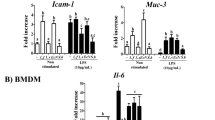
Abstract
Purpose
The pathogenesis of inflammatory bowel disease is thought to be a multifactorial process. One of the leading hypotheses is that an imbalance in normal gut flora induces an excessive immune response and contributes to inflammation in the gastrointestinal tract. Administration of probiotic bacteria reduces symptoms in patients suffering from inflammatory bowel diseases, probably via both manipulation of the microflora and stimulation of the intestinal immune system. In the current study the therapeutic potential of two different probiotics–Lactobacillus GG and a mixture of Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum (YO-MIX™ Y 109 FRO 1000)—in a rat model of colitis were evaluated.
Methods
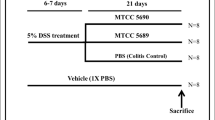
Male Wistar rats were administered probiotics for three days simultaneously with colitis induction. Colonic damage was evaluated histologically and biochemically and colonic tissues, as well as fecal samples, were used for bacterial studies using 16S rRNA gene primers.
Results
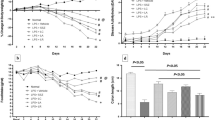
Probiotics administration reduced the relative amounts of the pathogenic bacteria Aeromonas and Escherichia coli in the colonic tissue. However, whereas both probiotics affected colon morphology, only Lactobacillus GG administration reduced myeloperoxidase activity.
Conclusions
We report the therapeutic rather than preventive potential of two different probiotics in an animal model of colitis.






Similar content being viewed by others
References
Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205.
Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29.
Thompson-Chagoyan OC, Maldonado J, Gil A. Aetiology of inflammatory bowel disease (IBD): role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin Nutr 2005;24:339–52.
Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54.
Greenberg GR. Antibiotics should be used as first-line therapy for Crohn’s disease. Inflamm Bowel Dis 2004;10:318–20.
Guarner F, Malagelada JR. Role of bacteria in experimental colitis. Best Pract Res Clin Gastroenterol 2003;17:793–804.
Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994;180:2359–64.
Alander M, Satokari R, Korpela R, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 1999;65:351–4.
Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics 1999;104:e64.
Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology 1993;105:1643–50.
Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 1999;116:1107–14.
Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in children with Crohn’ disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 2000;31:453–7.
Tannock GW. New perceptions of the gut microbiota: implications for future research. Gastroenterol Clin North Am 2005;34:361–82, vii.
Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 2004;53:821–8.
Menard S, Laharie D, Asensio C, et al. Bifidobacterium breve and Streptococcus thermophilus secretion products enhance T helper 1 immune response and intestinal barrier in mice. Exp Biol Med (Maywood) 2005;230:749–56.
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989;96:795–803.
Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 1998;114:385–91.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982;78:206–9.
Zhu XY, Zhong T, Pandya Y, Joerger RD. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl Environ Microbiol 2002;68:124–37.
Amit-Romach E, Sklan D, Uni Z. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poult Sci 2004;83:1093–8.
Wang RF, Cao WW, Cerniglia CE. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl Environ Microbiol 1996;62:1242–7.
Langendijk PS, Schut F, Jansen GJ, et al. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol 1995;61:3069–75.
Tsen HY, Lin CK, Chi WR. Development and use of 16S rRNA gene targeted PCR primers for the identification of Escherichia coli cells in water. J Appl Microbiol 1998;85:554–60.
Furet JP, Quenee P, Tailliez P. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol 2004;97:197–207.
Liu Y, Han JX, Huang HY, Zhu B. Development and evaluation of 16S rDNA microarray for detecting bacterial pathogens in cerebrospinal fluid. Exp Biol Med (Maywood) 2005;230:587–91.
Peran L, Camuesco D, Comalada M, et al. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol 2007;103:836–44.
Peran L, Sierra S, Comalada M, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr 2007;97:96–103.
Garcia-Lafuente A, Antolin M, Guarner F, et al. Incrimination of anaerobic bacteria in the induction of experimental colitis. Am J Physiol 1997;272(1 Pt 1)G10–5.
Fabia R, Ar’Rajab A, Johansson ML, et al. Impairment of bacterial flora in human ulcerative colitis and experimental colitis in the rat. Digestion 1993;54:248–55.
Chadwick VS, Anderson RP. Inflammatory products of commensal bacteria and gastro-intestinal disorders. Dig Dis 1990;8:253–68.
Rioux KP, Fedorak RN. Probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol 2006;40:260–3.
Venturi A, Gionchetti P, Rizzello F, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther 1999;13:1103–8.
Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding Panorama of species, disease presentations, and unanswered questions. Clin Infect Dis 1998;27:332–44.
Knight DJ, Girling KJ. Gut flora in health and disease. Lancet 2003;361:1831.
Hessle C, Hanson LA, Wold AE. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol 1999;116:276–82.
Maassen CB, van Holten-Neelen C, Balk F, et al. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 2000;18:2613–23.
Amit-Romach E, Uni Z, Cheled S, Berkovich Z, Reifen R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J Nutr Biochem (in press).
Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 2002;68:3401–7.
Versalovic J. Probiotics: intestinal gatekeeping, immunomodulation, and hepatic injury. Hepatology 2007;46:618–21.
Acknowledgment
The authors thank Mr. Eli Zinal, head of Tnuva R&D, for his help and constructive advice throughout the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reprints are not available.
About this article
Cite this article
Amit-Romach, E., Uni, Z. & Reifen, R. Therapeutic Potential of Two Probiotics in Inflammatory Bowel Disease as observed in the Trinitrobenzene Sulfonic Acid Model of Colitis. Dis Colon Rectum 51, 1828–1836 (2008). https://doi.org/10.1007/s10350-008-9394-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10350-008-9394-1