Abstract
Purpose
To study quantitative changes in anterior chamber angle (ACA) after laser peripheral iridotomy (LPI) in narrow-angle eyes using anterior segment optical coherence tomography (AS-OCT).
Methods
Eighteen subjects with narrow angles were imaged with AS-OCT for determining test–retest variability. Forty-six participants with narrow angles were scanned with AS-OCT before LPI and 4 weeks after LPI. The presence of ACA closure by both AS-OCT imaging and gonioscopy was compared before and after LPI. Three ACA parameters by AS-OCT, angle opening distance at 500 μm (AOD500), trabecular-ris space area at 500 μm (TISA500) and angle recess area at 500 μm (ARA500), at both nasal and temporal quadrants were incorporated for analysis. The increment of ACA parameters defined as exceeding the 95% confidence interval of test–retest variability was assessed after LPI.
Results
All 3 parameters obtained from the 18 eyes showed good measurement reproducibility (intraclass correlation coefficient 0.850–0.979). Persistent angle closure was detected in 23.9% of eyes by gonioscopy, and in 34.8% of eyes by AS-OCT images at temporal quadrant after LPI. When assessed by measurement variability criteria, the percentage of eyes that showed no significant change in ACA parameters ranged from 23.9% to 45.7% after LPI.
Conclusions
Overall, ACA parameters changed significantly after LPI; however, when assessed by AS-OCT, ACA remained unchanged in some narrow-angle eyes despite LPI. Our findings suggest that multiple causes other than pupillary block may contribute to narrow-angle closure following LPI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditional methods such as gonioscopy are used in the visualization of anterior chamber angle (ACA); however, gonioscopy has certain limitations such as high interobserver variability and difficulty in quantification of ACA [1, 2]. An ultrasound biomicroscope (UBM) is another imaging device used for ACA assessment; however, currently available UBM devices usually need ocular contact with the probe and patients should be tested in the supine position. The newly developed anterior segment optical coherence tomography (AS-OCT) has advantages over both gonioscopy and UBM since image acquisition and subsequent measurements can be performed objectively under controlled lighting conditions; it is a non-contact method performed in the sitting position and provides quantitative assessment of the ACA structure.
Primary angle closure (PAC) and subsequent angle-closure glaucoma is a leading cause of irreversible blindness [3].The main underlying mechanism of PAC is pupillary block and laser peripheral iridotomy (LPI) is considered the standard treatment for eliminating pupillary block [3]; however, a substantial portion of PAC eyes was reported to develop peripheral anterior synechiae (PAS) and/or an intraocular pressure (IOP) rise after LPI [4, 5]. A few studies report that LPI might not open all narrow angles [6, 7]. He et al. [7] showed that 20% of narrow-angle suspects remained closed despite LPI based on subjective gonioscopy. Therefore, incomplete or no angle change after LPI could explain progressive PAS despite patent LPI.
Although previous studies investigated ACA changes quantitatively both before and after LPI using AS-OCT [8–11], these changes were based on overall mean values of various parameters following LPI. In addition, these changes might have been affected by operator variability since image processing required the operator to define the scleral spur position on the image. The purpose of our study was to compare ACA changes both before and after LPI using the AS-OCT both quantitatively and qualitatively. In doing so, we tested the amount of operator-induced variability in various parameters among eyes with a narrow ACA. Based on our own variability data and arbitrary cut-off value, we attempted to evaluate the frequency of remained angle closure following LPI using AS-OCT.
Patients and methods
Subjects
PAC and PAC suspect (PACS) subjects were consecutively recruited at the glaucoma clinic of the Asan Medical Center, Seoul, Korea from March to October 2009. All subjects underwent a complete ophthalmic examination, including best-corrected visual acuity (BCVA) testing, slit-lamp examination, Goldmann applanation tonometry, gonioscopy and fundoscopy.
Informed consent was obtained from all participants. The study was approved by the Institutional Review Board of the Asan Medical Center, and we followed the tenets of the Declaration of Helsinki. PAC and PACS were diagnosed by gonioscopic examination. Subjects with appositional contact between the peripheral iris and the posterior trabecular meshwork of >270° were included in the PACS group [12]. Eyes with occludable angles and features indicating that trabecular obstruction by the peripheral iris has occurred, such as elevated IOP, iris whorling (distortion of the radially orientated iris fibers), ‘glaucomflecken’ lens opacities or excessive pigment deposition on the trabecular surface were defined as PAC. Since this study was meant to evaluate ACA changes after LPI, eyes with any PAS in their angle were excluded. We excluded from the study subjects with any history or current use of topical or systemic medication that could affect the angle or pupillary reflex, history of any previous intraocular surgery, laser trabeculoplasty, laser iridoplasty or laser iridotomy and inability to fixate for AS-OCT examination. If both eyes were qualified for inclusion criteria, one eye was randomly selected for analysis.
Slit-lamp examination and gonioscopy
Subjects underwent slit-lamp examination and gonioscopy by an independent observer (MSK) with extensive experience in performing gonioscopy prior to AS-OCT imaging. All subjects were examined with a Sussman lens in a controlled darkened room (0.5 cd/m2). A 1-mm light beam was reduced to a narrow slit. The vertical beam was offset horizontally for assessing superior and inferior angles and horizontal beam offset vertically for nasal and temporal angles. Both static and dynamic gonioscopy were performed using a Sussman lens, with the eye in the primary position of gaze. Indentation gonioscopy was performed to determine if angle closure was due to apposition or PAS. Care was taken to avoid light falling on the pupil. All four quadrants were assessed although nasal and temporal data were entered in the analysis. The ACA in each quadrant was graded using the Scheie [13] grading system according to the anatomic structures observed during gonioscopy. A grade IV angle > 70° at each quadrant was considered to be closed. Following LPI, gonioscopy was performed by the same glaucoma specialist (MSK).
AS-OCT imaging
All participants were imaged for nasal and temporal ACA with AS-OCT (Visante OCT, version 2.0; Carl Zeiss Meditec, Dublin, CA, USA) under controlled room lighting conditions (0.5 cd/m2) by a single well-trained operator (DYK). Two subject groups that met defined eligibility requirements were used in the current study. First, variability of ACA parameters by AS-OCT imaging was tested. We acquired 3 sets of images from the first 18 eyes of 18 consecutive subjects (PAC 8; PACS 10) with identifiable scleral spur on three different occasions and those data sets were analyzed by a single examiner (SYK). We obtained data on intraclass correlation coefficients (ICCs) and inter-session test–retest variability for the various ACA parameters [14].
The next group of consecutive subjects was with narrow angles. AS-OCT imaging and assessment of ACA parameters were performed both before and after LPI. Post-LPI imaging was performed at 4 weeks post-lasers. All LPI procedures were performed by an independent ophthalmologist (KSL) using sequential Argon and YAG laser in the superotemporal quadrant in all participants. Perforation of the iris and patency were confirmed by retroillumination during LPI and post-LPI AS-OCT measurement. ACA was evaluated in two ways based on AS-OCT imaging. An independent examiner (SYK) masked to the other test results and clinical information of participants and to whether the images were taken before or after LPI analyzed all images. First, the presence of angle closure defined as the contact between peripheral iris and angle wall anterior to scleral spur was evaluated. For the purpose of masking the status of LPI to the examiner, only the temporal and nasal angles were analyzed.
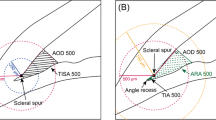
SYK used calipers to measure pupil size and anterior chamber depth (ACD; distance between corneal endothelium to anterior surface of lens capsule) and then assigned the scleral spur, which was defined as the point showing a change in curvature of the inner surface of the angle wall, often appearing as an inward protrusion of the sclera [15]. The AS-OCT software then automatically calculated angle opening distance at 500 μm (AOD500), angle recess area at 500 μm (ARA500), and trabecular−iris space area at 500 μm (TISA500). The AOD500 was defined as the linear distance between the point of the inner corneoscleral wall (500 μm anterior to the scleral spur) and the iris. The ARA500 was defined as the triangular area formed by the AOD500. The corners of the triangle were the angle recess (the apex), the iris surface and the inner corneoscleral wall. The TISA500 was defined as the trapezoidal area with the following boundaries: anteriorly, the AOD500; posteriorly, a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris; superiorly, the inner corneoscleral wall; and inferiorly, the iris surface (Fig. 1). These 3 ACA parameters were assessed both before and after LPI. If the scleral spur visibility could not be confirmed in a given image, the eye was excluded from analysis. Thus 18 subjects, tested for measurement variability assessment, were excluded for main analysis.
Anterior chamber angle parameters of anterior segment optical coherence tomography. The AOD500 is defined as the linear distance between the point of the inner corneoscleral wall (which was 500 μm anterior to the scleral spur) and the iris. The ARA500 is defined as the triangular area formed by the AOD500. The TISA500 is defined as the trapezoidal area with the following boundaries: anteriorly, the AOD500; posteriorly, a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the opposing iris; superiorly, the inner corneoscleral wall; and, inferiorly, the iris surface
Analysis
Parametric and non-parametric tests were used to compare variables according to data distribution (Gaussian vs. non-Gaussian). The presence of angle closure by AS-OCT and gonioscopic examination at each nasal and temporal quadrant both before and after LPI was determined and compared using chi-squared test. The ACA parameters were compared both before and after LPI using the Wilcoxon signed rank test. With the reproducibility data derived from 18 eyes, ICC and inter-session test–retest variability were calculated for ACA parameters. In our study, test–retest variability was defined at the 95% confidence interval (CI), determined as 1.96-times greater than the inter-session standard deviation (SD) for the 95% level. The increment of ACA parameters (AOD500, ARA500, TISA500) after LPI which exceeds the 95% CI of test–retest variability derived from the variability study group was classified as a significant angle change. Statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Eighty eyes of 80 PACS and PAC subjects were enrolled initially in the present study (PAC 48, PACS 32 eyes). Among the 80 initially enrolled subjects, 22 subjects were scanned in three different sessions for measurement reproducibility assessment. The remaining 58 subjects were scanned both before and after LPI. Four of the 22 subjects and 12 of the 58 subjects were excluded due to poor quality of AS-OCT image and thus difficult assignment of scleral spur. Therefore, the remaining 64 subjects were finally enrolled in both the variability (18 subjects) and change analysis (46 subjects) studies. Among the 18 eyes of 18 subjects analyzed for variability assessment, 4 eyes were treated by LPI after acute attack, 10 eyes were the contralateral eyes of acute attacks and 4 eyes were asymptomatic narrow angles. Among the 46 eyes of 46 subjects, 12 eyes were treated by LPI after acute attack, 24 eyes were the contralateral eyes of acute attacks, 10 eyes were asymptomatic narrow angles. Primary angle closure glaucoma patients were excluded and only PAC and PACS patients were included. Accordingly, none of the participants had any optic disc damage. There was no difference between participants and excluded subjects in terms of age, gender, baseline IOP and ACD based on AS-OCT.
Variability measurement
ACA parameters obtained from the 18 subjects showed good measurement reproducibility. ICC ranged from 0.850 to 0.992. Among the 4 parameters, ACD showed the highest ICC. Test–retest measurement variability of the three parameters as defined at the 95% CI was assessed in nasal and temporal quadrants for determination of significant angle change (Table 1).
Change analysis
The mean (±SD) age of the 46 participants was 63.4 ± 7.0 years (6 men, 40 women, all Asian). Since IOP and ACA data could not be described by Gaussian statistics, median value and range of data are summarized below. IOP was significantly reduced after LPI (median (range minimum, maximum), pre-LPI: 17.0 (12, 31) mmHg, post-LPI: 15 (10, 23) mmHg, p = 0.007). ACD was not significantly different between pre- and post-LPI [pre-LPI: 2.01 (1.47, 2.83) mm, post-LPI: 2.07 (1.50–2.84) mm, p = 0.165]. The other three parameters were significantly increased after LPI (Table 2).
By subjective assessment using gonioscopy, residual appositional angle closure was detected in 9 (19.6%) eyes at the nasal and in 11 (23.9%) eyes at the temporal quadrant after LPI while all eyes showed angle closure in both the nasal and temporal quadrants before LPI by gonioscopy. The frequency of angle closure by gonioscopic examination at each nasal and temporal quadrant before and after LPI was significantly different (p < 0.0001, 0.0001). AS-OCT angle images detected angle closure in 38 (82.6%) eyes at the nasal quadrant and in 35 (76.0%) eyes at the temporal quadrant before LPI. After LPI, from a total of 46 eyes, angle closure was detected in 22 (47.8%) eyes at the nasal quadrant and in 16 (34.8%) eyes at the temporal quadrant. The frequency of angle closure by AS-OCT at each nasal and temporal quadrant was also significantly different before and after LPI (p = 0.001, 0.0002).
By measurement variability criteria, the percentage of eyes which remained unchanged after LPI ranged from 26.1% to 43.5% in the nasal and 23.9% to 45.7% in the temporal quadrant (Table 3). Among 46 eyes, 38 eyes (82.6%) did not show significant change by measurement variability criteria. Figure 2 shows AS-OCT images of a narrow-angle eye in which no significant change of ACA parameters before LPI (a) and after LPI (b) can be seen.
Anterior segment optical coherence tomography images of narrow angle eye. a Anterior chamber angle parameters before laser peripheral iridotomy. b Anterior chamber angle parameters after laser peripheral iridotomy. There was no significant change of anterior chamber angle parameters before and after laser peripheral iridotomy
Discussion
Our variability study group showed good measurement reproducibility of ACA parameters (Table 1). From this dataset, test–retest variability was calculated and cut-off criteria for defining a significant angle change was arbitrarily determined. All diagnostic devices have inherent measurement variability. Therefore, we assumed that the change beyond test–retest variability could be regarded as a reasonable cut-off value for defining ‘true change’. This strategy for defining ‘true change’ as the ‘change beyond test–retest variability’ was implemented in numerous publications [16–18]. Furthermore, test–retest measurement variability derived from our own patient group would be a more objective way of setting cut-off criteria.
Overall, LPI significantly widened the ACA, and AS-OCT was capable of quantifying this change as shown in Table 2. We were able to confirm previous findings that LPI increased ACA parameters quantitatively after LPI using AS-OCT [8–11]; however, these changes were based on overall mean values of various parameters following LPI. These publications show overall changes in AS-OCT parameters both before and after LPI. However, in the current study, we specifically looked at each case and obtained the percentage of eyes which showed the changes in ACA by applying the cut-off criteria derived from our own reproducibility data. Among the parameters, AOD500 showed a higher percentage of angle change after LPI than the area-related parameters such as ARA500 or TISA500. In other words, more eyes showed significant quantitative changes of AOD500 than ARA500 or TISA500 following LPI based on our change criterion. The explanation for this finding remains unclear.
Before LPI, there were also differences in angle closure detection between AS-OCT and gonioscopy. Of the 46 eyes with a closed ACA on gonioscopy, AS-OCT detected closed ACA in 76.0% in the temporal and 82.6% in the nasal quadrant. This discrepancy in findings between gonioscopy and AS-OCT was also noted in the study by Wong et al. [20]. The explanation for this finding might include differences in the methods of assessing and interpreting the ACA configuration with different techniques.
Previous studies [21, 22] found that the prevalence of angle closure by qualitative AS-OCT analysis were higher than those by gonioscopy after LPI as we also noticed in our series following LPI. The possible explanation for this discrepancy is that inadvertent pressure during gonioscopic examination may artificially open the appositional angle. Nolan et al. suggested the possible causes for discrepancy between gonioscopy and AS-OCT were different lighting conditions and distortion of the anterior segment by gonioscopy. They also pointed out different landmarks to define angle closure using the 2 methods [20]. Sakata et al. also reported that steep iris profile or short irido-angle contact may explain the different percentage of angle closure between AS OCT and gonioscopy [22].
In an effort to overcome the aforementioned shortcomings associated with subjective assessment of ACA based on both gonioscopy and AS-OCT, quantitative analysis based on reproducibility of the single examiner (SYK) was performed. By using our own reproducibility data, the prevalence of eyes which did not show angle changes beyond the test retest variability was noted to be 23.9–45.8% depending on the location and ACA parameters (Table 3). Therefore, based on our AS-OCT study results, the significant percentage of ACA parameters were unchanged after LPI.
A few study results regarding the remained closure of ACA after LPI assessed by other techniques have been reported. He et al. [7] demonstrate that, although LPI induced a significant increase in the angle width in Chinese people with narrow angles, 19.4% of eyes still had 3 or more quadrants in which the posterior trabecular meshwork could not be seen after LPI when assessed by gonioscopy. Their incidence was similar to our findings of 19.6–23% when assessed by gonioscopy. However, Yeung et al. [19] reported that 42.9% of eyes had appositional angle closure after LPI assessed by gonioscopy, which was higher than our results and those by He et al. A different incidence of angle closure after LPI among different studies may reflect the inherent diagnostic variability of gonioscopy between observers and examination conditions. Yao et al. [23] used UBM for assessing ACA features qualitatively after LPI and reported that appositional angle closure was observed in at least 1 quadrant in 38.2% of the fellow narrow-angle eyes of acute PAC patients after LPI. Considering our results that angle closure was detected in 34.8–47.8% of eyes after LPI, the prevalence of angle closure assessed by AS-OCT and UBM was revealed to be similar, although it is problematic to compare different studies with different study designs and definition of angle closure.
Our study has certain limitations. One was the exclusion of superior and inferior quadrants for analysis. AS-OCT has difficulty in visualizing the superior quadrant due to the eye lid. Image quality of the inferior quadrant has been reported to be poor [15], which often leads to difficulties in assigning sclera spur and subsequent ACA parameter measurement. Another limitation was that the same examiner (MSK) performed the gonioscopy both before and after LPI. This might have introduced a certain examiner bias in the identification of residual angle closure following LPI based on gonioscopy; however, it is impossible to mask the examiner to the status of LPI during gonioscopy. For the purpose of exploring the causes of ACA crowding, detailed imaging of posterior chamber configuration or lens status might be needed. AS-OCT has limited tissue penetration and scan depth. Thus, we were not able to visualize the ciliary body or measure lens configuration and/or thickness. The ciliary body and crystalline lens are important anterior segment structures that may contribute to ACA changes. Plateau iris configuration may be responsible for persistent angle closure following LPI and this entity can be identified by confirmation of the disappearance of the ciliary sulcus with use of an imaging device such as UBM. Thus this could also be a limitation of the current study. However, the aim of our study was not to investigate the structural mechanism for the angle closure following LPI but to attempt to test the residual angle closure based on our predefined quantitative criterion using AS-OCT following LPI.
The overall conclusion of our study was that LPI led to statistically significant increases in ACA parameters when assessed by AS-OCT. However, using cut-off values derived from our own test–retest variability data, substantial portions of both PAC and PACS eyes showed no significant change of ACA parameters despite patent LPI. These findings coincided with previous qualitative evaluations by UBM or gonioscopy and with the clinical finding that a significant percentage of eyes showed progressive PAS formation despite patent LPI.
References
Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson JG, Bassanhu J. YAG laser iridotomy treatment for primary angle closure in East Asian eyes. Br J Ophthalmol. 2000;84:1255–9.
Foster PJ, Devereux JG, Alsbirk PH, Lee PS, Uranchimeg D, Machin D, et al. Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol. 2000;84:186–92.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Aung T, Ang LP, Chan SP, Chew PT. Acute primary angle-closure: long-term intraocular pressure outcome in Asian eyes. Am J Ophthalmol. 2001;131:7–12.
Ang LP, Aung T, Chew PT. Acute primary angle closure in an Asian population: long-term outcome of the fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology. 2000;107:2092–6.
Alsagoff Z, Aung T, Ang LP, Chew PT. Long-term clinical course of primary angle-closure glaucoma in an Asian population. Ophthalmology. 2000;107:2300–4.
He M, Friedman DS, Ge J, Huang W, Jin C, Lee PS, et al. Laser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology. 2007;114:494–500.
Lei K, Wang N, Wang L, Wang B. Morphological changes of the anterior segment after laser peripheral iridotomy in primary angle closure. Eye. 2009;23:345–50.
See JL, Chew PT, Smith SD, Nolan WP, Chan YH, Huang D, et al. Changes in anterior segment morphology in response to illumination and after laser iridotomy in Asian eyes: an anterior segment OCT study. Br J Ophthalmol. 2007;91:1485–9.
Wang N, Wang B, Zhai G, Lei K, Wang L, Congdon N. A method of measuring anterior chamber volume using the anterior segment optical coherence tomographer and specialized software. Am J Ophthalmol. 2007;143:879–81.
Memarzadeh F, Li Y, Chopra V, Varma R, Francis B, Huang D. Anterior segment optical coherence tomography for imaging the anterior chamber after laser peripheral iridotomy. Am J Ophthalmol. 2007;143:877–9.
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42.
Scheie HG. Width and pigmentation of the angle of the anterior chamber. A system of grading by gonioscopy. Arch Ophthalmol. 1957;58:510–2.
Kim DY, Sung KR, Kang SY, Cho JW, Lee KS, Park SB, et al. Characteristics and reproducibility of anterior chamber angle assessment by anterior segment optical coherence tomography. Acta Ophthalmol. 2009 (doi.10.1111/j.1755-3768.2009.01714.x).
Sakata LM, Lavanya R, Friedman DS, Aung HT, Seah SK, Foster PJ, et al. Assessment of the scleral spur in anterior segment optical coherence tomography images. Arch Ophthalmol. 2008;126:181–5.
Medeiros FA, Felipe A, Doshi R, Zanwill LM, Vasile C, Weinreb RN, et al. Long-term variability of GDx VCC retinal nerve fiber layer thickness measurements. J Glaucoma. 2007;16:277–81.
Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma: total versus pattern deviation analyses. Invest Ophthalmol Vis Sci. 2005;46:4600–6.
Wollstein G, Schuman JS, Price LL, Aydin A, Stark PC, Hertzmark E, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–70.
Yeung BYM, Ng PWC, Chiu TYH, Tsang CW, Lee FCH, Chi CC, et al. Prevalence and mechanism of appositional angle closure in acute primary angle closure after iridotomy. Clin Exp Ophthalmol. 2005;33:478–82.
Wong HT, Chua JL, Sakata LM, Wong MHY, Aung HT, Aung T. Comparison of slitlamp optical coherence tomography and scanning peripheral anterior chamber depth analyzer to evaluate angle closure in Asian eyes. Arch Ophthalmol. 2009;127:599–603.
Nolan WP, See JL, Chew PT, Friedman DS, Smith SD, Radhakrishnan S, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114:33–9.
Sakata LM, Lavanya R, Friedman DS, Aung HT, Gao H, Kumar RS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115:769–74.
Yao BQ, Wu LL, Zhang C, Wang X. Ultrasound biomicroscopic features associated with angle closure in fellow eyes of acute primary angle closure after laser iridotomy. Ophthalmology. 2009;116:444–8.
Conflict of interest
The authors have no proprietary interest or financial support in the development or marketing of instruments or equipment mentioned in this article, or any competing instruments or pieces of equipment. This study was not supported by any foundation or research grant.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lee, K.S., Sung, K.R., Kang, S.Y. et al. Residual anterior chamber angle closure in narrow-angle eyes following laser peripheral iridotomy: anterior segment optical coherence tomography quantitative study. Jpn J Ophthalmol 55, 213–219 (2011). https://doi.org/10.1007/s10384-011-0009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-011-0009-3