocoagulation for microaneurysm closure is assumed to be more effective than SMLP alone and less invasive than modified ETDRS photocoagulation. The IQ577® (Iridex, Mountain View, CA, USA) is a new apparatus capable of delivering a conventional laser at a 577-nm wavelength suitable for microaneurysm closure. The 577-nm wavelength is absorbed well by oxyhemoglobin and RPE [18]. This instrument can also deliver a micropulse laser at 577 nm. A literature search revealed few published reviews on the efficacy of pure yellow SMLP (577-nm MP) [19]. This study assessed the efficacy of a combination therapy of grid photocoagulation by SMLP and direct photocoagulation of microaneurysms by a continuous wave laser and compared 577-nm SMLP + 577-nm direct photocoagulation of microaneurysms and 810-nm SMLP + 561-nm direct photocoagulation of microaneurysms.
Methods
Study design
This study was a single-center, prospective, nonrandomized interventional case series.
Patient eligibility
Forty-nine consecutive patients (53 eyes) with type 2 diabetes and clinically significant macular edema per the ETDRS [1] criteria were recruited for the study. Twenty-two patients (24 eyes) received 810-nm MP (810-nm MP group) from 3 April 2010 through 25 July 2011; 27 patients (29 eyes) received 577-nm MP (577-nm MP group) from 25 September 2011 through 5 May 2012. We obtained approval from the Research Ethics Committee of St. Luke’s International Hospital prior to the study. Informed consent was obtained from all patients. The eligibility criteria included a diagnosis of mild, moderate or severe nonproliferative diabetic retinopathy or proliferative diabetic retinopathy with clinically significant macular edema (ETDRS criteria [1]) involving either the center of the macular region or a border involving the foveal avascular zone. Fluorescein angiography was performed at the time of recruitment to confirm diffuse dye leakage. We treated focal macular edema using a continuous wave laser and 41 out of 53 (77.4 %) eyes with focal dye leakage combined with a circulate ring in which microaneurysms are located at the center. In this study, the edge of the ring was located ≥1000 μm from the center of the fovea. Patients who had only focal fluorescein dye leakage from microaneurysms were excluded, whereas patients who had either single or multiple areas of focal dye leakage were included. The other exclusion criteria included a history of vitrectomy, a history of cataract surgery or any other intraocular surgery or panretinal photocoagulation, and previous therapy for macular edema (including subtenon injection of triamcinolone, intravitreal injection of any drug or macular laser photocoagulation) less than 3 months before the study. Patients on hemodialysis were also excluded.
SMLP and direct photocoagulation of microaneurysms were performed by a single surgeon; however, pre- and postoperative examinations as well as PRP for severe NPDR or PDR prior to the study were performed by other doctors.
For micropulse photocoagulation, an 810-nm diode laser photocoagulation device (Iris Medical OcuLight Slx®) or 577-nm laser photocoagulation device (IQ577®), both manufactured by Iridex Corp. (Mountain View, CA, USA), was used in the Micropulse operating mode. The laser was applied through a three-mirror contact lens to the thickened area of the macular region exhibiting diffuse fluorescein leakage. The laser power for SMLP was determined for each patient by creating a threshold burn with the lowest energy required to make a visible “test burn” at a nonedematous area outside the vascular arcade. The laser was then employed at 60 % of that energy level in the micropulse mode and applied to confluent spots up to 500 μm from the center of the fovea. The test burn was created with continuous wave laser energy for 0.1 s at a diameter of 200 μm. Subsequently, laser spots were applied using the 15 % duty cycle micropulse mode at 200 % of threshold energy for 0.2 s, which delivered 60 % of the threshold energy. The final mean energy was 954.9 mW (500–2000 mW) in the 810 MP group and 204.1 mW (180–400 mW) in the 577-nm MP group. If patients had focal macula edema secondary to microaneurysms, microaneurysm closure was attempted at the time of initial treatment as well as via SMLP within 1 week in the 810-nm MP group or simultaneously via SMLP in the 577-nm MP group. Microaneurysms located <1000 µm from the macula center were not closed using direct photocoagulation. In 20/24 (83.3 %) eyes in the 810-nm MP group, direct photocoagulation was performed on microaneurysms using a NOVAS Varia®(LUMINAS Corp., Mountain View, CA, USA) (561 nm, spot size 50–100 μm, time 0.05–0.1 s, 100–140 mW). In 21/29 (72.5 %) eyes in the 577-nm MP group, the IQ577® (577 nm, spot size 50–100 μm, time 0.05–0.1 s, 80–110 mW) was used for microaneurysm closure.
Best-corrected visual acuity (BCVA) and macular parameters were examined at the time of enrollment as well as at 1, 2, 3, 6 and 12 months after treatment. Visual acuity was determined using a Snellen chart, and logarithm of the minimum angle of resolution (logMAR) values were calculated for statistical analyses. Central macular thickness (CMT) was measured using a Cirrus apparatus (Zeiss Humphry Instruments, Dublin, CA, USA) using the “Cube scan” mode. Color fundus photographs were taken at enrollment, immediately after treatment, and at 1, 3, 6 and 12 months after treatment. Fluorescein angiography was performed at the time of enrollment and repeated when considered to be clinically necessary.
Patients were followed up at monthly intervals for at least 3 months without any additional treatment. Subsequently, further SMLP or additional pharmacological treatment was provided for persistent macular edema and/or a decrease in visual acuity since the last visit, as reported previously [13, 16]. Macular edema that was stable or increased CMT in comparison to values measured at the last visit was defined as persistent macular edema. Patients who received further SMLP were evaluated at the final visit, while BCVA and CMT were not evaluated again in patients who received additional pharmacologic treatment.
Pearson’s chi-squared test was used to compare the gender and type of diabetes mellitus between groups. Student’s t test was used to compare age and HbA1c between groups. The primary endpoint of this study was the change in CMT at 3 months; secondary endpoints included the change in BCVA (logMAR) at 3 months. The Friedman test and Wilcoxon signed-rank test for post hoc testing were used to evaluate changes in CMT and BCVA within each treatment group throughout the study. Changes in CMT and BCVA from baseline were compared between groups using the Mann-Whitney test. All analyses were performed using SPSS software version 22 (Chicago, IL, USA).
Results
Demographic data and baseline characteristics
The demographic data and baseline characteristics for the study patients are shown in Table 1. The study included 32 men (35 eyes) and 17 women (18 eyes). Mean age (SD) was 65.9 (9.2) years in the 810-nm MP group and 65.3 (7.9) years in the 577-nm MP group. Among all subjects, diabetic retinopathy was classified as mild or moderate nonproliferative retinopathy in 30/53 eyes (56.6 %), severe nonproliferative retinopathy in 11/53 eyes (20.8 %) and early proliferative retinopathy in 12/53 eyes (22.6 %). No significant differences between groups were found for mean age, preoperative HbA1c level, diabetic retinopathy severity or CMT. Panretinal photocoagulation was not performed during the follow-up period. Panretinal photocoagulation was performed more than 3 months before the study in 8/24 eyes in the 810 MP group (severe nonproliferative retinopathy in 5 eyes, early proliferative retinopathy in 3 eyes) and 10/29 eyes in the 577 MP group (severe nonproliferative retinopathy in 1 eye, early proliferative retinopathy in 9 eyes). The final mean energy required for SMLP was 204.1 mW in the 577 MP group, which was significantly lower than the 954.1 mW required in the 810 MP group (p < 0.0001).
Further treatment
The additional treatment administered after micropulse photocoagulation is shown in Table 2. All patients completed 3 months of follow-up prior to receiving additional treatment. Treatment for persistent macular edema was performed within 12 months in 4/24 eyes (16.7 %) in the 810-nm MP group and 1/29 eyes (3.4 %) in the 577-nm MP group. SMLP was performed within 12 months for 3/24 eyes (12.5 %) in the 810-nm MP group. For persistent subfoveal retinal detachment, intravitreal bevacizumab injections were administered to 1/24 eyes (4.2 %) in the 810-nm MP group within 12 months from inclusion in the study. Overall, 20/24 eyes (83.3 %) in the 810-nm MP group and 28/29 eyes (96.6 %) in the 577-nm MP group required no additional treatment within the first 12 months after the study than subthreshold micropulse diode laser photocoagulation. Panretinal photocoagulation was not performed in any of the eyes during this 12-month period.
Effect of treatment on central macular thickness
The CMT changes in both groups are shown in Fig. 1. For all patients, significant reductions (p < 0.01) in CMT remained stable for 3–12 months after treatment. At baseline, mean CMT was not significantly different (p = 0.161) between groups. At 1 month, there was a significant reduction (p < 0.01) in CMT in the 577-nm MP group. After 3 months, there was a decrease in CMT (p < 0.01) in both groups. These significant reductions (p < 0.05) in CMT remained stable after treatment for 3–12 months after treatment in both groups. There was no significant difference in CMT between groups within up to 12 months after the study began. In the 810-nm MP group, a CMT reduction of ≥20 % was observed in 9/22 eyes (40.9 %) at 3 months and 10/24 (41.7 %) eyes at 12 months. The data obtained at 3 months for 2 patients (2 eyes) in the 810-nm MP group were excluded owing to a lack of CMT data. In the 577-nm MP group, a CMT reduction of ≥20 % was seen in 10/27 eyes (37.0 %) at 3 months and 10/27 eyes (37.0 %) at 12 months. Data for 2 patients (2 eyes) in the 577-nm MP group were excluded owing to a lack of CMT data at 3 and 12 months.
Comparison of central macular thickness (CMT) in the 810-nm micropulse photocoagulation (MP) group vs. the 577-nm MP group. At 1 month, there was a significant difference (*p < 0.05) in CMT between groups. The significant difference in CMT between groups was stable from 3 to 12 months after treatment. There was no significant difference in CMT at 12 months. Asterisk: significantly different compared with baseline
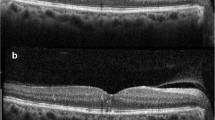
A representative case treated with 577-nm MP combined with direct photocoagulation is shown in Fig. 2.
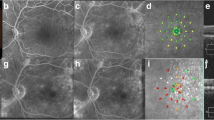
A patient with diabetic macular edema treated by 577-nm micropulse photocoagulation (MP) combined with direct photocoagulation for microaneurysms. a Fundus color photograph at 1 h after photocoagulation shows cystoid macular edema. Fundus color photograph at 1 h after photocoagulation shows direct photocoagulation laser scars but no obvious micropulse laser scars. Optical coherence tomography at baseline (b), 3 months (c) and 6 months (d) after treatment. Foveal thickness was 347 μm at baseline and 320 μm at 3 months. Visual acuity was 20/29 before 577-nm MP and 20/29 at 3 months. e Baseline fluorescein angiography reveals diffuse dye leakage in the macular area; 577-nm MP was applied to the area of diffuse dye leakage, and direct photocoagulation was applied to the area of leakage for microaneurysms. The area treated with 577-nm MP is enclosed by the dotted line. f Fluorescein angiography at 3 months. Diffuse dye leakage was decreased in comparison to baseline
Effect of treatment on visual acuity
Changes in visual acuity are shown in Fig. 3. Because baseline BCVA differed significantly between groups, intergroup differences at subsequent time points were not evaluated. In both groups, mean BCVA remained stable until 12 months after treatment. BCVA was either improved or maintained within 0.2 logMAR in 91.7 % of eyes in the 810-nm MP group and 93.1 % in the 577-nm MP group at 3 months. At 12 months, BCVA was improved or maintained within 0.2 logMAR in 87.5 % of eyes in the 810-nm MP group and 89.7 % in the 577-nm MP group.
Change in BCVA in the 810-nm MP group and the 577-nm MP group. In both groups as well as the overall patient population, mean BCVA was stable up to 12 months after treatment. Because baseline BCVA was significantly different between groups, intergroup differences at subsequent time points were not evaluated
Adverse events and macular changes
When pre- and postoperative fundus color photographs were compared, no laser scars due to SMLP were detected. No patient developed a subretinal neovascular membrane, subretinal fibrosis, foveal distortion or any macular complication of laser therapy, and none of the patients complained of scotoma.
Discussion
In this study, 89.7 % of patients treated with 577-nm MP and 87.5 % of those treated with 810-nm MP maintained a relatively constant level of visual acuity for 12 months. DME was significantly decreased at 3 months after surgery in both groups.
The 577-nm MP is easy to perform and results in successful microaneurysm closure, thereby suggesting that the technique can be used to control DME. The efficacy of treatment as assessed at the 12-month follow-up was not significantly different between groups.
In 2011, Lavinsky et al. [9] reported a prospective randomized study comparing 810-nm MP and the modified ETDRS laser. In the study, high-density SMLP was reported to be superior to conventional modified ETDRS laser therapy in the reduction of DME. In 2010, Ohkoshi et al. [13] reported that 810-nm MP reduced DME and maintained visual acuity for the ensuing 12 months. In 2012, Inagaki et al. [16] followed patients for 12 months and reported that a combination of 810-nm MP therapy and 561-nm continuous direct photocoagulation for microaneurysm closure was effective in the treatment of DME.
SMLP is designed to produce lesions that do not extend beyond the RPE. This treatment does not produce any visible scar or tissue damage that can be detected on OCT images [19]. However, 20.9 % of patients treated with SMLP required direct photocoagulation to microaneurysms or other adjuvant therapy within 12 months after treatment [13]. The present study showed that combination therapy with SMLP and direct photocoagulation reduced the rate of additional treatment within 12 months [810-nm MP group, 4/24 eyes (16.7 %) vs. 577-nm MP group, 1/29 eyes (3.4 %)].
The major pathogenic mechanism of DME is serum leakage into the extravascular space. In particular, microaneurysms are a major source of leakage, frequently resulting in the extravasation of serum lipoprotein and associated circinate rings. It is difficult to control macular edema presenting with a circinate ring without applying direct photocoagulation, as this type of macular edema is typically associated with multiple leaking microaneurysms. Direct photocoagulation to leaking microaneurysms effectively controls macular edema in most patients [17]. Thus, combined therapy is a better approach than SMLP alone for diffuse macular edema with microaneurysms.
In 2007, the Diabetic Retinopathy Clinical Research network reported that patients who had undergone modified ETDRS laser treatments for macular edema exhibited outcomes superior to those with mild macular edema who did not undergo microaneurysm closure [17]. The results of that study suggest that grid photocoagulation targeting the RPE and outer retina was insufficient for the treatment of patients with leaking microaneurysms. Therefore, the combination of grid photocoagulation to diffuse leakage areas and microaneurysm closure is considered the most effective strategy for DME treatment. However, performing grid photocoagulation with a modified ETDRS laser creates a visible scar, leading to irreversible macular damage.
The pattern scan laser was recently presented as a less invasive modality [20, 21]. This type of laser selectively damages the outer retina, allowing for photoreceptor recovery at the ellipsoid line several months after surgery [20, 21]. The portion of the retina to which the laser was applied was visualized on OCT scans long after surgery, revealing irreversible damage to the outer retina. In contrast, combined SMLP and direct photocoagulation for diffuse macular edema with circinate rings create several scars at the site of microaneurysm ablation, but do not create any scar in the area of grid laser application.
The rate of absorption by melanin and oxyhemoglobin is higher for the 577-nm wavelength as compared to the 810-nm wavelength [18]. In this study, substantially lower power was required when micropulses were applied at 577 vs. 810 nm. Thus, the 577-nm laser appears to be more suitable for microaneurysm coagulation as well as micropulse ablation.
In conclusion, there were no statistically significant differences in efficacy between the two SMLP wavelengths. However, the IQ577® was easier to manipulate given its more stable titration power than the 810-nm instrument, which allowed microaneurysm coagulation and SMLP to be performed simultaneously in this study. The limitations of this study include the lack of a control group, nonrandomization and differences in BCVA between the groups. The 577-nm group exhibited significantly higher BCVA than the 810-nm group at baseline. Therefore, the improvement in BCVA presented here might have limited relevance. We expect that future well-designed, randomized studies will corroborate the efficacy of this combination technique.
References
Early Treatment Diabetic Retinopathy Study Reseach Group. Photocoagulation for diabetic macular edema: ETDRS report number 1. Arch Ophthalmol. 1985;103:1796–806.
Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–51.
Guyer DR, D’Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113:652–6.
Rutledge BK, Wallow IH, Poulsen GL. Sub-pigment epithelial membranes after photocoagulation for diabetic macular edema. Arch Ophthalmol. 1993;111:608.
Varley MP, Frank E, Purnell EW. Subretinal neovascularization after focal argon laser for diabetic macular edema. Ophthalmology. 1988;95:567–73.
Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–4.
Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997;104:2030–8.
Laursen M, Moeller F, Sander B, Sjoelie A. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–9.
Lavinsky D, Cardillo JA, Melo LA, Dare A, Farah ME, Belfort R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011;52:4314–23.
Luttrull J, Musch D, Mainster M. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80.
Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2006;37:370.
Moorman C, Hamilton A. Clinical applications of the micropulse diode laser. Eye. 1999;13:145–50.
Ohkoshi K, Yamaguchi T. Subthreshold micropulse diode laser photocoagulation for diabetic macular edema in Japanese patients. Am J Ophthalmol. 2010;149(133–9):e1.
Stanga PE, Reck AC, Hamilton AMP. Micropulse laser in the treatment of diabetic macular edema. Seminars in ophthalmology, vol. 4. London: Informa UK Ltd; 1999. p. 210–3.
Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010;30:908–16.
Inagaki K, Iseda A, Ohkoshi K. Subthreshold micropulse diode laser photocoagulation combined with direct photocoagulation for diabetic macular edema in Japanese patients. NipponGankaGakkaiZasshi. 2012;116:568–74 (in Japanese).
Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, et al. Comparison of the modified early treatment diabetic retinopathy study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80.
Mainster MA. Wavelength selection in macular photocoagulation. Ophthalmology. 1986;93:952–8.
Vujosevic S, Martini F, Convento E, Longhin E, Kotsafti O, Parrozzani R, et al. Subthreshold laser therapy for diabetic macular edema: metabolic and safety issues. Curr Med Chem. 2013;20:3267–71.
Inagaki K, Ohkoshi K, Ohde S. Spectral-domain optical coherence tomography imaging of retinal changes after conventional multicolor laser, subthreshold micropulse diode laser, or pattern scanning laser therapy in Japanese with macular edema. Retina. 2012;32:1592–600.
Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Patton N, et al. In vivo laser-tissue interactions and healing responses from 20 vs. 100-millisecond pulse Pascal photocoagulation burns. Arch Ophthalmol. 2010;128:448–55.
Conflicts of interest
K. Inagaki, None; K. Ohkoshi, None; S. Ohde, None; G. A. Deshpande, None; N. Ebihara, None; A. Murakami, None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Inagaki, K., Ohkoshi, K., Ohde, S. et al. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561–577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol 59, 21–28 (2015). https://doi.org/10.1007/s10384-014-0361-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-014-0361-1