Abstract
Purpose
We evaluated central retinal thickness (CRT) in diabetic macular edema (DME) using two different spectral-domain (SD) optical coherence tomography (OCT) instruments: the Cirrus and Spectralis OCTs.
Methods
CRT was measured in 63 eyes of 32 patients with DME using both instruments on the same day.
Results
CRT measurements were significantly greater for the Spectralis than the Cirrus (p < 0.0003, n = 63, paired t test); mean CRT values were 382 ± 136 μm with the Cirrus and 394 ± 139 μm with the Spectralis. In eyes with poor best-corrected visual acuity (BCVA) (<20/40), mean CRT was 378 ± 130 μm with the Cirrus and 395 ± 139 μm with the Spectralis (p < 0.007, n = 29). In eyes with good acuity (equal to or better than 20/40), the mean CRT was 385 ± 142 μm with the Cirrus and 393 ± 140 μm with the Spectralis (p < 0.005, n = 34).
Conclusions
Foveal retinal thickness measurements may vary among SD-OCT devices. The differences between instruments could affect the results of a multicenter study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optical coherence tomography (OCT) evaluation of macular structure is important in diagnosing and monitoring patients with diabetic macular edema (DME). Automated measurements of retinal thickness and volume in the macula using retinal tomograms are useful in observing patients longitudinally in clinical practice and making comparisons in clinical trials. The time-domain OCT using the Stratus OCT (Carl Zeiss Meditec, Inc., Oberkochen, Germany) is the most widely used technique for obtaining macular measurements [1–3]. However, a new class of OCT instruments employing spectral (Fourier)-domain technology has recently been developed. The development of imaging technology for OCT has been remarkable with regard to both speed and methods of analysis. Therefore, it is important to understand the characteristics of the different OCT devices. Wolf-Schnurrbusch et al. [4] report the differences in macular thickness measurements in healthy eyes using six different OCT instruments. Forooghian et al. [5] compared the time-domain Stratus with the spectral-domain (SD) Cirrus OCT (Carl Zeiss Meditec, Inc.) for measuring DME. Two groups reported comparisons of different SD-OCT instruments for retinal thickness measurements in healthy and diseased eyes [6, 7]. However, to our knowledge, no previous reports have compared macular thickness in >50 eyes with DME as measured by different SD-OCT devices, such as the Cirrus and the Spectralis® (Heidelberg Engineering, Inc., Heidelberg, Germany) [8]. Accordingly, we compared the mean foveal retinal thickness in DME measured using the Cirrus and Spectralis to compare reliability across instruments.
Methods
The study involved 22 men and ten women (63 eyes) who were referred for examination with OCT when DME was identified during routine fundus examination at the Department of Ophthalmology, Nagasaki University Hospital. Patient age ranged from 33 to 83 (mean 61.2 ± 10) years. Patients underwent complete ophthalmic examinations, including best-corrected visual acuity (BCVA) measurement, slit-lamp biomicroscopy, indirect ophthalmoscopy, color fundus photography, and fluorescein angiography. Patients with conditions such as refractive error > ±5 D, dense media opacity, and other ocular diseases with macular involvement (e.g., retinal-vein occlusion, age-related macular degeneration, uveitis) were excluded. Patients were fully informed of the purpose of our study and signed written consent forms. The study was conducted under a protocol approved by the local institutional review board in accordance with the ethics stated in the Declaration of Helsinki. In eligible patients, both eyes were studied.
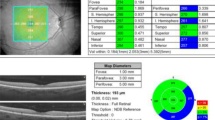
Two different SD-OCT instruments were used to assess central retinal thickness (CRT; mean thickness in the central 1,000-μm diameter area) within 15 min on the same day. OCT instruments were operated by the same trained operators. Before retinal thickness measurements, the eyes were dilated with 2.5% phenylephrine hydrochloride and 1% tropicamide. In the Cirrus, we used a 200 × 200-scan pattern (200 lines; 200 A-scans per line) in a 6-mm2 scan area; and in the Spectralis, we used a 512 × 19-scan pattern (19 lines; 512 A-scans per line with nine scans were averaged to produce one line scan used as the volume scan.) in a 6 × 4.5-mm scan area. Following each examination, the images were deemed acceptable if the retina was clearly visible and distinguishable in every B scan, no eye movement or blinking artifacts occurred during the examination, the full depth and extent of the retina was visualized in each B-scan image, scan signal strength was >6/10 in the Cirrus, and scan qualify factor was >25/40 decibels in the Spectralis. Each CRT value was obtained by a single session. Replicates were only taken if the OCT scans were of insufficient quality. From examinations, thickness maps were calculated with the builtin analysis software of the OCT. For analysis, the means and standard deviations (SD) were calculated for the CRT measured with each instrument. Results are expressed as mean ± SD unless otherwise indicated. The paired Student’s t test was used to compare CRT measurements, unless otherwise indicated. A p value of <0.05 was considered statistically significant. For further analysis, eyes were divided into two groups based on BCVA: good (≥20/40) or poor (<20/40).
Results
Mean CRT for all eyes was 382 ± 136 μm with the Cirrus and 394 ± 139 μm with the Spectralis. Values measured with the Spectralis were significantly greater than those with the Cirrus (Fig. 1a, p < 0.0003, n = 63). Scatterplots were created to illustrate the association between CRT measurements by both OCTs in each eye (Fig. 1b for all eyes). Spearman’s rank correlation coefficient showed a significant correlation (r = 0.95501, p < 0.001). Linear regression analysis was y = 1.0032x + 11.203, R 2 = 0.961. In eyes with poor BCVA (<20/40), mean CRT was 378 ± 130 μm with the Cirrus and 395 ± 139 μm with the Spectralis. For these eyes, the values measured by the Spectralis were significantly greater than those obtained with the Cirrus (Fig. 2, p < 0.007, n = 29). Spearman’s rank correlation coefficient showed a significant correlation (r = 0.94077, p < 0.001). Linear regression analysis was y = 1.0337x + 4.2374, R 2 = 0.9363. In eyes with good acuity (BCVA ≥20/40), mean CRT was 385 ± 142 μm with the Cirrus and 393 ± 140 μm with the Spectralis. Measured values of the Spectralis were significantly greater than those of the Cirrus for these eyes as well (Fig. 3, p < 0.005, n = 34). Spearman’s rank correlation coefficient showed a significant correlation (r = 0.98037, p < 0.001). Linear regression analysis was y = 0.9823x + 15.22, R 2 = 0.985.
a Mean central retinal thickness (CRT) for all eyes with Cirrus and Spectralis optical coherence tomography (OCT). Measured values with the Spectralis were significantly greater than those with the Cirrus (*p < 0.0003, n = 63). b Scatterplot showing the relationship between CRT values of both devices for each eye examined. Linear regression analysis was y = 1.0032x + 11.203, R 2 = 0.961
a Mean central retinal thickness (CRT) in eyes with poorer best-corrected visual acuity (BCVA; <20/40) with Cirrus and Spectralis optical coherence tomography (OCT). Measured values with the Spectralis were significantly greater than those with the Cirrus (**p < 0.007, n = 29). b Scatterplot comparing CRT values between devices in each eye with BCVA <20/40. Linear regression analysis was y = 1.0337x + 4.2374, R 2 = 0.9363
a Mean central retinal thickness (CRT) in eyes with better best-corrected visual acuity (BCVA) ≥20/40) with Cirrus and Spectralis optical coherence tomography (OCT). Measured values with the Spectralis were significantly greater than those with the Cirrus (***p < 0.005, n = 34). b Association between CRT values with both devices in eyes with good visual acuity. Linear regression analysis was y = 0.9823x + 15.22, R 2 = 0.985
As expected from the mean measurements, values for individual eyes were predominantly above the unity diagonal in the scatterplots, indicating that the Spectralis device had a larger CRT estimate. Comparing Fig. 3b with Fig. 2b, the difference between devices appears to be smaller in eyes with good BCVA than in eyes with poor BCVA (mean difference 8.4 μm in eyes with good BCVA; 17.0 μm in eyes with poor BCVA), but the difference between groups was not statistically significant (p < 0.108, unpaired t test). However, correlation coefficients of both groups were significantly different (p < 0.006).
Discussion
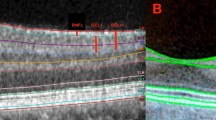
Macular edema is commonly associated with diabetic retinopathy, retinal vein occlusion, uveitis, and cataract extraction. Although fluorescein angiography is highly sensitive in detecting fluid leakage, increases in retinal thickness and loss of corrected visual acuity can occur in the absence of detectable fluorescein leakage. OCT provides quantitative measurements of retinal thickness and is reported as being useful for evaluating various retinal disorders [9, 10]. Davis et al. [11] report that there was a correlation between the Stratus OCT and stereoscopic fundus photograph assessments of macular thickness in patients with DME, suggesting that OCT provides a sensitive and reproducible measurement of retinal thickness. Browning et al. report that central subfield mean thickness (mean thickness in the central 1,000-μm diameter area, equivalent to CRT in this study) is the preferred Stratus measurement for the central macula in patients with DME because of its high reproducibility and correlation with other measurements of the central macula [12]. Accordingly, it is also reasonable to use CRT measurements to compare mean foveal retinal thickness in DME using one of the two SD-OCT instruments: the Cirrus or the Spectralis. Wolf-Schnurrbusch et al. [4] report differences in macular thickness measurements in healthy eyes using six different OCT instruments. In their report, all instruments identified the vitreoretinal interface as the inner retinal border. In contrast, segmentation of the outer retinal border differed substantially among instruments. For example, the Stratus , SOCT Copernicus (Reichert/Optopol Technology, Inc., Depew, NY, USA), Spectral OCT/SLO (Opko/OTI, Inc., Miami, FL, USA), and RTVue-100 (Optovue Corporation, Freemont, CA, USA) all use the junction of the photoreceptor outer (OS) and inner (IS) segments as the outer border of the retina. The Cirrus and Spectralis identify the most outer reflective band (RPE) as the outer border of the retina [4]. Thus, the Cirrus and Spectralis devices should yield higher measurements. Notably, differences in mean CRT between the Cirrus and the Spectralis were not statistically significant in healthy eyes. However, Giani et al. [6] reported that mean CRT values measured with the Spectralis were significantly greater than those obtained with the Cirrus.
In our study, CRT values measured with the Spectralis were significantly greater than those measured with the Cirrus for all eyes. Differences in CRT values between the two devices appeared to be smaller in eyes with good acuity, but this trend was not statistically significant. We attempted to use similar acquisition protocols on each instrument. However, the difference in measured CRT values between the devices may primarily have been the result of the picture intervals between devices, as they each use different numbers of B-scan slices and A-scan pieces [4]. In the Cirrus, we used a 200 × 200-scan pattern (200 lines, 200 A scans per line) in a 6-mm2 scan area; whereas in the Spectralis, we used a 512 × 19-scan pattern (19 lines; 512 A scans per line, nine scans were averaged to produce one line scan to be used as the volume scan) in a 6 × 4.5-mm scan area. In the Cirrus, the intervals were equal for both A and B scans. However, in the Spectralis, the smallest area between the B scans might not capture irregular changes of retinal thickness associated with DME. The number of slices can be changed with the Spectralis, although it is difficult to completely match the acquisition protocol used by the Cirrus. Accordingly, greater similarities between the two devices may be seen if the number of B-scan slices is increased in the Spectralis. Further studies are necessary to clarify the effects of the scan pattern on retinal thickness measurements [13].
The differences of the measured CRT values between the Cirrus and the Spectralis were small enough that they may have been the result of the image processing software algorithm or may have arisen from the different B-scan noise-reduction techniques. Han et al. report that the outer segmentation line for the Cirrus can be seen lying superior to the retinal pigment epithelium/Bruch’s membrane interface. In contrast, the Spectralis segmentation line likely overlies the retinal pigment epithelium/Bruch’s membrane interface [7]. As the Spectralis has an eye-tracking system and follow-up mode to scan exactly the same area as previous scans, it might provide better accuracy when observing patients longitudinally in clinical practice.
Our study is limited by the relatively small number of patients. Macular edema associated with diseases other than DME, such as retinal-vein occlusion, uveitis, and cataract extraction, was not tested. SD-OCTs other than the Cirrus and Spectralis were not evaluated.
In summary, CRT values measured with two different SD-OCT instruments (Cirrus and Spectralis) were significantly different in DME. It is possible that measured values of foveal retinal thickness vary among different SD-OCT devices, and we should therefore be careful with instrument selection when planning a multicenter study to evaluate macular edema using OCT.
References
Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004;137:156–69.
Sanchez-Tocino H, Alvarez-Vidal A, Maldonado MJ, Moreno-Montanes J, Garcia-Layana A. Retinal thickness study with optical coherence tomography in patients with diabetes. Invest Ophthalmol Vis Sci. 2002;43:1588–94.
Krzystolik MG, Strauber SF, Aiello LP, Beck RW, Berger BB, Bressler NM, et al. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114:1520–5.
Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–7.
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:4290–6.
Giani A, Cigada M, Choudhry N, Deiro AP, Oldani M, Pellegrini M, et al. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am J Ophthalmol. 2010;150:815–24.
Han IC, Jaffe GJ. Comparison of spectral- and time-domain optical coherence tomography for retinal thickness measurements in healthy and diseased eyes. Am J Ophthalmol. 2009;147:847–58, 58 e1.
Grover S, Murthy RK, Brar VS, Chalam KV. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (spectralis). Am J Ophthalmol. 2009;148:266–71.
Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Rutledge B, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113:1019–29.
Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29.
Davis MD, Bressler SB, Aiello LP, Bressler NM, Browning DJ, Flaxel CJ, et al. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:1745–52.
Browning DJ, Glassman AR, Aiello LP, Bressler NM, Bressler SB, Danis RP, et al. Optical coherence tomography measurements and analysis methods in optical coherence tomography studies of diabetic macular edema. Ophthalmology. 2008;115:1366–71, 71 e1.
Sayanagi K, Sharma S, Kaiser PK. Comparison of retinal thickness measurements between three-dimensional and radial scans on spectral-domain optical coherence tomography. Am J Ophthalmol. 2009;148:431–8.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture (No. 21592234, KS, TK), by the Japan National Society for the Prevention of Blindness (KS), and by the Takeda Science Foundation (KS).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suzuma, K., Yamada, Y., Liu, M. et al. Comparing central retinal thickness in diabetic macular edema measured by two different spectral-domain optical coherence tomography devices. Jpn J Ophthalmol 55, 620–624 (2011). https://doi.org/10.1007/s10384-011-0085-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-011-0085-4