Abstract
Purpose
To investigate the effects of vitreomacular adhesion (VMA) on intravitreal ranibizumab treatment in Japanese patients with exudative age-related macular degeneration (AMD).
Methods
This was a retrospective comparative study that included 123 eyes from 123 patients with exudative AMD. The presence or absence of VMA was examined by spectral domain optical coherence tomography. The association of VMA with best-corrected visual acuity (BCVA) and central retinal thickness (CRT) at 3, 6, and 12 months after ranibizumab treatment was evaluated.
Results
In the group of eyes without VMA [VMA(−)], the mean BCVA was 0.41 logMAR at baseline and significantly improved to 0.28, 0.30, and 0.29 logMAR at 3, 6, and 12 months following the initiation of treatment (P < 0.0001, <0.0001, <0.0001), respectively. In the group of eyes with VMA [VMA(+)], the mean BCVA was 0.42 logMAR at baseline, and there was no improvement at any of the measurement time-points during the follow-up period [0.39, 0.40, and 0.39 logMAR at 3, 6, and 12 months (P = 0.53, 0.75, 0.67), respectively]. The mean baseline CRT in the VMA(−) and VMA(+) groups was 326 and 370 µm, respectively, decreasing to 195 and 293 µm (P < 0.0001 and P = 0.0070), respectively, at 12 months. A better baseline BCVA was associated with poor visual response to intravitreal ranibizumab.
Conclusions
Our study of Japanese patients with AMD managed in real-world clinical practice revealed that both VMA and BCVA at baseline were associated with a poor visual response to intravitreal ranibizumab. These results are in agreement with previously reported findings for other ethnic groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The possible association between vitreomacular interface status and exudative age-related macular degeneration (AMD) has been extensively investigated, revealing a number of interesting results. First, posterior vitreous attachment to the posterior pole has become generally recognized as being more frequent in eyes with exudative AMD than in those without AMD [1–5]. A previous study from our group also demonstrated that complete posterior vitreous detachment (PVD) is less frequent in typical AMD than in controls. In that study, a larger lesion size was associated with the absence of PVD in typical AMD [6]. Second, a number of investigators have suggested that PVD protects against exudative AMD, arguing that intervention to induce PVD may be a treatment option for exudative AMD [4, 7]. The results of various studies which examined the visual and anatomic outcomes of patients treated with off-label use of bevacizumab and ranibizumab support this possibility, demonstrating that incomplete PVD or vitreomacular adhesion (VMA) may have a significantly adverse effect on the efficacy of intravitreal anti-vascular endothelial growth factor (VEGF) treatment for exudative AMD [7–11].
However, to the best of our knowledge, the association between VMA and treatment outcome has not been investigated in Japanese patients with AMD. Ethnic differences do exist in the clinical findings of exudative AMD. For example, in Japanese patients, the proportion of typical AMD ranges from 30 to 40 % of newly diagnosed exudative AMD cases, whereas PCV comprises 30–50 % of these same cases [12–14]; in comparison, in white patients, most exudative AMD cases are categorized as typical AMD [15]. In the study reported here, we corroborate and extend the findings of previous clinical studies, focusing on Japanese patients.
Patients and methods
Patients
The patient group comprised 123 consecutive patients who visited the outpatient macular clinic of the University of Tokyo Hospital and were diagnosed as having exudative AMD between June 2009 and April 2011 and followed up for at least 12 months after the initial treatment. Only those patients whose best-corrected visual acuity (BCVA) data and spectral domain optical coherence tomography (SD-OCT) images were available at baseline and at 3, 6, and 12 months after the initiation of treatment were initially enrolled in the study. All clinical examination data on these patients were collected and retrospectively reviewed. Ultimately, only patients with a definite diagnosis of exudative AMD based on the fundus examination, fluorescein angiography (FA), and indocyanine green angiography (ICGA) were included. The following patients were excluded from the study: (1) patients with a history of treatment for AMD, such as laser photocoagulation, verteporfin photodynamic therapy (PDT), and off-label use of intravitreal bevacizumab; (2) patients who switched to treatments other than intravitreal ranibizumab, such as photocoagulation and PDT, during follow-up; (3) patients who had undergone vitrectomy or had other pathologic eye conditions known to affect the vitreomacular interface, such as retinal vascular disease, pathologic myopia, diabetic retinopathy, and retinal breaks; (4) patients who were unable to receive or refused the recommended treatment for non-medical-related (e.g., socioeconomic) reasons.
Institutional review board approval was obtained from the University of Tokyo.
Diagnostic criteria
All patients with exudative AMD were diagnosed by slit lamp biomicroscopy and both FA and ICGA. If elevated orange–red lesions were observed by fundus examination or if a polypoidal structure of the choroidal vessels with or without abnormal vascular networks was observed on ICGA, polypoidal choroidal vasculopathy (PCV) was diagnosed [12–14].
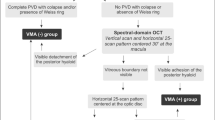
Vitreomacular adhesion
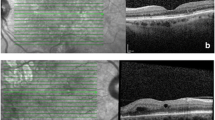
Vitreomacular adhesion was examined by SD-OCT (model 3D OCT-1000; Topcon, Tokyo, Japan) as part of the routine eye examinations. The data obtained with the raster scan protocol were used for the evaluation of VMA. The data consisted of 128 horizontal B-scan images; each image was composed of 512 axial scans, covering a 6 × 6-mm area of the posterior pole. The investigator who retrospectively analyzed the SD-OCT findings was blinded to the clinical diagnosis. All B-scan images were inspected and, as in previous studies [4, 16], VMA was defined as an elevation of the cortical vitreous above the retinal surface, with the vitreous remaining attached within a 3-mm radius of the fovea. If VMA was observed, the eyes were categorized into VMA(+) group; otherwise the eyes were categorized into VMA(−) group. Two independent investigators assessed the cases in a blinded fashion. Assessment of six cases originally differed between the two investigators; agreement was reached upon subsequent re-examination of the OCT images by both investigators and further discussion.
Ranibizumab treatment
Ranibizumab (0.5 mg/0.05 mL; Lucentis; Genentech, South San Francisco, CA) was injected into the vitreous cavity using a standard protocol. After three intravitreal injections of ranibizumab at 0, 1, and 2 months, additional injections were administered at the discretion of the attending physician. Basically, the patients were observed on a monthly basis, and retreatment was recommended if persistent or recurrent fluid was observed on OCT. The total number of injections within 12 months of the initial treatment was recorded.
Treatment outcomes
Visual acuity measurement
The BCVA was measured using a Landolt C chart at baseline and at 3, 6, and 12 months after the initial treatment and converted to the logarithm of the minimum angle of resolution (logMAR) values for statistical analysis.
Central retinal thickness
Central retinal thickness (CRT) was defined as the retinal thickness from the inner limiting membrane to the surface of the retinal pigment epithelium (RPE) of the central points in the horizontal scanning lines. CRT was measured using the electronic calipers of the SD-OCT at baseline and at 3, 6, and 12 months after the initial treatment.
Statistical analysis
Statistical analysis was performed using JMP version 9.0 software (SAS Institute, Cary, NC). Categorical data were assessed using the Chi-square test, and continuous variables were compared using the t test. Paired t tests were used to compare the BCVA and CRT at follow-up with the data at baseline in each group. The changes in BCVA and CRT over time of the two groups were compared using repeated-measures analysis of variance (ANOVA). The association between the changes in BCVA outcomes and each background factor (such as age, BCVA at baseline, and CRT at baseline) was tested using the Spearman rank correlation. The differences in the BCVA changes between male and female patients were tested using the t test. The association between the changes in CRT and BCVA was assessed using the Spearman rank correlation. Probability values of <0.05 were considered to be significant.
Results
Demographic data
Vitreomacular adhesion was observed in 16 eyes at baseline. In one eye, the VMA had resolved 2 months after the initial treatment and this eye was therefore excluded from the analysis. As for the other eyes, the VMA persisted until 12 months. Vitreomacular traction [7] was not detected in any of the VMA cases. The patients’ demographic characteristics are summarized in Table 1. Sex, age, proportion of PCV, baseline logMAR BCVA, and baseline CRT did not differ between the 2 groups.
Visual outcome
The mean changes in BCVA after the initial ranibizumab treatments are shown in Fig. 1. In the VMA(−) group, the mean BCVA was 0.41 logMAR at baseline, with a subsequent significant improvement to 0.28, 0.30, and 0.29 logMAR at 3, 6, and 12 months (P < 0.0001, <0.0001, and <0.0001), respectively. In the VMA(+) group, the mean BCVA was 0.42 logMAR at baseline, with no subsequent improvement at any of the measurement time-points during the follow-up period [0.39, 0.40, and 0.39 logMAR at 3, 6, and 12 months (P = 0.53, 0.75, and 0.67), respectively]. The mean logMAR BCVA was significantly worse in the VMA(+) group than in the VMA(−) group (P = 0.012, repeated-measures ANOVA). Sex, age, and baseline CRT were not associated with changes in the BCVA from baseline to 12 months of follow-up (P = 0.63, 0.23, and 0.39, respectively). The baseline BCVA was strongly associated with the changes in BCVA at 3, 6, and 12 months (ρ = −0.49, P < 0.0001; ρ = −0.30, P = 0.0006; ρ = −0.37, P < 0.0001, respectively; Spearman rank correlation). The average number of injections during the 12 months of follow-up was 5.2 and 5.1 in the VMA(−) and VMA(+) groups, respectively (P = 0.83).
Mean changes in best-corrected visual acuity (BCVA) (logMAR) after initial ranibizumab treatment. In the eyes without vitreomacular adhesion [VMA(−); filled circles], the mean BCVA was significantly improved at 3, 6, and 12 months following the initiation of treatment. In the eyes with VMA [VMA(+); filled squares], the mean BCVA (logMAR) did not improve at any measurement time-point during the follow-up. Asterisks significant improvement in BCVA as compared with the baseline values, bar standard deviation (SD). logMAR Logarithm of the minimum angle of resolution
OCT outcomes
The mean changes in CRT after the initial ranibizumab treatment are shown in Fig. 2. In the VMA(−) group, the mean CRT was 326 μm at baseline and significantly decreased to 186, 199, and 195 μm at 3, 6, and 12 months after the initiation of treatment (P < 0.0001, <0.0001, and <0.0001), respectively. In the VMA(+) group, the mean CRT was 370 μm at baseline, subsequently decreasing to 191, 252, and 293 μm at 3, 6, and 12 months (P = 0.0002, 0.0095, and 0.0070), respectively. The changes in the mean CRT over time did not differ between the two groups (P = 0.83, repeated-measures ANOVA). The decrease in CRT and the improvement in BCVA at 3 and 12 months were significantly correlated (ρ = 0.27, P = 0.0053 and ρ = 0.26, P = 0.0073, respectively; Spearman rank correlation) and showed a tendency to correlate at 6 months in the VMA(−) group (ρ = 0.17, P = 0.075; Spearman rank correlation). In contrast, the decrease in CRT and the improvement in BCVA at 3, 6, and 12 months were not correlated in the VMA(+) group (P = 0.46, 0.92, and 0.14, respectively).
Mean changes in central retinal thickness (CRT) after initial ranibizumab treatment. In both the VMA(−) group (filled circles) and the VMA(+) group (filled squares), the mean CRT was significantly decreased at 3, 6, and 12 months after treatment initiation. Asterisks Significant improvement in CRT as compared with the baseline values, bar SD
Discussion
Results from earlier studies have demonstrated a lower frequency of PVD and a higher frequency of VMA in patients with exudative AMD than in controls or those with nonexudative AMD [1–5]. These observations indicate a pathologic association between posterior vitreous attachment and exudative AMD. In the present study, BCVA increased in the VMA(−) group and this increase persisted for 12 months; in contrast, BCVA did not increase in the VMA(+) group. Thus, our results indicate that VMA is associated with a poor visual response to intravitreal ranibizumab in Japanese patients with AMD, confirming the findings of previous studies showing that visual prognosis after anti-VEGF treatment for exudative AMD was worse in eyes with posterior VMA [8–11]. A subanalysis of the “Randomized, double-masked, active-controlled, multi-center study comparing the efficacy and safety of ranibizumab administered as two dosing regimens in patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration” (EXCITE) study found that in AMD patients with PVD, the therapeutic efficacy of ranibizumab for patients treated quarterly was similar to that in patients treated monthly. However, the outcome of the VMA group was totally different, with monthly treatment being clearly superior to quarterly treatment [10]. In the present study, the average number of injections during the 12 months of follow-up was 5.2 and 5.1 in the VMA(−) and VMA(+) groups, respectively, which is fewer than the average number of injections in the CATT (n = 6.9) and IVAN (n = 7) trials [17, 18] during a 12-month follow-up. A better baseline BCVA was associated with less improvement. As previously reported, this may due to a “ceiling effect”; i.e., eyes with better BCVA have fewer options to potentially improve [19].
In the present study, CRT had significantly decreased in both the VMA(−) and VMA(+) groups at each measurement time-point during the follow-up period. In the VMA(+) group, ranibizumab treatment resulted in decreased CRT, but not in increased BCVA. Moreover, in the VMA(+) group, the improvement in BCVA did not correlate with a concomitant decrease in CRT at any of the time-points during the follow-up period—in contrast to the findings in the VMA(−) group—thereby confirming the results of a previous study [8]. Why functional gain was not obtained despite the anatomic improvement was unclear. One plausible explanation may be that functional impairment in the retinal and RPE cells was due to the presence of VMA, but further investigations are needed to clarify the mechanism(s).
A previous study by our group failed to support the theory that tractional force, similar to that observed in macular hole formation, exacerbates exudative AMD [6]; i.e., vitreomacular traction was only rarely observed. In the present study we confirmed this observation. Unexpectedly, in branch retinal vein occlusion (BRVO), the presence of VMA was found to be associated with superior visual and anatomic outcomes for intravitreal bevacizumab treatment [20], which seems to contradict the finding of our present study. However, this discrepancy can be reconciled by the different effects of VMA on AMD and BRVO described in our previous study [20].
There are several limitations to our study. First, it was a retrospective study, and the results are from real-world clinical settings where there were no pre-established protocols. As B-mode ultrasonography was not performed, some eyes with vitreous completely attached to the retina might have been categorized into the VMA(−) group in the analysis. However, as the International Vitreomacular Traction Study (IVTS) group mentioned, VMA represents a specific stage of vitreous separation wherein partial detachment of the vitreous in the perifoveal area has occurred [16]. Similarly to the current study, recent reports examined the status of the vitreomacular interface with OCT, assuming that OCT is the best clinical instrument to detect VMA [7, 8]. Second, the patients were from a single institution and, therefore, the results do not present a general overview of exudative AMD in Japanese patients. In the present study, eyes with typical AMD and PCV were not analyzed separately because of the limited number of cases and selection bias; e.g., a certain number of patients with PCV were recommended PDT treatment and, consequently, were excluded from the current analysis.
The findings of this study confirmed the results of previous studies [8–11] and support previous observations showing a poor visual response in eyes with VMA to anti-VEGF treatment, also among Japanese patients. These findings suggest that the effect of VMA on anti-VEGF treatment in exudative AMD is ethnicity-independent.
References
Weber-Krause B, Eckardt U. Incidence of posterior vitreous detachment in eyes with and without age-related macular degeneration: an ultrasonic study [in German]. Ophthalmologe. 1996;93:660–5.
Ondes F, Yilmaz G, Acar MA, Unlu N, Kocaoglan H, Arsan AK. Role of the vitreous in age-related macular degeneration. Jpn J Ophthalmol. 2000;44:91–3.
Quaranta-El Maftouhi M, Mauget-Faysse M. Anomalous vitreoretinal adhesions in patients with exudative age-related macular degeneration: an OCT study. Eur J Ophthalmol. 2006;16:134–7.
Krebs I, Brannath W, Glittenberg C, Zeiler F, Sebag J, Binder S. Posterior vitreomacular adhesion: a potential risk factor for exudative age-related macular degeneration? Am J Ophthalmol. 2007;144:741–6.
Robison CD, Krebs I, Binder S, Barbazetto IA, Kotsolis AI, Yannuzzi LA, et al. Vitreomacular adhesion in active and end-stage age-related macular degeneration. Am J Ophthalmol. 2009;148(79–82):e72.
Nomura Y, Ueta T, Iriyama A, Inoue Y, Obata R, Tamaki Y, et al. Vitreomacular interface in typical exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology. 2011;118:853–9.
Mojana F, Cheng L, Bartsch DU, Silva GA, Kozak I, Nigam N, et al. The role of abnormal vitreomacular adhesion in age-related macular degeneration: spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008;146:218–27.
Lee SJ, Koh HJ. Effects of vitreomacular adhesion on anti-vascular endothelial growth factor treatment for exudative age-related macular degeneration. Ophthalmology. 2011;118:101–10.
Cho HJ, Baek JS, Lee DW, Cho SW, Kim CG, Kim JW. Effects of vitreomacular adhesion on anti-vascular endothelial growth factor treatment for polypoidal choroidal vasculopathy. Retina. 2013;33:2126–32.
Mayr-Sponer U, Waldstein SM, Kundi M, Ritter M, Golbaz I, Heiling U, et al. Influence of the vitreomacular interface on outcomes of ranibizumab therapy in neovascular age-related macular degeneration. Ophthalmology. 2013;120:2620–9.
Uney GO, Unlu N, Acar MA, Hazirolan D, Altiparmak UE, Yalniz-Akkaya Z, et al. Role of posterior vitreous detachment on outcome of anti-vascular endothelial growth factor treatment in age-related macular degeneration. Retina. 2014;34:32–7.
Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392–6.
Obata R, Yanagi Y, Kami J, Takahashi H, Inoue Y, Tamaki Y. Polypoidal choroidal vasculopathy and retinochoroidal anastomosis in Japanese patients eligible for photodynamic therapy for exudative age-related macular degeneration. Jpn J Ophthalmol. 2006;50:354–60.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22.
Iranmanesh R, Eandi CM, Peiretti E, Klais CM, Garuti S, Goldberg DE, et al. The nature and frequency of neovascular age-related macular degeneration. Eur J Ophthalmol. 2007;17:75–83.
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–9.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411.
Williams TA, Blyth CP. Outcome of ranibizumab treatment in neovascular age related macula degeneration in eyes with baseline visual acuity better than 6/12. Eye (Lond). 2011;25:1617–21.
Terao R, Yuda K, Kure K, Inoue T, Ohtsu H, Yanagi Y. Effect of vitreomacular adhesion on antivascular endothelial growth factor therapy for macular edema secondary to branch retinal vein occlusion. Jpn J Ophthalmol. 2014;58:139–45.
Conflicts of interest
Y. Nomura, None; H. Takahashi, None; X. Tan, None; S. Fujimura, None; R. Obata, None; Y. Yanagi, None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nomura, Y., Takahashi, H., Tan, X. et al. Effects of vitreomacular adhesion on ranibizumab treatment in Japanese patients with age-related macular degeneration. Jpn J Ophthalmol 58, 443–447 (2014). https://doi.org/10.1007/s10384-014-0333-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-014-0333-5