Abstract
Live-collected samples of four common reef-building coral genera (Acropora, Pocillopora, Goniastrea, Porites) from subtidal and intertidal settings of Heron Reef, Great Barrier Reef, show extensive early marine diagenesis where parts of the coralla less than 3 years old contain abundant macro- and microborings and aragonite, high-Mg calcite, low-Mg calcite, and brucite cements. Many types of cement are associated directly with microendoliths and endobionts that inhabit parts of the corallum recently abandoned by coral polyps. The occurrence of cements that generally do not precipitate in normal shallow seawater (e.g., brucite, low-Mg calcite) highlights the importance of microenvironments in coral diagenesis. Cements precipitated in microenvironments may not reflect ambient seawater chemistry. Hence, geochemical sampling of these cements will contaminate trace-element and stable-isotope inventories used for palaeoclimate and dating analysis. Thus, great care must be taken in vetting samples for both bulk and microanalysis of geochemistry. Visual inspection using scanning electron microscopy may be required for vetting in many cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skeletons of scleractinian corals are commonly analyzed for archives of geochemical data relevant to modern and palaeoclimate (e.g., Marshall and McCulloch 2002; Corrège 2006; Gaetani and Cohen 2006; Grottoli and Eakin 2007; Lewis et al. 2007). Scleractinian corals form exoskeletons of aragonite (CaCO3) beneath a layer of organic material secreted by cells in the basal ectoderm of polyps (Sorauf 1972). Individual aragonite ‘crystals’ are precipitated in a ‘hydro-organic gel’ (Cuif et al. 2004; Cuif and Dauphin 2005) and are arranged into macroscopic skeletal elements, such as walls, septa, and dissepiments as controlled by the distribution of the ectoderm and organic molecules at various scales of interaction down to nanometer-scale organization within individual ‘crystals’ (Cuif and Sorauf 2001; Cuif and Dauphin 2005; Stolarski and Mazur 2005; Przenioslo et al. 2008). Trace elements within coral skeletons that are of interest as palaeoenvironmental proxies are thought to be derived primarily from ambient seawater, but with acknowledged vital effects of the polyp and/or symbiotic algae (e.g., Marshall 2002). Regardless, the experimental correlation of stable isotopes and elemental ratios such as Sr/Ca, Mg/Ca, and U/Ca between coral skeletons and the temperature of ambient seawater forms the basis for sea surface temperature (SST) proxies (e.g., Beck et al. 1992; de Villiers et al. 1995; Mitsuguchi et al. 1996; Grottoli and Eakin 2007). Skeletal Ba concentrations in coral skeleton have been used to trace sediment-laden plumes related to terrestrial runoff events (McCulloch et al. 2003; Sinclair and McCulloch 2004; Sinclair 2005), and rare earth element (REE) and Mn concentrations have been used as indicators of marine productivity (Wyndham et al. 2004).
Although evidence increasingly suggests that trace-element distributions in coral skeletons are heterogeneous at very fine scales, possibly reflecting poorly understood vital effects in part (Cohen et al. 2002; Marshall 2002; Meibom et al. 2003, 2004, 2006, 2008; Allison et al. 2005; Shirai et al. 2005; Sinclair 2005; Stolarski and Mazur 2005; Gaetani and Cohen 2006; Sinclair and Risk 2006; Nothdurft et al. 2007), coral skeletons can provide useful palaeoenvironmental proxies provided: (1) original trace-element and stable-isotope inventories have not been altered by diagenesis; and (2) sample sizes and spatial resolution are adequately understood (e.g., Nothdurft and Webb 2007). Hence, coral skeletons are routinely vetted prior to analysis to check for diagenetic alteration, such as recrystallization of skeletal aragonite to calcite (e.g., Enmar et al. 2000; McGregor and Gagan 2003; Quinn and Taylor 2006), on the general assumption that coral skeleton that has not been exposed to freshwater (meteoric) diagenesis should retain reliable marine trace-element inventories. However, it also has been shown that early marine aragonite cement in coral skeletons generally has higher Sr and lower Mg content than the biogenic aragonite of the coral skeleton (Dauphin et al. 1990; Enmar et al. 2000; Quinn and Taylor 2006; Allison et al. 2007; Hendy et al. 2007). Regardless, many previous sampling approaches have not addressed the occurrence of early marine cements within analyzed samples.
Recent efforts in sampling have focused increasingly on achieving high resolution temporal palaeoclimate records, and that has required increased spatial resolution commonly by means of sampling techniques such as laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) (e.g., Sinclair et al. 1998; Fallon et al. 1999; McCulloch et al. 2003; Runnalls and Coleman 2003; Sinclair and McCulloch 2004), ion microprobe (Allison and Tudhope 1992; Allison 1996a, b; Cohen et al. 2001, 2002; Meibom et al. 2003; Rollion-Bard et al. 2003), and secondary ion mass spectrometry (NanoSIMS) (Meibom et al. 2004). These very fine-scale sampling techniques emphasize the natural heterogeneity in the coral record and place a greater responsibility on the researcher to constrain exactly what material is being sampled (e.g., Nothdurft et al. 2007). As recent investigations have found early marine cements within corals, and a large variety of endobionts are known to inhabit coral skeletons (e.g., cyanobacteria, green algae, fungi and sponges), there is a great need to understand what effects these biota have on the geochemistry of preserved coral skeleton. The purpose of this paper is to describe the products of very early diagenesis (i.e., diagenesis within a few years of skeletal biomineralization), including cementation and bioerosion, in modern reefal coral skeletons that could potentially influence geochemical data collected for palaeoclimate analysis. This study specifically tests the hypothesis that live-collected coral skeletons preserve pristine records of ambient seawater in their skeletal aragonite.
Early diagenesis in scleractinian corals
The diagenesis of scleractinian corals has received considerable attention (for review see Sorauf and Cuif 2001), but cement has been illustrated in the skeletons of live corals by few authors since the early 1970s (e.g., Hubbard 1972; 1975; Potthast 1992; Le Campion-Alsumard et al. 1995b; Enmar et al. 2000; Müller et al. 2001; 2004; Perrin and Cuif 2001; Perrin 2004; Nothdurft et al. 2005; Reuter et al. 2005; Buster and Holmes 2006; Quinn and Taylor 2006; Allison et al. 2007; Hendy et al. 2007; Nothdurft and Webb 2007; Nothdurft et al. 2007; Perrin and Smith 2007). Hubbard (1972, 1975) demonstrated the occurrence of intra-skeletal aragonite needle cements in live coral skeletons in both aquarium-grown and natural samples from the Caribbean, East Africa and the Great Barrier Reef (GBR). Macintyre (1977) noted, without illustration, that secondary aragonite commonly occurs in skeletal cavities a few millimeters below the tissue of living hermatypic corals. Potthast (1992) described early marine aragonite cement that precipitated syntaxially on coral aragonite surfaces in seawater within ten to 12 years of growth of Porites samples from water depths ranging from 1.5 to 4.5 m in fringing reefs at Mauritius Island. However, acicular aragonite cements 50 μm thick have been described in parts of Porites coralla that are only 2–3 years old (Le Campion-Alsumard et al. 1995a). Müller et al. (2001) investigated secondary aragonite at the base of a 1.9 m-long core through a colony of Porites lobata recovered from ~8 m of water offshore from Rabaul, PNG, and showed a positive shift in δ18O and Sr/Ca in the cemented, basal section of the core relative to non-cemented sections. Palaeothermometry analysis of the cement-rich skeleton yielded SST estimates that were ~4–5°C cooler than unaltered parts of the skeleton.
Endolithic and endobiotic microbes have long been of concern to geochemists seeking pristine coral skeleton for analysis because some microbes induce and/or localize the precipitation of secondary aragonite, high-Mg calcite (Schroeder 1972), low-Mg calcite (Nothdurft et al. 2007) or even different mineral phases, such as brucite [Mg(OH)2] (Smith and De Long 1978; Nothdurft et al. 2005; Buster and Holmes 2006). Exotic cements (e.g., brucite; Nothdurft et al. 2005) associated with organic biofilms in live collected corals highlight the presence and importance of intra-skeletal micro-environments that reflect chemistry that is significantly different from ambient seawater. Microenvironmental precipitates are common in the abandoned cavities of scleractinian coralla (Fig. 1), but they less commonly fill borings within the skeleton itself (e.g., Allison and Tudhope 1992; Nothdurft et al. 2007). Additionally, bioeroding sponges are among the most important internal excavating biotas in tropical environments (Rützler 1975) and may occur in very young parts of coralla (e.g., Schönberg 2002) where they may alter internal fluid flow or diffusion characteristics (Schroeder 1972; Scherer 1974; Land and Moore 1980).
Scanning electron microscope (SEM) image of longitudinal section of the upper centimeters of a Goniastrea favulus corallum illustrating common features referred to in the text: dissepiments (d), theca (t), pali (p), and columella (c). The calice was previously occupied by the coral polyp and is defined by the area above the last-formed dissepiment. Interseptal spaces occur naturally beneath the last-formed dissepiment when the coral moves distally and no longer occupies that part of the skeleton
Methods
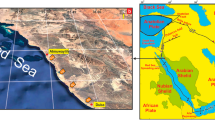
Four common species of scleractinian coral, Acropora hyacinthus, Pocillopora damicornis, Goniastrea favulus, and Porites lobata were collected live from Heron Reef, an enclosed elongate lagoonal platform reef just south of the Tropic of Capricorn and 70 km from the mainland in the southern GBR, Australia. Fifteen ~5 cm specimens were collected of each species within 20 m of the reef margin, including five samples each from environments: (1) on the reef flat within 10 m of the reef edge in positions that were exposed completely at low tide; (2) on the reef flat within 10 m of the edge that remained continuously submerged in 10–30 cm of ponded reef flat water at low tide; and (3) in a protected subtidal pool over the reef edge in ~2–3 m water depth at low tide (Fig. 2). Samples of A. cuneata were also collected, but a full survey of the three environments was not carried out for those samples. Coralla were washed thoroughly in freshwater to remove most surface organic matter and allowed to air dry. Sections of samples were immersed in sodium hypochlorite (12.5% NaOCl) for one to two days to remove remaining organic matter prior to analysis and rinsed thoroughly with deionized water. Parts of some samples were left untreated.
Sample locations and environments on the leeward margin of Heron Reef, Great Barrier Reef. a Northward view toward the reef margin showing the proximity of the three sampled environments. b The reef flat at low tide illustrating exposed corals and ponded pools of water on the reef flat. c Corals from the subtidal environment at the edge of the reef
Samples were cut in longitudinal and transverse sections, polished, etched and carbon coated or left uncoated for observation using an FEI Quanta 200 environmental scanning electron microscope (SEM) with energy dispersive spectroscopy (EDS). SEM observations were made in several operation modes, including operation at high vacuum (15 and 20 kV) on samples coated with carbon and at 2–10 kV for uncoated samples using both secondary and backscattered electron imaging. Secondary electron images were made from freshly broken and external surfaces, with some specimens etched with dilute formic acid (2%) for 20 s prior to being carbon coated. Transverse and longitudinal ultra-thin sections (‘lame a faces polies’ of Lafuste 1970) were prepared for observation using optical microscopy.
Carbonate polymorphs and other minerals were identified in situ on broken, sawn and polished sections using Raman microspectroscopy (e.g., Vénec-Peyré and Boyer 1978; Frost et al. 1999; Perrin and Smith 2002) on a Renishaw Raman microscope system 3000 (Renishaw, Gloucestershire, UK) equipped with a 1,200 lines/mm grating and a charge-coupled device (CCD) detector and incorporating a Leica optical microscope with ×50 objective lens. Spectra were excited by the 785 nm line of a 200-mW Renishaw diode laser operating in line focus mode. Spectra were recorded with the grating static and centered at 900 cm−1, giving a spectral range of approximately 1,200–600 cm−1. The presence of a 712 nm peak was used to distinguish calcite from aragonite. Other samples were analyzed using infrared (IR) microspectroscopy on a NEXUS 870 FTIR (Fourier transform infrared) microscope. Repeat acquisitions (256 scans) were collected in the range between 650 and 4,000 cm−1 at a resolution of 8 cm−1.
Resin casts (e.g., Golubic et al. 1970; Golubic et al. 2005) were made of selected bioerosion fabrics using thin sections of coral skeleton made with commercial super-glue. The coral skeleton then was dissolved completely in dilute formic acid (2%) leaving behind resin-cast replicas of microbial borings from within the coral skeleton. Replicas were observed using SEM.
Additional experiments were run to test the effects of different methods of removing organic matter from samples. Cements were identified prior to treatment in two air-dried, untreated, uncoated samples of G. favulus on the basis of morphology and composition using SEM with EDS. Samples reflect the same species from the same environment (exposed) and contain several different cements. One sample was then immersed in sodium hypochlorite (12.5% NaOCl) for 24 h and the other in hydrogen peroxide (30% H2O2), both at room temperature. Samples were then gently rinsed in deionized water, air-dried and observed again using SEM to identify any changes to previously observed cements.
Results
Scanning electron microscope observation revealed a range of diagenetic products in all samples, including early marine cements consisting of different minerals, and a range of bioerosion products associated with endolithic sponges and microbes. Cements include aragonite, high-magnesian calcite (HMC), low-magnesian calcite (LMC), and brucite on the basis of combined Raman and infrared spectroscopy, EDS and crystal morphology. Figure 3 is a summary of the occurrence of each cement type within the sample set. However, before the results can be discussed it is important to ensure that they do not reflect artifacts of the treatments for removal of organic matter.
Summary of results. a Scanning electron microscope images of the different bioerosion products and cement types. b Schematic cross section of the three different environments from which the coral samples were collected. c Table summarizing the distribution of bioerosion processes and cement types in the different environments. Spots representing abundance refer to presence-absence in numbers of samples, not to volumetric abundance within coralla
Sample pretreatment experiment results and interpretation
The vast majority of geochemical analyses of coral skeleton are done after removal of organic matter by bleaching. The most common bleaching treatments use hydrogen peroxide (H2O2) or sodium hypochlorite (NaClO) (Grottoli et al. 2005). Aragonite, HMC and brucite cements were present in both samples prior to treatment. HMC splays and aragonite needles were unaffected by treatment with H2O2 under observation at 8,000–10,000× magnification (Fig. 4). Aragonite needle and botryoid cements and HMC calcite splay cements were also unaffected by treatment with NaOCl (Fig. 5). Only brucite cements behaved differently in the two treatments. Minor etching of brucite occurred in NaOCl (Fig. 5b), but, it is unclear if this was caused entirely by dissolution of brucite, removal of entrapped organic matter, or mechanical fracturing resulting from wetting and desiccation. By contrast, treatment with H2O2 resulted in complete dissolution of brucite (Fig. 4b, d).
Scanning electron microscope images of G. favulus (04-9-6—exposed reef flat). Comparison of untreated sample (a, c) with sample after treatment with H2O2 (b, d). Aragonite needle cement (a) and HMC splay (c) cement showed no difference after treatment. Brucite cement (b) was completely removed during the treatment (b*). The brucite cement that encrusted the microbial filament in (c) has been removed leaving behind part of the organic filament shown in (d). Halite (h) that precipitated during the initial desiccation of the sample immediately after collection has also been removed by the treatment
Scanning electron microscope images of G. favulus (04-9-7—exposed reef flat). Comparison of untreated sample (a, c) with sample after treatment with NaClO (b, d). Aragonite botryoid cement (a) shown in a and b and aragonite needle cement (a) shown in c and d was unaffected by the treatment. HMC splay cement (c) was also unaffected. Brucite cements (b) showed little change after the treatment apart from some minor etching shown in b. Note also in d that removal of organic matter has better exposed microbial borings
Preferential dissolution of brucite in H2O2 relative to NaClO may reflect the difference in pH of the two solutions (~pH 9–13 in 12.5% NaClO and ~pH 4.2 in 5% H2O2). Brucite is the most soluble of the presently documented cements and commonly forms only in solutions with pH above 9 (e.g., Jordan and Rammensee 1996). Hence, it was removed completely in the acidic H2O2. Although 24 h immersion in NaOCl or H2O2 had no detectable affect on the appearance of aragonite and HMC cement, more prolonged exposure to H2O2 may result in dissolution of HMC and aragonite cements. Halimeda (aragonite) and echinoid plates (HMC) treated with H2O2 and NaClO by Gaffey and Bronnimann (1993) showed marked dissolution after 5 days in H2O2 but no detectable dissolution after 11 days in NaClO. Grottoli et al. (2005) concluded that H2O2 also causes the most significant and variable change in coral δ13C and δ18O values, and H2O2 may etch the scleractinian coral skeleton itself (Mitsuguchi et al. 2001; Clode and Marshall 2003).
Although, studies of scleractinian coral skeletons necessitate the removal of organic matter to visualize the skeleton underneath, pretreatment should be avoided or limited to NaClO for microstructural and geochemical analysis of early marine cements. In the case of the present study it is clear that the cements described below do not reflect artifacts of the preparation techniques employed and that significant cements were not removed prior to observation.
Bioerosion
Sponges
Sponge galleries (borings) occur in all coral species surveyed, being most abundant in Porites. Their abundance does not favor a particular environment. Some galleries occur in spaces immediately below the zone of the living coral polyp (i.e., immediately below the most recently deposited dissepiments), and the presence of spicules and organic tissue in galleries in desiccated samples shows that living sponges were present when the samples were collected (Fig. 6). Sponge borings are characterized by the presence of distinctive scalloped edges in the galleries (Fig. 6e) and, in most cases, surviving siliceous spicules. Accumulations of distinctive-shaped chips (Fig. 7) (i.e., ‘sponge chips’) occur at the base of some galleries. In most cases the type of sponge is unidentifiable, but tylostyle-headed spicules (Fig. 6d), smooth-faced scallops and small cavities close to the surface of the corallum suggest that most galleries were made by clionaid sponges (Macintyre 1985; Schönberg 2002; Calcinai et al. 2003). Excavated galleries in the most recently formed portions of coralla do not contain early marine cement. However, areas immediately surrounding the galleries commonly contain particularly abundant syntaxial acicular aragonite cement (Fig. 6e, f), which will be discussed below. Sponge chips also commonly are coated by syntaxial aragonite cement (Fig. 7d). As expected, the occurrence of sponge chips mirrors that of the galleries with the interesting exception that they were, for some reason, not observed in Acropora even where galleries were identified.
Scanning electron microscope images of sponge galleries in skeletons of Porites lobata. a Longitudinal section through the upper part of the corallum (03-10-66—subtidal—bleached) showing sponge gallery (identifiable from rough, scalloped edges) within 1 mm of the active growth surface of the coral. b Elongate, flat sponge gallery parallel to the growth surface of the corallum in unbleached sample (04-9-35-subtidal -unbleached) with desiccated organic material in the chamber. c Gallery with organic material removed by NaClO leaving behind meshwork of spicules (03-10-66). d Desiccated organic mater in unbleached sample (04-9-34—subtidal—unbleached) with embedded spicules with distinctive ‘pin’ shape (tylostyle) and siliceous composition. This is most likely the remains of a clionaid sponge. e, f Examples of sponge galleries immediately adjacent to abundant aragonite needle cement in bleached (03-10-75) and unbleached (04-9-35) samples
Scanning electron microscope images of sponge chips in skeletons of Porites lobata. a Sponge gallery with single sponge chip, several spicules and scallop-shaped excavations (03-10-89—bleached–ponded reef flat). b Sponge chips that have accumulated at the base of interseptal spaces. c Accumulation of cemented sponge chips. d Sponge chip with syntaxial aragonite needle cement covering the original scalloped surface. b–d are sample 03-10-75 (bleached-exposed reef flat)
Although sponge borings appear to be completely isolated below living coral tissues in some small coralla, they are most abundant below regions where the corallites are no longer occupied (i.e., under ‘dead’ sections of the corallum). In some cases, those regions were encrusted by other calcifying organisms (Fig. 8) (e.g., foraminifers, coralline red algae), but pores through the encrusters connected the galleries to external seawater. Where sponges occur beneath living coral polyps it is not clear how they maintained communication with external seawater.
Sponge borings in live-collected Porites lobata. a Photographs of the top and underside of dried specimen 04-9-35 (subtidal). Corallum is partially encrusted by coralline red algae. Note scattered holes through the algae, which represent incurrent and excurrent canals of underlying sponges. b Underside of corallum 04-9-35 showing abundance of sponge galleries beneath sections covered by coralline red algae. c Scanning electron microscope image of longitudinal section of unbleached sample 04-9-34 (subtidal). d Trace of image c showing the distribution of sponge galleries and desiccated sponge tissue
Microborings
Sixty-five percent of samples, including samples from all three environments, contain microbial borings within the skeleton (Fig. 3) based on SEM observation of random sections from all samples. The vast majority of samples from the exposed environment have microbial borings (~90%), decreasing in abundance with increasing water depth (50% in ponded reef flat samples and 40% in subtidal samples). Of the coral genera, Goniastrea specimens are most heavily bored with all samples surveyed having micro-borings. Porites also has a high abundance of borings (~80%). However, branching corals appear to be far less affected by microbial bioerosion (Acropora ~ 27% and Pocillopora ~ 33%), at least during the life of a branch. Green to black/green colored endobiont-rich bands (e.g., Le Campion-Alsumard et al. 1995b; Golubic et al. 2005) occur in cross sections of Goniastrea and Porites coralla.
Microborings are generally <10 μm in diameter and are consistent with a number of green algae, cyanobacteria and fungi (Fig. 9). They occur in all parts of the coral skeleton, including the most recently deposited areas that are enveloped by living coral polyps, but are most concentrated in distinctive green to grey stained bands. Resin casts suggest that several microborers coexist, including Ostreobium quekettii, endolithic fungi, and the cyanobacterium Mastigocoleus testarum (Le Campion-Alsumard et al. 1995a; Gektidis et al. 2006). The majority of the microborings can be attributed to O. quekettii, which occurs in high densities in some samples. Fungal hyphae occur attached to some Ostreobium filaments suggesting possible parasitism (Fig. 9d) (Golubic et al. 2005).
Scanning electron microscope images and thin section photomicrographs of microborings and resin casts in coral skeletons. a Polished and etched longitudinal section of Porites lobata (03-9-75—exposed reef flat—bleached) with filamentous microborings ~10–15 μm in diameter throughout the skeleton including in dissepiments. b Resin cast of Porites lobata (03-10-89—ponded reef flat). The larger filaments are most likely borings of Ostreobium quekettii (O) (trace Ichnoreticulina elegans), and the smaller diameter borings are probably caused by endolithic fungi (f). The texture on the surface of the resin is the result of a slight etch with formic acid on the coral skeleton prior to impregnation. c Resin cast of tunnel network of O. quekettii (O) with some fungal hyphae (f) in G. favulus (04-9-7—exposed reef flat). d Dense network of resin casts in G. favulus (04-9-7) located in a grey/green band. Individual species are difficult to identify, but borings were probably made by O. quekettii, the cyanobacteria Plectonema terebrans (P) (trace Scoletia filosa), and fungal hyphae (f). e Resin casts in G. favulus (04-9-7) with borings of the cyanobacteria M. testarum (M) (trace Eurygonum nodosum) and fungal hyphae. f Thin-section photomicrograph of Porites lobata (03-10-75) at the growth tip of a septum with fungal hyphae having bored to with a few micrometer of the growth surface. The hypha displays a typical bulbous terminal swelling (e.g., Gektidis et al. 2006)
In some cases, microbial filaments penetrated through the skeletons into the tissue of the living polyp, as previously observed by Le Campion-Alsumard et al. (1995b) and Bentis et al. (2000). Regular tubular to dome-shaped protuberences of the coral skeleton up to 400 μm in length, but generally <30 μm long, occur in all four coral species (Fig. 10). In some cases, dissepiments moulded around the protuberances. Protuberances may have smooth unbroken surfaces and may contain one or multiple borings through to the surface that are generally less than 5 μm in diameter. Protuberances are most abundant in Goniastrea (i.e., in 93% of samples) where they may be very dense (Fig. 10g). Protuberences were far less common in the other species, occurring in only one sample of each of the remaining three species from the exposed reef flat.
Scanning electron microscope images and thin section photomicrograph of fungal protuberances within the coral skeleton. a–e show a range of morphologies of the fungal protuberances (arrows) in the different species of coral. a Simple and branching morphology in G. favulus (04-9-5—exposed reef flat—bleached). b Large branching protuberances in G. favulus (04-9-5—exposed reef flat—bleached). Note the apertures in the centers of the protuberances. c Large ‘hook-shaped’ protuberance in Acropora hyacinthus (03-10-54—exposed reef flat—bleached). d Straight, narrow, elongate protuberance in Pocillopora damicornis (03-10-82—exposed reef flat—bleached). e Thin-section photomicrograph of protuberance in Porites lobata (03-10-75—exposed reef flat—bleached). Dark line through the middle of the protuberance is area occupied by the fungal hypha. Protuberance extends into the calice and would have been in contact with the coral polyp. f An individual dome-shaped protuberance in G. favulus (04-9-6—exposed reef flat—bleached). Note the aperture in the center. Protuberance also acted as a nucleation point for subsequent aragonite needle cement. g Interseptal space in G. favulus (04-9-6—bleached). The face of the septum is riddled with fungal protuberances of various shapes and sizes. The dissepiment at the top of the cavity moulds around the protuberances suggesting that they were present in the calice while the polyp was actively building the skeleton. h Resin cast of a septal face of G. favulus (04-9-7—exposed reef flat). Image represent the inverse of an area similar to that depicted in g. Protuberances are shown with hyphae extending through the center (arrows). Hyphae casts show some details with segments ~0.5 μm in length, possibly representing fungal form with a chain-like terminal swelling as illustrated by Gektidis et al. (2006—their Fig. 6)
One Goniastrea sample from the exposed reef flat contains a different type of protuberance that projects into the calice of the living polyp (Fig. 11). These structures may or may not represent material deposited by the coral, but EDS spectra suggest an aragonite composition. The tubes have a much larger diameter than the more common protuberances, with the aperture diameter of ~130 μm and outer diameter of the larger tube ~400 μm. It is not clear if the structures opened to seawater or within the gastric cavity of the polyp.
Atypical protuberances into the calice of G. favulus (04-9-7—exposed reef flat—bleached). a Photograph of corallite with two protuberances projecting into the calice. b Scanning electron microscope image of the same view as a. Protuberances are shown with arrows. Structures that surround them are pali and septa (as described by Nothdurft and Webb 2007). c Higher magnification SEM image of the nearer protuberance. Note the large aperture that presumably opened within or outside the coelenteron of the polyp
Three samples of Porites contain cement-filled borings including one sample from each of the three sampled environments (Fig. 12). These borings were previously described in detail by Nothdurft et al. (2007) who used Raman microspectroscopy to determine that the boring-filling cements are low-Mg calcite. Cement-filled borings are most abundant in samples from the ponded reef flat and least abundant in subtidal samples. Individual borings are cylindrical in cross section with diameters ranging between 6 and 15 μm (mean 9 μm), and they tend to be concentrated near the central regions of skeletal elements where they commonly are aligned more or less with the growth direction of the coral. Cement-filled borings occupy as much as 60% of the skeletal volume in random longitudinal sections (Fig. 12d) and rarely extend within micrometers of the growing tips of walls and septa at the most distal (youngest) surface of the corallum (Fig. 12b, c). The cement generally fills most of the boring except for: (1) minor porosity that defines concavo-convex bands; (2) irregular microporosity that separates calcite cement from the skeletal aragonite along the boring walls (Fig. 12e); and (3) small equidimensional cavities at the ends of some borings, particularly at the most distal edges of the skeleton (Fig. 12c). The LMC cement is described in more detail below.
Scanning electron microscope images and thin section photomicrograph of LMC cement-filled microborings in skeletons of Porites lobata. a Polished and etched longitudinal section of strongly bored skeleton (03-10-76—exposed reef flat—bleached). The majority of microborings above the dashed line are filled with calcite cement, whereas below those are mostly empty. b Thin-section photomicrograph of the upper portion of a corallum (03-10-89—ponded reef flat—bleached). LMC cement-filled microborings occur very near the growth tip of the skeleton. c Growth tip of a septum with LMC cement-filled microborings within micrometers of the growth surface (03-10-89—bleached). d Cement-filled microborings occupy as much 60% of the skeleton in this longitudinal section (03-10-89—bleached). Box indicates the location of f. e LMC cement in a boring with concavo-convex bands defined by irregular pores. Arrow indicates direction of movement of the microbe. f Coalesced LMC cement-filled borings with etching pattern showing coarse fabric cement. Note remnants of fibrous coral skeleton aragonite (a). (c–e after Nothdurft et al. 2007)
Early cements
Aragonite cements
Early aragonite cement occurs in several different morphologies, including aragonite needles, rods, botryoids, and filament encrustations. Aragonite needle cement, which is acicular with more or less sharp terminations, grows as fringes on the surface of the coral skeleton (Fig. 13). Needles are as much as 80 μm long (typically <30 μm) and <1 μm in diameter. Needle cements are generally syntaxial on coral skeleton, but may form as clumps of parallel needles 5–10 μm in diameter within an overall syntaxial fabric (Fig. 13a–f). The result may appear as a meshwork of needles, and the edges of these regions may terminate abruptly (Fig. 13d–f). However, isopachous aragonite fringes can cover relatively large areas of coral surface in some cavities, although they generally do not encrust entire pore systems (Fig. 13g, h). This cement type occurs in 58% of coral samples surveyed, and was present in 75% of samples from exposed reef flat corals, but was notably absent in Acropora sampled from ponded reef flat and subtidal environments (Fig. 3). The distribution of aragonite needle cement is patchy within Acropora and Pocillopora and is generally associated with brucite cement (described below). In Porites and Goniastrea, aragonite needle cement is also associated with brucite, but it is also common in areas immediately adjacent to sponge galleries, where the density may be greatest. Also in Goniastrea, aragonite needle cements tend to concentrate on and around dissepiments, particularly where the dissepiment abuts the septal wall.
Scanning electron microscope images of aragonite needle cement. a, b Aragonite needle cement lining an inter-septal space in Porites lobata (04-9-35—subtidal—unbleached). c Aragonite needle cement in G. favulus (04-9-6—exposed reef flat—bleached). Cement forms clumps of parallel needles 5–10 μm in diameter, within an overall syntaxial fabric. Clumps appear to gradually increase in size to the left. d–f show aragonite needle cement in P. lobata (98-6-12G—bleached) with a distinct boundary, where its growth appears to have been inhibited by something (possibly organic matter, now removed). f is a broken section of septa with syntaxial needle cement growing on it. Note that the boundary between coral and cement is very difficult to distinguish. g Image of a larger area of G. favulus (04-9-11—ponded reef flat—unbleached) showing volumes of the interseptal spaces in-filled with aragonite needle cement. h Heavily bored polished and etched longitudinal section of P. lobata (03-9-76—exposed reef flat—bleached) with syntaxial aragonite needle cement (arrows). Note that some microborings have excavated parts of the cement and that the cement distribution is patchy
Aragonite needle cement also encrusts filaments of microbial endobionts within the natural cavities of the coralla (Fig. 14). Needles generally radiate outward from the filament. However, some occurrences have a splay morphology with bundles of individual needles nucleating from an isolated point on the filament (Fig. 14c, d). Encrusted filaments range in diameter from 2 to 20 μm. Aragonite-encrusted filaments are present in 20% of samples surveyed, decreasing in abundance from exposed reef flat, to ponded reef flat through to subtidal environments (Fig. 3). Additionally, aragonite needle cements occur preferentially near the tips of fungal protuberances (Fig. 10f). As with microbial borings, acicular aragonite cement is more abundant in the massive corals Porites and Goniastrea.
Scanning electron microscope images of aragonite needle cement encrusting filaments of microbial endobionts within coralla. a Section through aragonite needle cement showing a space in the center previously occupied by microbial filament. b Aragonite needle cement encrusting a microbial filament on the underside of a dissepiment. Note that needles radiate from the filament (a, b are both from Pocillopora damicornis—03-10-83—exposed reef flat—bleached). c, d Encrusted filaments in G. favulus (sample 04-9-33—subtidal and 04-9-4—exposed reef flat respectively—unbleached) that exhibit a splay morphology of bundles of individual needles
Aragonite rods are acicular, but with broader, flat crystal terminations. Individual crystals are typically hexagonal and ~1–2 μm in diameter (Fig. 15). However, individual crystals tend to clump into larger 5–10 μm diameter groups (Fig. 15b, c). Growth fabric is commonly syntaxial on coral aragonite. Although, not as widely distributed as aragonite needle cement, rods also are most common in exposed reef flat samples and occur more abundantly in Goniastrea and Porites. Where rods occur, they tend to cover large areas of the coral skeleton extending over several hundreds of square micrometers. Like aragonite needle cement, rods are commonly abundant adjacent to sponge galleries, but unlike needle cements, they are not generally associated with other cements, like brucite. Some aragonite rods were subsequently cut by microborings (Fig. 15a).
Scanning electron microscope images of aragonite rod cement. a Aragonite rod cement in Porites lobata (03-10-75—exposed reef flat—bleached). Cement is truncated by sponge gallery and microboring (b). The location of b is shown by the box. b Oblique and transverse views of aragonite rods. Individual crystals tend to clump into larger 5–10 μm diameter growths. c Aragonite rods in P. lobata (98-6-11D—bleached) with flat crystal terminations. d Aragonite rod cement coating large areas of the coral surface of G. favulus specimen (04-9-9—ponded reef flat—unbleached)
Aragonite botryoids are spherulitic, composed of dense, splayed fibrous aragonite crystals (Fig 16). Aragonite mineralogy was confirmed with Raman microspectroscopy and a lack of Mg in the EDS spectra (Fig. 17). Botryoids generally have a domal or bulbous shape. They may occur isolated but generally coalesce into groups as much as 300 μm in diameter. Growth lines are visible through botryoids in polished etched sections (Fig. 18) and on their outer growth surfaces (Fig. 16a, b). Growth lines are spaced ~2–4 μm apart. Radiating aragonite fibers have characteristic sweeping extinction under cross-polarized light (Fig. 18c, d), and fiber density varies, but botryoids are typically solid, dense growths. Botryoids are less common than other aragonite cement types but can be volumetrically significant within individual cavities. They occur in only 18% of surveyed samples, but that includes 53% of Goniastrea samples, which is far greater than in any other coral species. They are generally associated with other aragonite cements and brucite. Botryoids were also observed to have microborings on their outer edges (Fig. 16a) and cutting across growth lines (Fig. 18).
Scanning electron microscope images of aragonite botryoids. Images on the right are high-resolution views of the images on the left. a, b Densely packed botryoid in G. favulus (04-9-7—exposed reef flat—bleached) showing growth bands on the outer surface and microborings. c, d Series of coalesced splays (partial botryoids) of densely packed aragonite needle crystals with pointed terminations (G. favulus 04-9-13—exposed reef flat—unbleached). e, f Densely packed aragonite botryoids that appear to have grown coevally or subsequent to large aragonite needle cement. Note that the coral skeleton substrate is heavily bioeroded (G. favulus 04-9-4—exposed reef flat—bleached)
Semi-quantitative energy dispersive spectra (EDS) data obtained during SEM analysis of the various cement types described herein. Relevant elements are highlighted with dashed lines. While minerals cannot be identified solely on the basis of the EDS elemental data, those data combined with crystal morphology were sufficient to identify most cement minerals following ground-truthing with Raman microspectroscopy
Images of a section through aragonite botryoids. a, b Scanning electron microscope images of polished and etched section through an aragonite botryoid in Porites lobata (03-10-76—exposed reef flat—bleached). Cement consists of a series of dome-shaped coalesced growths on the coral surface. Growth bands occur in each of the individual growths. Note that microborings penetrate through the coral and the botryoids. c plane-polarized and d crossed polarized light photomicrographs of thin-section of the same botryoids. Note the clarity of the cement compared to the coral skeleton in c and the sweeping extinction in d
Magnesium calcite (HMC) cements
Elevated Mg levels indicated by EDS spectra (Fig. 17) clearly distinguish HMC from other carbonate phases, and the phase was confirmed using Raman micro-spectroscopy. These cements are characterized by distinctive splayed accumulations of flattened elongate crystals with triangular terminations (Fig. 19). EDS counts allow estimation of MgCO3 content of the splays to between 8 and 15% MgCO3. Individual splays make domes of roughly triangular shape, and crystals tend to splay from a single point, commonly on a microbial filament (Fig. 19b–d). Individual crystals are difficult to distinguish, but single accumulations can range in diameter from 15 to 200 μm. They also occur as “butterfly” structures with two splays on either side of a point of nucleation (i.e., on a microbial filament) (Fig. 19d), but they do not form continuous isopachous crusts. Regardless, many single splays form in close proximity to each other, and they may be inter-grown with other cements, including aragonite needles and brucite. HMC splays are equally abundant in subtidal and intertidal environments, occurring in 22% of samples. HMC was not observed in Acropora and was observed in only one exposed Pocillopora sample and one subtidal Porites sample. However, HMC splays were present in 73% of Goniastrea samples.
Scanning electron microscope images of high-magnesium calcite (HMC) splays. a HMC cement splay accumulations of flattened elongate crystals with triangular terminations in G. favulus (04-9-6—exposed reef flat—unbleached). b (04-9-5—exposed reef flat—unbleached) and c (04-9-6—unbleached) Dome-shaped HMC splay cement that nucleated from a point, possibly an organic filament. d “Butterfly” structure of HMC splay cement in G. favulus (04-9-11—ponded reef flat—unbleached) with two splays on either side of a point of nucleation. In this case the point of nucleation is clearly a microbial filament ~8–10 μm in diameter. e, f Natural cavities in G. favulus (04-9-5—exposed reef flat—unbleached and 04-9-11—ponded subtidal—unbleached) filled with many single splays in close proximity to each other. e is covered with a layer of organic matter (possibly biofilm) and intergrown with aragonite needle cement. f has many single splays that appear to have been broken off leaving circular marks on the surface of skeleton that are ~10 μm in diameter (arrows)
Very fine acicular (needle-fiber) crystals were confirmed as HMC using Raman microspectroscopy, and EDS data suggest that approximately 20–22% MgCO3 is present (Fig. 17). Needle-fiber HMC is characterized by irregular mesh-works of acicular calcite crystals, typically encrusting a filamentous endobiont resulting in a tubular structure (Fig. 20a–d). Crystals are fairly uniform in size with a width of <500 nm and lengths up to 5 μm, although exact measurements are difficult due to the small size and random orientation of the crystals (Fig. 20e, f). The diameters of the encrusted filaments average between 13 and 20 μm, but some are as large as 40 μm. The inner edges of the tubes are relatively smooth, but the outer surfaces are typically irregular, and immediately adjacent tube structures merge in some cases to form one mass or a thick crust (Fig. 20d–f). HMC-fiber cements were observed in all three environments, but only in massive coralla (27% of Porites and 40% of Goniastrea).
Scanning electron microscope images of high-magnesium calcite (HMC) ‘whisker’cements. a, b Irregular mesh-works of acicular HMC crystals that encrusted a filamentous endobiont resulting in a tubular structure. Inner surface of the tube is smooth whereas outer surface is irregular (Porites lobata—03-10-89—ponded reef flat—bleached). The box shows the location of b. c Isolated tube of ‘whisker’ HMC in P. lobata (03-10-76—exposed reef flat—bleached). e Several tubes of ‘whisker’ HMC filling a cavity in P. lobata (03-9-89—bleached). Individual tubes are indicated by arrows. Note how the HMC of closely adjacent filaments has merged to form a crust. e, f Broken section through a crust of ‘whisker’ HMC in G. favulus (04-9-7—exposed reef flat—bleached) showing disorderd mesh-work of fine acicular crystals
Typical scalanohedral HMC cement also encrusts microbial filaments. Mineralogy was identified on the basis of EDS spectra (~18% MgCO3) and crystal morphology. The crusts have textured microcrystalline outer surfaces with scalanohedral crystal terminations on the inner parts of the filaments (Fig. 21). Scalenohedra are <5 μm in diameter and were observed in only one specimen of A. cuneata. Hence, it is not tabulated on Fig. 3 where that species is not included. The scalenohedral HMC cements that are typical of more mature reef rocks (e.g., Macintyre 1977) were not observed in the other four genera.
a, b Scanning electron microscope images of HMC scalanohedra cement in Acropora cuneata (04-9-17—bleached), showing ~5 μm scalanohedral crystals on the inside of the filament and fine dense crust also made of HMC on the outside of the filament. c Tube encrusted by two layers of HMC calcite cement. The inner layer, possibly needle-fiber HMC (nf), is covered by another layer of micritic HMC (mc). The layers are separated by many small tubular cavities (arrows). It is difficult to determine if these cavities represent microborings through the cement, or filaments encrusted by the cement
Low-Mg calcite (LMC) cement
Borings containing low-Mg calcite (LMC) cement were observed in one corallum of Porites from each of the three environments (20% of Porites). It was not observed in the other three genera. Cement in the borings is relatively coarse-grained compared to the skeletal aragonite fibers and contains concavo-convex bands defined by irregular minor porosity in many cases (Fig. 12e, f). The weakly banded microstructure is similar in appearance to that of aragonite cement that fills borings produced by migrating coccoid cyanobacteria in foraminifer tests and ooids (Macintyre et al. 2000; Reid and Macintyre 2000). Where banding is not apparent, etching patterns commonly are aligned suggesting that lengths of cement share the same crystallographic orientation or represent single crystals. The calcite contains elevated (but still very low) Mg content relative to host aragonite on the basis of EDS. Raman microspectrometry mapping confirms that the cement is calcite with a vibration peak at wave number 712 cm−1 and that the coral skeleton is aragonite with a vibration peak at wave number 704 cm−1 (Nothdurft et al. 2007).
Brucite cements
Brucite [Mg(OH)2] cement is present in 57% of samples and does not favor any one environment. Brucite morphologies include plates (i.e., individual crystals with rounded edges); rosettes composed of radiating plates; tubular structures composed of coalesced plates growing on filaments; and dense crusts on surfaces consisting of plates or coalesced rosettes (Fig. 22). Brucite cement has a patchy distribution within each corallum and does not appear to increase in volume with depth below living coral tissue. Brucite crystals commonly radiate from organic filaments to form tubes composed of coalesced plates. In poorly bleached samples crusts and tubes are commonly covered by organic films (Fig. 22d, e). Brucite appears reddish or pink in color in normal light, and contrasting density bands are visible in some cross sections of brucite structures (Figs. 22k, l, 23a, c). Fine-scale, ~400 nm banding occurs, and larger-scale banding is also present in some samples, showing greater contrast on a scale of ~5 μm.
Scanning electron microscope images of brucite cements. a–d Brucite crust on organic filaments. a, b Cavity filling mass of brucite-encrusted filaments in G. favulus (04-9-4—exposed reef flat—bleached) and Acropora hyacinthus (03-10-52—exposed reef flat—bleached). c Brucite crust on desiccated filament (arrow) in G. favulus (04-9-30—subtidal—bleached). d String of rosette-shaped brucite cement that has encrusted a microbial filament in Pocillopora damicornis (03-10-83—exposed reef flat—bleached). Note some organic filaments still remain after bleaching (arrow). e, f Irregular brucite crust of flat, rounded plate-shaped crystals on the underside of a dissepiment in P. damicornis (03-10-83—bleached). Some HMC splay cement is also present. f Plate-shaped brucite crystals under preserved organic coating, presumably representing a microbial biofilm. g Flakey brucite rosette-shaped cement in G. favulus (04-9-30—bleached). h Cluster of dense brucite rosette cement in P. damicornis (03-10-72—subtidal—bleached). i, j Thick crust of brucite filling a cavity in Porites lobata (03-10-65—bleached). At the top of the cavity the brucite has grown around a microbial filament (possibly green alga—Ostreobium). A view of the wavy nature of the crystals in the crust is shown in j. h Polished and etched section through rosettes of brucite in Porites lobata (03-10-65—subtidal—bleached). Rosettes display at least six distinct growth lines (arrows). l Broken section through a crust of brucite cement with numerous growth lines <0.5 μm apart (G. favulus—04-9-7—exposed reef flat—unbleached)
Scanning electron microscope images of associated brucite and carbonate cements. a Brucite cement crust (b) intergrown with aragonite botryoid cement (a) in Porites lobata (03-10-65—subtidal—bleached). Dashed line illustrates contact between two cement types. Growth lines are shown in aragonite (black arrows) and brucite (white arrows). b–d Brucite cement crusts on microbial filaments (b) and adjacent aragonite needle cement (a). b Cements in a cavity of Pocillopora damicornis (03-10-90—ponded reef flat—bleached). Microborings occur parallel to the surface of the coral skeleton (m). c Broken section through brucite cement with growth lines (arrows) surrounded by aragonite needle cement in cavity of G. favulus (4-9-7—exposed reef flat—unbleached). b Broken section through brucite cement with very fine needle of aragonite cement growing on the surface of P. damicornis skeleton (03-10-72—subtidal—bleached). e Brucite cement rosettes (b) grown around aragonite rods and needle cements (a). f Brucite cement (b) that has encrusted a microbial filament (f) that has bored through HMC splay (c) in a cavity of G. favulus (04-9-10—ponded reef flat—unbleached). g Brucite cement plates and rosettes (b) surrounded by aragonite needle cement (a) in P. lobata (03-10-65—bleached). h ‘Ghost’ structures of brucite cement, where brucite was apparently removed and the surrounding aragonite cement has left an outline of the brucite crystals (arrows) (G. favulus 04-9-30—subtidal—bleached)
Brucite cements appear to be closely associated with aragonite cement (Fig. 23). Aragonite rods and botryoids occur immediately adjacent to brucite, whereas HMC is less commonly associated (Fig. 23a, e). ‘Ghost’ structures of brucite are also present, where brucite apparently has been removed, and the surrounding aragonite cement has left an outline of the brucite crystals (Fig. 23h). In some cases it is not clear if dissolution occurred in seawater prior to collection or if the brucite was removed (dissolved or broken free) during the treatment prior to analysis (see discussion above).
Discussion
Bioerosion
Sponges
The abundance of sponge galleries is not unexpected in these samples as excavating sponges are common bioeroders in reef environments (e.g., Neumann 1966; Rützler and Rieger 1973; Acker and Risk 1985; Schönberg 2002; Schönberg 2008), and even in deeper coral environments (Beuck et al. 2007). Cliona, a common boring sponge in live scleractinian coral skeletons (e.g., Hubbard and Swart 1982; Pandolfi and Greenstein 1997; Holmes et al. 2000), forms a complicated network of connected galleries 1–15 mm in diameter that generally penetrate no more than 20 mm below the coral surface. There is no obvious pattern in the distribution of sponge galleries in the present samples, although sponge galleries are absent in Acropora from the ponded reef flat and subtidal environments. The rate and pattern of sponge borings in modern corals is partly controlled by the skeletal architecture of the host coral (Risk and MacGeachy 1978). For example, sponge borings are preferentially located in more porous corallites of massive Montastrea annularis, where thin septa were bored within corallites before thicker theca walls between neighboring corallites (Ward and Risk 1977; Schönberg 2000). Dead areas of the corallum increase the available substrate for sponge invasion, and thus increase the extent of sponge boring (McKenna 1997). However, sponges observed in this study invaded small Porites and Goniastrea colonies even where the entire upper surfaces were covered by living coral polyps. It is not clear how the sponges invaded the coralla, as the galleries do not extend to the corallum bases where they were in contact with the substrate. Regardless, high light intensity, strong currents and, possibly, low temperatures have been found to accelerate sponge bioerosion (Rützler 1975). The relatively uniform distribution in the present samples, excepting Acropora branches, suggests that the differences in these parameters afforded by the 2–3 m of water depth in the subtidal samples was insufficient to affect the abundance of the sponges.
Microborings
Microbial borers identified from resin casts are typical of shallow marine carbonate environments (e.g., Holmes et al. 2000; Vogel et al. 2000; Perry and Macdonald 2002; Golubic et al. 2005; Gektidis et al. 2006). The green alga Ostreobium is ubiquitous in skeletons of live corals and is generally associated with Plectonema terebrans (Lukas 1974). O. quekettii is the most commonly identified species with filaments 1–25 (mostly 2–5) μm in diameter, but swelling to 40 μm. Other species, such as O. constrictum, which is commonly associated with O. quekettii, can swell to 200 μm (Lukas 1974). The green bands observed in Goniastrea and Porites (Fig. 25) probably represent high concentrations of these endoliths (Lukas 1974; Le Campion-Alsumard et al. 1995b). However, where the bands are more black/grey in color they may reflect extensive parasitism of the algae by endolithic fungi (Priess et al. 2000). Fungal attack is accompanied by the release of a dark tannin-like substance that stains hyphae, algal filaments and coral skeleton resulting in a black band (Golubic et al. 2005).
Pandolfi and Greenstein (1997) stated that massive corals suffer greater degrees of taphonomic alteration by macroborers than free living and branching corals. The greater degree of microboring in massive corals (i.e., Porites and Goniastrea) than in branching corals (i.e., Acropora and Pocillopora) in this study may relate to skeletal surface area and density distribution of live tissue, all of which may affect light penetration (e.g., Highsmith 1980). However, another factor could be linear skeletal extension rate. As endoliths first penetrate coral skeletons from the base shortly after metamorphosis and then grow upwards paralleling coral extension (Lukas 1974), the slower growth rate of the massive corals compared to branching forms may allow endolithic microbes to better keep pace with the rate of coral skeletal extension. However, endoliths may not keep up as well in faster growing coral branches, leading to reduced boring density. Porites has recorded growth rates between ~8.8 and 21.7 mm/yr with a maximum of 21.7 mm/year, and Goniastrea has recorded growth rates between 3.8 and 9.9 mm/year (for review see Nothdurft and Webb 2007, their Table 2). The branching forms Pocillopora and Acropora, by contrast, have growth rates of as much as 72 and 185 mm/year, respectively. A reduction of coral growth rate, for example associated with a coral bleaching event or other environmental stress, also may result in a concentration of endolithic algae and parasitic fungi in the skeleton around or just below live-polyps (Golubic et al. 2005), resulting in concentric green or grey/black bands (Le Campion-Alsumard et al. 1995b).
Environmental stress could also be responsible for severe fungal attack on the coral polyps themselves as indicated by the abundant skeletal protuberances in the present samples (e.g., Bentis et al. 2000; Golubic et al. 2005). Such structures have been interpreted as a defense response to invasion by fungal hyphae into the calice wherein the coral deposits dense carbonate over or around boring apertures (see Le Campion-Alsumard et al. 1995b—their Fig. 17). It is not clear if the aberrant skeleton is deposited in the same way as normal coral skeleton, but examples where dissepiments mould around the protuberances (Fig. 10g) indicate that they formed in the presence of the living polyp. Regardless, their outer textural appearance differs slightly from adjacent skeleton. Previous studies of these structures focused on simple conical structures in massive Porites species, although Domart-Coulon et al. (2004) produced such structures using cultured strains of fungi in P. damicornis. The presence of protuberances in three other coral genera from the GBR suggests that their occurrence may be far more widespread. Additionally, some of the structures in the present material are much larger (up to 400 μm in length), more complex (e.g., multiple divergent protuberances) and more numerous in a given coral (e.g., Fig. 10g) than has been documented previously. Whether the great diversity and wide distribution of these structures in the present material is normal in the intertidal setting or reflects increased recent environmental stress (i.e., thermal stress from warm sea surface temperature) remains to be tested.
Cements
The range of early marine cements identified in coralla of live-collected corals in this study, including several different mineral species, is surprising. Previously, live-collected skeletons have been assumed to be relatively free of marine cements. Of the few authors that have documented cement precipitated in the skeletons of live coral colonies, most have described only aragonite needle cement (e.g., Hubbard 1972, 1975; Potthast 1992; Le Campion-Alsumard et al. 1995b; Enmar et al. 2000; Perrin and Cuif 2001; Müller et al. 2001; 2004; Perrin 2004; Reuter et al. 2005; Quinn and Taylor 2006). Reports of other cements are much less common (e.g., LMC—Nothdurft and Webb 2007; brucite—Nothdurft et al. 2005; Buster and Holmes 2006). All other cement varieties reported here have not been reported previously in live coral colonies, although many of them have been documented in older Holocene corals and reef rock or in other non-reefal environments.
Despite the extensive record of Holocene reef lithification and cementation, few papers have tried to constrain the timing of cementation within scleractinian corals. Most authors found little alteration in living coral specimens and inferred that most cementation begins after the death and removal of the polyps (e.g., Gvirtzman and Friedman 1976; Hubbard and Swart 1982; Macintyre 1984). Enmar et al. (2000) estimated that aragonite cements in coral skeletons could account for 0.15% of the total weight per year, filling as much as 2% of the initial porosity within 100 years. However, other authors have documented early diagenesis in scleractinian coral skeletons within a few years after living polyps ceased to occupy those skeletal parts (e.g., Potthast 1992; Le Campion-Alsumard et al. 1995b). Those observations are consistent with the relatively rapid cementation that can occur in seawater. Grammer et al. (1993) showed aragonite needle cement developed around a sediment trap within 2 years at 158 m water depth on the Bahamian slope, and Grammer et al. (1999) demonstrated partial lithification of ooids suspended above the sea floor within eight months at depths of up to 60 m. Moberly (1970, 1973) determined that cement could infill coralline red algae in as few as 3 years, and Nothdurft et al. (2007) found that LMC cements could be precipitated within days to weeks within the specialized microenvironments of the enclosed microborings described above. Hence, it is clear that marine cements can precipitate on short time frames within weeks or days of skeletal formation. The controls on such cementation are only partly understood.
Water chemistry is a fundamental control on diagenetic processes, but the physico-chemical parameters that govern carbonate precipitation and dissolution are highly complex and, although extensively studied, are not entirely understood (see reviews by Morse and Mackenzie 1990; Morse and Arvidson 2002). Primary abiological factors that affect cementation include: (1) the saturation state of ambient seawater with respect to carbonate minerals; (2) presence of appropriate nucleation sites; and (3) the presence/absence of specific nucleation inhibitors (Webb 2001). Shallow tropical marine environments, such as those targeted in this study, commonly support volumetrically significant cementation where water agitation results in CO2 degassing and thus increased pH (Hanor 1978; Given and Wilkinson 1985; Gischler and Lomando 1997; Webb et al. 1999), and solar heating, particularly of ponded waters, decreases carbonate solubility and promotes CO2 degassing (Revelle and Emery 1957; Hanor 1978). Additionally, evaporation increases ionic concentrations of ponded waters. The exposed and ponded samples of the present study would have been affected by all of those factors, although the subtidal samples would have been affected less so, and the exposed samples also would have experienced a greater degree of evaporation and higher temperature extremes. Sea surface temperature data are not available for the exact years when the present samples grew, but Potts and Swart (1984) reported mean monthly seawater temperatures at Heron Reef over a 15 month period to range from 20.8°C in June to 29.4°C in January, and maximum–minimum thermometers recorded 13.5°C on inner and outer reef flats and 37°C on inner reef flat. The largest daily range recorded by Potts and Swart (1984) over a 24-h period was 12°C. Similar temperature ranges were measured by Davies and Kinsey (1973) in isolated pools in the beachrock on Heron Island at low tide where water temperature ranged from 34.8–20.7°C within a low tide cycle (5 h). Temperatures were likely to be higher rather than lower during growth of the present samples (i.e., relatively warm years from 1993 to 2004).
Aside from ambient physico-chemical effects, localized biological factors are also capable of inducing carbonate precipitation through increasing alkalinity or pH, providing appropriate nucleation sites or removing nucleation inhibitors (for reviews see Ehrlich 1990; Castanier et al. 1999; Riding 2000; Webb 2001). Biologically induced precipitation may occur in microenvironments within organic matter or in semi-confined pore spaces below organic communities in settings where ambient water volumes are large and well mixed. However, natural cavities in coralla may be partially closed systems already and thus, somewhat isolated from ambient seawater. Such conditions may facilitate far greater influence of endolithic communities on the semi-confined water chemistry, thus generating conditions that favor precipitation within the entire localized fluid volume (Webb 2001; Nothdurft et al. 2005). This may result in precipitation of apparently abiotic cement (e.g., isopachous aragonite needle cement) that is not physically confined to the communities that induced precipitation. For example, photosynthetic removal of CO2 by phototrophic endobionts could increase the pH of semi-confined waters throughout the corallum. Additionally enhanced cementation could involve organic material derived from the coral itself. Gautret (2000) suggested that the diagenetic state of intraskeletal organic matrices influences the development of cements directly on the surface of skeletal substrates. Thus, aside from the physico-chemically ‘active’ shallow setting of reef corals, the high diversity and volume of endobionts provides many potential opportunities for mediation of intracorallum water chemistry in microenvironments.
Aragonite cements
As with most previous reports of early marine cement in living corals (e.g., Potthast 1992; Le Campion-Alsumard et al. 1995a; Enmar et al. 2000) the most abundant and widely distributed cement in the present samples is syntaxial aragonite needle cement. Based on mean δ18O and δ13C values, Quinn and Taylor (2006) calculated that parts of live-collected coral skeleton from Papua New Guinea contained as much as 20% early marine aragonite cement, and Müller et al. (2006) estimated that aragonite cement accounted for as much as 30% of the aragonite in a coral from Ningaloo Reef, Western Australia. Although the present material does not contain volumes as great as those, our samples represent only a few years of potential growth. We interpret the syntaxial aragonite to have grown on clean aragonite surfaces due to fluid supersaturation. Precipitation rates may have been enhanced by biological activity within the semi-confined environment of the corallum. Aragonite rods previously have not been documented in live-collected corals, but Constantz (1986) illustrated similar aragonite crystals in older Diploria labyrinthiformis from Barbados (his Fig. 4E, p. 60). Rods could be considered very coarse aragonite needles, but they may require greater localized supersaturation. The irregular distribution of rods may reflect nucleation on scattered organic matter. Acicular aragonite is generally among the most common cements in Holocene reefs (e.g., Ginsburg et al. 1971; Macintyre 1977; Macintyre and Marshall 1988), including the GBR, where it was interpreted to be the earliest cement formed (Marshall 1983). Isopachous aragonite needle cement also occurs in beachrock from Heron Island, and needle- to lath-shaped aragonite cements radiate from, and encapsulate, filament molds in beachrock microbialites (Webb et al. 1999). Webb et al. (1999) suggested that much of the beachrock cement nucleated on organic matter, as it is not syntaxial in most cases. Hence, the association of aragonite needle cements with organic filaments and organic films in 20% of the present samples is consistent with other reports from Holocene carbonate environments. However, although aragonite cement is ubiquitous in such environments, its occurrence was localized in some cases by organic matter associated with endobionts, and broad isopachous crusts are the exception rather than the rule. However, that relationship may change through time as coralla age and organic matter is lost. Then, more pervasive isopachous cement may develop due to continued physico-chemical forcing, leading to the higher cement volumes reported by other authors (e.g., Müller et al. 2006; Quinn and Taylor 2006).
Aragonite needle cements also show a clear relationship with sponge borings in the present samples. Sponge borings previously have been shown to affect subsequent carbonate diagenesis by providing conduits through which reactive diagenetic fluids were introduced (Rehman et al. 1994). However, close association of occupied sponge galleries and dense aragonite needle cement has not been reported previously. The mechanism for increased cementation is unclear, but several hypotheses can be proposed. Increased syntaxial cementation in the immediate vicinity of the sponges may reflect a microenvironment with increased alkalinity, possibly due to sponge metabolism or boring activity, or increased water throughput near the sponge. As filter feeders, sponges primarily obtain nutrients by pumping seawater through their internal structures and expelling it through excurrent canals. Depending on the position of incurrent and excurrent canals this pumping could alter the internal hydrodynamics within a corallum. As previously mentioned, the positions of incurrent and excurrent canals are unknown in some of the observed sponges, especially where coralla were entirely covered by coral tissues. Regardless, increased seawater circulation through the corallum would make available abundant ions for precipitation. At larger scales, early marine cementation is concentrated where active pumping mechanisms, such as wave or tidal pumping, force large volumes of water through the pore system (e.g., a reef crest) (e.g., Lighty 1985). The same principle could apply to internal circulation within a corallum. The creation of galleries within the coralla must have changed internal fluid pathways, presumably by increasing flow potential, and their presence clearly increases the rate of subsequent diagenesis (Rehman et al. 1994).
Sponge metabolism also may have directly or indirectly affected the local alkalinity. In order for sponges to produce their burrows specialized cells (archaeocytes) dislodge silt-sized, lenticular fragments by etching fissures into the carbonate substrate using carbonic anhydrase and acid phosphatase (Pomponi 1980). They are capable of removing relatively large quantities of carbonate. Schönberg (2002) calculated the erosion rate of Cliona orientalis on coral substrates to be 3.4–10.3 kg of dry CaCO3 m−2 of sponge tissue per year. The higher values were from massive Porites and G. retiformis samples. Zundelevich et al. (2007) estimated the total amount of CaCO3 removed by Piona vastifica to be ~340 ± 170 gm−2 of sponge per year. The amount of dissolved CaCO3 produced by the sponges was calculated to be 260 g m−2 sponge year−1 on the basis of increased tank-seawater alkalinity over time. However, their results contrast many other studies of bioerosion rates in sponges. For example, Rützler and Rieger (1973) linked the carbonate dissolution rate to the total substrate removal rate by clionaid sponges suggesting that only 2–3% of substrate removed represents dissolved carbonate, the rest being chips. Zundelevich et al. (2007) suggested that the sponge chips represent a smaller portion of the total bioerosion capacity and that sponges were responsible for one-half of the total alkalinity increase in their experiments. Regardless of the exact mass balance, sponges clearly increase local alkalinity due to chemical dissolution of aragonite while boring. Scherer (1974) stated that carbonate dissolved in the vicinity of microborers is apparently reprecipitated near the place of dissolution. Such a model also may apply to macroboring sponges, thus accounting for the localized enhanced aragonite precipitation in our samples.
Other metabolic processes of the sponges and their endosymbionts and related bacteria also may affect cement precipitation. Sponge tissue provides a habitat for a variety of microorganisms (see review by Diaz and Rützler 2001), including phototrophs, such as cyanobacteria and zooxanthellae (Pang 1973; Hill 1996; Schönberg et al. 2005). Photosynthetic uptake of CO2 by endosymbionts would elevate carbonate ion activity, thus favoring aragonite precipitation. Sponge tissues may also contain large amounts of heterotrophic bacteria including, archaeobacteria, nitrifying bacteria, and methanotrophic bacteria. These bacteria may play a role during polysaccharide mucus formation and could promote ammonification during degradation of dead sponge tissue, thus increasing the pH and favoring calcification (e.g., Reitner 1993; Reitner et al. 1995). Hence, a variety of mechanisms may have served to enhance aragonite precipitation in the vicinity of living sponges.
Preferential precipitation of aragonite needle cement at the tips of fungal protuberances (Fig. 10f) occurred after the coral polyp vacated the space, and hence, is not related to the construction of the protuberances themselves. If fungi remained alive after withdrawal of the coral polyp, they may have created a microenvironment suitable for aragonite precipitation in the immediate vicinity. Alternatively, organic material left upon death or retraction of the fungus may have acted as a nucleation template for aragonite growth. It is also possible that the skeleton precipitated by the coral as a defense against rapidly growing fungi contained increased organic matrix to enhance aragonite precipitation rates and that the organic matter remained active after withdrawal of the polyp and fungus. Organic matrix material has been shown to retain chemical activity in scleractinian skeletons long after withdrawal of polyps (e.g., Cuif et al. 1992; Gautret and Marin 1993). Increased aragonite cement around the margins where dissepiments meet walls could also reflect the presence of relict organic matter left there when the polyp retracted to move up within the corallite.
Aragonite botryoids have not been described previously in live coralla. However, large aragonite botryoids (also termed spherulites or mamelons) have been described in Holocene reef rock from the GBR (Marshall 1986), Belize (Ginsburg and James 1976) and the Persian Gulf (Shinn 1969), and in Pleistocene limestone (Aissaoui 1985). Grammer et al. (1993) described large botryoids from steep marginal slope deposits (45–85 m depth) from the Bahamas and Belize with fibers that were a few hundred micrometers to 32 mm in length. Botryoid growth had average synsedimentary growth rates of 80–100 μm/year (Grammer et al. 1993). Density bands in botryoidal cement in the present study suggest more rapid growth (400 μm/year assuming one band represents 1 day’s growth). The increased growth rates may reflect enhanced precipitation within the corallum microenvironment, possibly mediated by the daily photosynthetic cycles of endobiotic phototrophs. Regardless, their irregular distribution (e.g., Fig. 16a) and smooth surfaces probably reflect enclosure in a biofilm, but the biofilm was not observed in place.
High-Mg calcite (HMC) cement
Scalanohedral HMC cements are very common in Holocene reefs making up the bulk of peloidal fabrics and microbialite crusts (e.g., Macintyre 1977; Lighty 1985; Webb et al. 1998). Regardless, scalenohedral HMC cements were not abundant in the corals studied here. Instead, HMC splay morpholgies are better developed, but they are otherwise less well known in Holocene reef rock. Morphologically they are similar to Holocene cements that are associated with algal filaments described by Schroeder (1972) in Bermuda reefs. He described HMC cements with frequent twinning parallel to the rhomb-face and an oblique orientation of the c-axis with respect to the substrate (his Fig. 6a) as well as bladed palisade cement (his Fig. 10b). HMC cements with identical textures and similar sizes that are intergrown with aragonite needle cement have also been documented in fossil Porites from Hawaii (Allison et al. 2007), and similar cements also were illustrated in non-reefal environments, including Holocene delta deposits from Texas, USA (Weiss and Wilkinson 1988) and Holocene beachrock from Brazil (Vieira and De Ros 2006, their Fig. 2e).
Controls on the morphology of HMC cements are poorly constrained, and experimental work and observations from natural settings are difficult to reconcile (for a review see Mackenzie et al. 1983). Morphologies similar to the HMC splay cement described above have been generated experimentally in the presence of the organic acid malic acid (Meldrum and Hyde 2001, their Fig. 2). A variety of organic acids may be released by degradation of cyanobacteria or polymeric materials in EPS (e.g., Anderson et al. 1987). Additionally, HMC splay cements of much smaller size were shown to nucleate directly on EPS associated with benthic cyanobacterial biofilms by Défarge et al. (1994). Hence, such cements may be indicative of processes within microbial biofilms.
The HMC needle-fiber cements are more enigmatic. Needle-fiber or ‘whisker-fiber’ cement (e.g., Supko 1971; Whittle et al. 1993) is very rare in marine settings, but is more common in meteoric environments where it occurs in the pores of calcarenite, calcretes and eolinites (e.g., Supko 1971; James 1972; Ward 1975; Knox 1977; Longman 1980; Whittle et al. 1993). LMC is the most common polymorph in needle-fiber cements in caliche environments, but aragonite also has been observed. HMC is rare as needle-fiber cement (e.g., Coniglio and Harrison 1983 from Verrecchia and Verrecchia 1994—their Table 1). Previous marine records include Bentis et al. (2000), who illustrated a calcified filament with a meshwork of acicular crystals with a ~5 μm aperture in Montipora from Johnston Atoll, but the composition of the crystals was not determined.
Controls on needle-fiber growth are unknown. Folk (1974) suggested that high Mg2+ contents selectively poison lateral growth thus forming fibrous or elongate crystals. Lahann (1978) proposed an opposing model wherein elongation is controlled by differential growth on crystal faces due to changes in surface charge. Given and Wilkinson (1985) suggested that rapid CO2-degassing in vadose waters with accompanying rapid increase in the saturation state of the water with respect to calcite could lead to needle-fiber growth. All three physico-chemical models could have affected precipitation within sampled corals, but none explain its restriction to filaments, and rapid degassing in vadose waters could have occurred only in the exposed samples, yet needle-fiber cement occurs in all three environments. Hence, some biological mediation is likely.
Needle-fiber calcite was documented within fungal hyphae in terrestrial soils (see Verrecchia and Verrecchia 1994, their fig. 11), and isotopic data indicate a significant incorporation of organically derived CO2 (e.g., Verrecchia et al. 2003; Bajnoczi and Kovacs-Kis 2006). However, it is unclear if marine fungi could have induced the precipitation of the needle-fiber cement in the present samples. Fungi were clearly active in the samples, but the 10–40 μm internal diameter of the HMC needle-fiber tubes is larger than typical for fungal hyphae, being more typical of cyanobacteria. However, fungal hyphae that extend into pore spaces in corals may swell to much larger diameters (Le Campion-Alsumard et al. 1995a). Additionally, the filaments could represent degradational structures wherein cementation was induced by fungi that attacked or degraded larger cyanobacterial filaments (Fig. 9c, d). However, a possible mechanism for fungi-induced calcification is unknown. Burford et al. (2006) suggested that CaCO3 could nucleate on fungal cell walls and that precipitation might be favored by increased alkalinity produced by dissolution of the host carbonate by excretion of H+ and/or organic acids.
Low-Mg calcite (LMC) cement
Boring-filling LMC cements represent a diagenetic paradox in a shallow marine environment (for extended discussion see Nothdurft et al. 2007). Although aragonite would normally be favored by the high Mg:Ca ratio of seawater (e.g., Bischoff and Fyfe 1968; Folk 1974; Berner 1975), Mg:Ca ratios were very low within the borings described above because precipitation occurred in an enclosed environment where minor elements were sourced almost entirely from dissolved aragonite. The low Mg content of the original aragonite allowed LMC to precipitate in the largely closed system (Nothdurft et al. 2007). Hence, these cements are clearly the products of a microbial microenvironment. A boring coccoid cyanobacterium, possibly similar to Solentia, dissolved skeletal aragonite at one end of the boring, and calcite precipitated in the space left as the microbe moved laterally into the newly dissolved tunnel. Protons released by calcite precipitation were apparently transported by the cyanobacterium to the dissolution end of the boring to convert HCO -3 to CO2 for photosynthesis (Nothdurft et al. 2007). Similar boring with concomitant infilling activity has been demonstrated in stromatolite and foraminifer substrates by coccoid cyanobacteria (Macintyre et al. 2000; Reid and Macintyre 2000). The occurrence of LMC-filled borings in the most recently deposited skeletal aragonite at the distal tips of skeleton still occupied by living polyps suggests that these LMC cements may reflect the earliest cements observed in the present study.
Brucite cement
Nothdurft et al. (2005) described brucite cement in live-collected Acropora, Pocillopora and Porites from Heron Reef, GBR (the present samples) and Montastrea from Florida. The present study adds brucite occurrences in Goniastrea, where morphologies are similar to those previously described in the other genera. Although brucite occurred in all three sampled environments in Pocillopora, Porites or Goniastrea, it occurred in Acropora samples only in the exposed setting. Buster and Holmes (2006) noted abundant brucite in green bands in Montastrea, and its distribution is clearly related to the distribution of microbial endoliths in the present material (Fig. 3). Nothdurft et al. (2005) suggested the following model to account for brucite precipitation in coral skeletons. (1) Semiconfined intracorallum spaces contain dead organic matter and live algae, cyanobacteria, and fungi. (2) Photosynthetic removal of CO2 lowers the pCO2 and increases the pH, leading to aragonite precipitation, which (3) lowers Ca2+ activity, thereby increasing the Mg:Ca ratio. (4) Increased pH, possibly due to bacterial ammonia production from decaying organic matter (e.g., Reitner 1993), produces excess OH-, leading to localized brucite precipitation. (5) Preferential nucleation of brucite on organic filaments may reflect the preferential adsorption of Mg2+ on organic matter. It is also possible that filamentous boring cyanobacteria that had little access to CO2 for photosynthesis may have utilized HCO -3 generated within their borings by aragonite dissolution and transported OH- along the filament to be released within the intracorallum spaces where the brucite formed. Regardless, the sheaths of the cyanobacterium Schizothrix calciola have Mg:Ca ratios three to four times greater than the seawater containing them (Gebelein and Hoffman 1973). Thus, high Mg2+ activity combined with high pH and low pCO2 led to localized brucite precipitation beneath organic biofilms.
Cement distribution
Distribution of cement within live coralla is controlled by both abiotic and biological processes, but the latter processes are clearly more important in the present samples. The more or less isopachous aragonite needle cement that is commonly syntaxial on coral aragonite is consistent with abiotic precipitation reflecting increased carbonate saturation typical of the warm and well-agitated ambient waters of the reef environment. However, nucleation of aragonite cement on organic matter, including algal or cyanobacterial filaments, and the increased cementation associated with boring sponges suggests that some aragonite cementation was biologically induced or at least biologically localized. The lack of isopachous aragonite cements in some cavities in the midst of better cemented parts of coralla may also reflect biological control wherein nucleation was inhibited by organic matter coating these surfaces (e.g., Fig. 13d–f). The distributions of all other cement types appear to be controlled entirely by microbially mediated microenvironments, although broader physico-chemical forcing may have enhanced precipitation rates.
Specific processes that control biologically induced mineralization have been reviewed and discussed by numerous authors (reviews by Ehrlich 1990; Castanier et al. 1999; Riding 2000; Webb 2001), but few environments have previously been documented that support biologically induced precipitation of so many different minerals with such diverse morphologies in such close proximity to each other as in the present live-collected coral skeletons (Figs. 5c, 24). The non-uniform distribution of the various minerals at fine scales, commonly occupying only small parts of available surfaces within a given cavity, is consistent with their formation in microenvironments constrained by biofilms. Additionally, organic films still partly cover many cements in partially bleached samples (Figs. 19e, 22f). Different minerals precipitated within the same intracorallum cavity, in some cases with cross cutting relationships and mutual growth interference. Ambient water within such a cavity could not have produced aragonite, brucite and HMC over the same time frames, without some of the cements being isolated within biofilm-controlled microenvironments. The brucite (Nothdurft et al. 2005) and LMC (Nothdurft et al. 2007) cements particularly highlight the importance of microenvironments in these settings and the role played by microbes in altering confined water chemistry relative to ambient seawater. Neither mineral should precipitate naturally in shallow seawater.
Scanning electron microscope images of interseptal spaces in G. favulus showing extensive cementation. a shows distribution of cement types and has been traced in b. The location of c–e is shown in a. c is aragonite botryoid cement. b is HMC whisker cement. e is an accumulation of sponge chips at the base a cavity
Despite the clear positive correlation of cements and microbial endoliths documented in this study and the ubiquitous occurrence of endobionts in coral skeletons in general (Risk et al. 1987; Le Campion-Alsumard et al. 1995a, b; Allison 1996b; Bentis et al. 2000; Golubic et al. 2005), there has been little discussion of causal links between bioerosion and cementation in corals (e.g., Scherer 1974; Rehman et al. 1994). Research has focused more on coral dissolution (e.g., Barnes and Lough 1993; Le Campion-Alsumard et al. 1995a). However, skeletal dissolution may increase alkalinity and lead to enhanced precipitation as discussed previously for sponges. Green or grey bands in coralla may have more than 25% of skeletal carbonate dissolved by borers (Le Campion-Alsumard et al. 1995a), and skeletal density has commonly been shown to be affected by as much as 8–9% (e.g., Barnes and Lough 1993; Le Campion-Alsumard et al. 1995a). Such density loss almost certainly affects the hydrodynamics within the coral skeleton, and resulting alkalinity may promote local cement precipitation (e.g., Scherer 1974). As microboring assemblages in reef environments relate to bathymetry and light penetration (e.g., Budd and Perkins 1980; May et al. 1982; Vogel et al. 2000), cementation might be expected to vary in a similar way. Additionally, microbial borings are more abundant in corals during the summer months, thus allowing annual cyclicity to be identified in some cases (Risk et al. 1987), and microborings may be more abundant in corals when growth rates are slowed due to stress, such as during bleaching events (e.g., Golubic et al. 2005). If the correlation between microborers and cementation observed in this study holds in those cases as well, it may be possible to use cement types to recognize intervals of stress or changes in growth rates in Pleistocene or older corals where organic matter is no longer preserved.
Of the three environments sampled, cementation and bioerosion are more abundant in coralla from the exposed reef flat and less so in the ponded and subtidal settings. It is unlikely that this variation is caused by differences in water depth, as major differences in bioerosion rates have not been documented over such small bathymetric gradients (2–3 m). The increased bioerosion more likely reflects environmental stress. Although scleractinian corals avoid desiccation during periods of tidal exposure by excreting mucus, they are more susceptible to thermal stress during exposure. Thermal stress may result in reduced growth rate and partial or total loss of zooxanthellae (i.e., bleaching) (Hoegh-Guldberg et al. 2005). Loss of zooxanthellae allows more light to penetrate the skeleton, which results in greater rates of photosynthesis by endoliths and thus, increased bioerosion (Fine and Loya 2002). Additionally, decreased coral growth rates resulting from stress result in greater concentration of endolithic algae in the skeleton near live polyps, and increased concentrations of parasitic fungi may also result. If the coral recovers from such a crisis, it leaves concentric green or black bands, which mark the occurrence of the crisis (Fig. 25) (Le Campion-Alsumard et al. 1995a; Golubic et al. 2005). Where stressed polyps die, dead corallum surfaces expose the skeleton to additional infestation of endoliths/endobionts (Fig. 25) (e.g., Scoffin and Bradshaw 2000; Hendy et al. 2003). The greatest cementation occurs under such dead portions of otherwise living coralla.
Diagram showing the progression of growth of a corallum of G. favulus (04-9-6—exposed reef flat) illustrated at the top. Line traces on the right follow green/grey bands, which represent specific time intervals. These lines suggest that sections of the coral have died at different stages of corallum development while the rest of the corallum continued growing. After a period coral polyps extended back over dead, encrusted parts of the skeleton (shown on the left). These temporarily exposed sections of skeleton are areas of increased diagenetic change. The removal of tissue exposed the skeleton to increased bioerosion by providing a point of access for bioeroding (micro- and macroborers) and encrusting (e.g., coralline red algae, foraminifera) organisms to infest previously protected areas. Note the high abundance of cements (e.g., area shown in Fig. 24)
As with endolithic activity in general, there is a correlation between cements and corallum morphology, with greater cement volume and diversity in the massive coralla (Porites, Goniastrea). As suggested above, much of this correlation reflects the distribution and abundance of microbes, and their abundance was previously related to the relative extension rates of the coralla. The slower extension rates would also provide more time for cement growth per surface area of abandoned intracorallum spaces. However, it is also likely that the greater volume to surface area ratios of the massive coralla compared to those of the branching corals retarded exchange of intracorallum fluids with fresh seawater to a greater degree, thus allowing the massive coralla to become more effective microenvironments in their own right. With lower fluid exchange rates, the fluids in massive coralla could be more heavily affected by internal processes that drive up pH and alkalinity (e.g., photosynthetic removal of CO2 and ammonification of degrading organic matter), thus enhancing cementation. Fluids in the more rapidly growing coral branches would be exchanged with external seawater far more easily, possibly explaining the absence of isopachous aragonite fringe cements, which occurred in all other samples, in subtidal and ponded Acropora.
Are the present samples representative of older Holocene corals?
Despite the high degree of diagenetic alteration observed in coralla in this study, it is clear from published SEM images and personal observation of some coral cores that some scleractinian coral skeletons have been less affected by the early diagenetic processes documented above (e.g., Meibom et al. 2003). Hence, the question arises as to how representative the present samples are of corals typically used for palaeoclimate studies. Long sections of core from large diameter, living Porites coralla have been observed wherein cements and boring are minimal or absent. Better preservation could reflect the large size of the coralla (up to several meters in diameter, representing centuries of continuous coral growth) if these corals reached a critical point where they were able to outgrow microbial infestations. However, they all must have started as small coralla similar to those studied here. Alternately, the larger coral heads generally grew in deeper water (5–10 m) than the corals sampled in this study where light penetration is less and borers may have been less active. Boring activity, and to a lesser degree cementation, was clearly less abundant in the subtidal samples examined from Heron Reef. Hence, endolith activity in the very shallow-water samples may have enhanced early cementation by increasing fluid throughput in coralla, increasing pH due to photosynthetic removal of CO2, and directly increasing alkalinity by dissolving coral aragonite. Additionally, those effects were enhanced in exposed coralla due to extrinsic parameters, such as solar heating of water, evaporation, CO2 degassing due to wave agitation and heating and increased fluid flow due to active wave and tidal pumping. Hence, observed differences may reflect only water depth and exposure.
However, an alternate hypothesis must also be considered. The present samples may have grown during a warmer interval than many older Holocene corals. Thus, the high endolithic activity, and resulting volume of cement and diversity of cement-producing microenvironments, may reflect the warmer environment and increased stress levels of very recent corals. Coral bleaching events are undocumented prior to the 1970s but have been expanding since then in frequency, severity, and geographic scale (Hoegh-Guldberg 1999). Major bleaching events affected coral populations on the GBR in 1998 and 2002. In each case, coral bleaching spread to more than 50% of the Great Barrier Reef Marine Park (Berkelmans and Oliver 1999; Berkelmans 2002; Dennis 2002). It is unknown to what extent the studied corals may have been affected by those events, as there are no records of bleaching specifically for corals in the study area. Regardless, the samples collected for this study could be atypical of longer Holocene reef history, due to recent increased thermal stress.
Implications for geochemical analysis and palaeoclimate research
Early diagenetic cements are an acknowledged source of error in geochemical analyses of coral skeletons, and aragonite cements contain distinct isotopic and trace-element compositions that differ from those of coral aragonite (e.g., Enmar et al. 2000; Quinn and Taylor 2006). Enmar et al. (2000) suggested that inclusion of secondary aragonite in coral analyses in the Gulf of Aqaba could lower estimated SST by ~2°C using conventional Sr/Ca thermometry. Similarly, Lazar et al. (2004) found significantly higher uranium concentrations in secondary aragonite than in skeletal aragonite (17.3 + −0.6 nmol g−1 and U/Ca = 1.79 nmol/mol compared to 11.9 + −0.3 nmol g−1 and U/Ca = 1.23 nmol/mol, respectively), resulting in a small but measurable effect on U/Ca thermometry and U/Th age determination. Aragonite and HMC cements also are enriched in 13C relative to coral aragonite (Müller et al. 2006). Regardless, most previous studies have attributed geochemical differences between coral skeleton and cements to differences in trace-element incorporation or isotopic equilibria related to coral skeletogenesis (i.e., biological effects). In both cases, the minerals are inferred to basically reflect ambient seawater. However, the present study suggests that many of the early cements in coral skeletons grew in microenvironments wherein the water chemistry did not reflect that of ambient shallow seawater. That observation has important implications for palaeoclimate archives.
The LMC cement in borings does not reflect precipitation from ambient seawater and contains significantly lower Sr/Ca and higher Mg/Ca ratios than the source aragonite (Sr/Cacalcite = 6.3 ± 1.3 mmol/mol, compared to Sr/Cacoral aragonite = 9.9 ± 1.2 mmol/mol; Mg/Cacalcite = 12.0 ± 5.1 mmol/mol, compared to Mg/Cacoral aragonite = 4.5 ± 2.3) (Nothdurft et al. 2007). Those authors used Sr palaeothermometry equations from Corrège (2006) to calculate that incorporation of 1% of the LMC into an analysis of coral aragonite increases the calculated mean SST by 0.5°C; 5% boring calcite increases the calculated mean SST by 2.5°C; and 60% boring calcite, the maximum percentage observed in our samples, increases the calculated mean SST by 30.3°C. Hence, even a single 10 μm calcite-filled boring contained within a typical 20–200 μm-diameter laser ablation or ion microprobe sample would provide a significantly faulty SST calculation. As some of the cements were emplaced in skeleton that was only days to weeks old, such cements could theoretically occur in any coral skeleton.
Similarly, abundant brucite cement does not reflect precipitation from ambient seawater. Owing to the high Mg content of brucite, even small amounts included in a geochemical analysis could greatly increase measured Mg concentrations (Buster and Holmes 2006), and inadvertent sampling of brucite could be responsible for reported anomalies in Mg/Ca versus SST plots in corals (e.g., discussion in Corrège 2006). Defining the source of such anomalies is critical for high-resolution, high-precision microsampling techniques.
Although HMC cements have been identified rarely in live-collected scleractinian coral skeletons they should be easily identified in high resolution geochemical analyses, owing to their anomalously high Mg:Ca ratios (e.g., 144 mmol/mol—Allison et al. 2007). However, with coarser sampling resolution (i.e., mm) such contaminants might be far less obvious. Allison et al. (2007) calculated that 1% HMC contamination alters calculated SST values by +1°C, and U contamination alters SST values by +3°C. Aragonite cement contamination of 5% would have a similar effect. Screening by XRD may not detect such small, but geochemically significant, amounts of calcite in corals and will also miss the aragonite cements, even where they are very abundant near sponge borings and near the tips of fungal protuberances.
Areas affected by protuberances have the potential to affect fine-scale geochemical data in two ways. Firstly, the secondary aragonite may be deposited with temporal disjunction from the adjacent skeletal aragonite. Thus, the anomalous skeletal aragonite may be significantly younger than the wall that was penetrated, potentially disturbing high resolution time-series data (e.g., see Nothdurft and Webb 2007). Secondly, as the protuberances may represent a somewhat different biomineralization process, possibly with fungal influence, they could contain different trace-element or stable-isotope characteristics. Additionally, organic matter contained within borings, representing either remains of the boring microbes or secondary degradational communities of microbes, could contain trace elements out of equilibrium with the surrounding aragonite (e.g., Allison and Tudhope 1992).
In summary, the resolution and technique employed for geochemical sampling of coral skeletons requires a specific degree of vetting to identify the early diagenetic features documented in live-collected corals from Heron Reef. While XRD may detect large-scale meteoric diagenesis, it would not be able to detect diagenetic contaminants at small concentrations that could still affect high resolution geochemical data. Large-scale, low temporal resolution sampling techniques, such as solution ICP-MS on powders generated by scraping or drilling (e.g., Yu et al. 2005; Calvo et al. 2007; Morimoto et al. 2007) and even LA–ICP-MS where spot sizes are greater than ~50 μm (e.g., Fallon et al. 2003) could potentially sample all of the diagenetic cement types documented above. Techniques with smaller spot sizes (e.g., LA-ICP-MS, NanoSIMS, ion microprobe) can target individual skeletal structures and so avoid cements that occur on the surfaces of cavities. However, they must still avoid cements and organic matter that occurs within microborings, and that may be difficult in cases such as the LMC-filled borings because they are difficult to see on polished coral sections (Nothdurft et al. 2007). Hence, petrographic examination by optical or, preferably, scanning electron microscopy on polished etched sections is necessary to confirm that samples are pristine.
References
Acker KL, Risk MJ (1985) Substrate destruction and sediment production by the boring sponge Cliona caribbaea on Grand Cayman Island. J Sediment Petrol 55:705–711
Aissaoui DM (1985) Botryoidal aragonite and its diagenesis. Sedimentology 32:345–361. doi:10.1111/j.1365-3091.1985.tb00516.x
Allison N (1996a) Comparative determinations of trace elements in coral aragonite by ion microprobe analysis, with preliminary results from Phucket, southern Thailand. Geochim Cosmochim Acta 60:3457–3470. doi:10.1016/0016-7037(96)00171-8
Allison N (1996b) Geochemical anomalies in coral skeletons and their possible implications for palaeoenvironmental analyses. Mar Chem 55:367–379. doi:10.1016/S0304-4203(96)00060-6
Allison N, Finch AA, Newville M, Sutton SR (2005) Strontium in coral aragonite: 3. Sr coordination and geochemistry in relation to skeletal architecture. Geochim Cosmochim Acta 69:3801–3811. doi:10.1016/j.gca.2005.01.026
Allison N, Finch AA, Webster JM, Clague DA (2007) Palaeoenvironmental records from fossil corals: the effects of submarine diagenesis on temperature and climate estimates. Geochim Cosmochim Acta 71:4693–4703. doi:10.1016/j.gca.2007.07.026
Allison N, Tudhope AW (1992) Nature and significance of geochemical variation in coral skeletons as determined by ion microprobe analysis. Proceedings of 7th international coral reef symposium 1:173–178
Anderson KL, Tayne TA, Ward DM (1987) Formation and fate of fermentation products in hot spring cyanobacterial mats. Appl Environ Microbiol 53:2343–2352
Bajnoczi B, Kovacs-Kis V (2006) Origin of pedogenic needle-fiber calcite revealed by micromorphology and stable isotope composition—a case study of a quaternary paleosol from Hungary. Chem Erde 66:203–212. doi:10.1016/j.chemer.2005.11.002
Barnes DJ, Lough JM (1993) On the nature and causes of density banding in massive coral skeletons. J Exp Mar Biol Ecol 167:91–108. doi:10.1016/0022-0981(93)90186-R
Beck JW, Edwards RL, Ito E, Taylor FW, Récy J, Rougerie F, Joannot P, Henin C (1992) Sea-surface temperature from coral skeletal strontium/calcium ratios. Science 257:644–647. doi:10.1126/science.257.5070.644
Bentis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol Bull 198:254–260. doi:10.2307/1542528
Berkelmans R (2002) Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar Ecol Prog Ser 229:73–82. doi:10.3354/meps229073
Berkelmans R, Oliver JK (1999) Large-scale bleaching of corals on the Great Barrier Reef. Coral Reefs 18:55–60. doi:10.1007/s003380050154
Berner RA (1975) The role of magnesium in the crystal growth of calcite and aragonite in seawater. Geochim Cosmochim Acta 39:489–504. doi:10.1016/0016-7037(75)90102-7
Beuck L, Vertino A, Stepina E, Karolczak M, Pfannkuche O (2007) Skeletal response of Lophelia pertusa (Scleractinia) to bioeroding sponge infestation visualised with micro-computed tomography. Facies 53:157–176. doi:10.1007/s10347-006-0094-9
Bischoff JL, Fyfe WS (1968) Catalysis, inhibition, and the calcite–aragonite problem. I. The aragonite–calcite transformation. Am J Sci 266:65–79
Budd DA, Perkins RD (1980) Bathymetric zonation and paleoecological significance of microborings in Puerto Rican shelf and slope sediments. J Sediment Petrol 50:881–904
Burford EP, Hillier S, Gadd GM (2006) Biomineralization of fungal hyphae with calcite (CaCO3) and calcium oxalate mono- and dihydrate in Carboniferous limestone microcosms. Geomicrobiol J 23:599–611. doi:10.1080/01490450600964375
Buster NA, Holmes CW (2006) Magnesium content within the skeletal architecture of the coral Montastraea faveolata: locations of brucite precipitation and implications to fine-scale data fluctuations. Coral Reefs 25:243–253. doi:10.1007/s00338-006-0092-y
Calcinai B, Arillo A, Cerrano C, Bavestrello G (2003) Taxonomy-related differences in the excavating micro-patterns of boring sponges. J Mar Biol Assoc UK 83:37–39
Calvo E, Marshall JF, Pelejero C, McCulloch MT, Gagan MK, Lough JM (2007) Interdecadal climate variability in the Coral Sea since 1708 A.D. Palaeogeogr Palaeoclimatol Palaeoecol 248:190–201. doi:10.1016/j.palaeo.2006.12.003
Castanier S, Le Metayer-Levrel G, Perthuisot J-P (1999) Ca-carbonate precipitation and limestone genesis—the microbiogeologist point of view. Sediment Geol 126:9–23. doi:10.1016/S0037-0738(99)00028-7
Clode PL, Marshall AT (2003) Variation in skeletal microstructure of the coral Galaxea fascicularis: effects of an aquarium environment and preparatory techniques. Biol Bull 204:138–145. doi:10.2307/1543549
Cohen AL, Layne GD, Hart SR (2001) Kinetic control of skeletal Sr/Ca in a symbiotic coral: implications for the paleotemperature proxy. Paleoceanography 16:20–26. doi:10.1029/1999PA000478
Cohen AL, Owens KE, Layne GD, Shimizu N (2002) The effect of algal symbionts on the accuracy of Sr/Ca paleotemperatures from coral. Science 296:331–333. doi:10.1126/science.1069330
Coniglio M, Harrison RS (1983) Holocene and Pleistocene caliche from Big Pine Key. Bull Can Pet Geol 31:3–13
Constantz BR (1986) The primary surface area of corals and variations in their susceptibility to diagenesis. In: Shroeder JH, Purser BH (eds) Reef diagenesis. Springer, Berlin, pp 53–76
Corrège T (2006) Sea surface temperature and salinity reconstruction from coral geochemical tracers. Palaeogeogr Palaeoclimatol Palaeoecol 232:408–428. doi:10.1016/j.palaeo.2005.10.014
Cuif J-P, Dauphin Y (2005) The environmental recording unit in coral skeletons—a synthesis of structural and chemical evidences for a biochemically driven, stepping-growth process in fibres. Biogeosciences 2:67–73
Cuif J-P, Denis A, Gautret P, Marin F, Mastandrea A, Russo F (1992) Recherches sur l’alteration diagenetique des biomineralisations carbonatees: evolution de la phase organique intrasquelettique dans des polypiers aragonitiques de Madreporaires du Cenozoique (Bassin de Paris) et du Trias superieur (Dolomites et Turquie). C R Acad Sci Paris 314:1097–1102
Cuif J-P, Dauphin Y, Berthet P, Jegoudez J (2004) Associated water and organic compounds in coral skeletons: quantitative thermogravimetry coupled to infrared absorption spectrometry. Geochem Geophys Geosyst 5. doi:10.1029/2004GC000783
Cuif J-P, Sorauf JE (2001) Biomineralization and diagenesis in the Scleractinia: part 1, biominerlization. Bull Tohoku Univ Mus 1:144–151
Dauphin Y, Marin F, Gautret P, Cuif J-P (1990) Discimination des biominéralisations aragonitiques fibreuses des spongiaires, cnidaires et mollusques, par l’indice de substitution des éléments mineurs dans le reseau carbonate. CR Acad Sci Paris 314:1111–1116
Davies PJ, Kinsey DW (1973) Organic and inorganic factors in recent beach rock formation, Heron Island, Great Barrier Reef. J Sediment Petrol 43:59–81
Dennis C (2002) Reef under threat from bleaching outbreak. Nature 415:947. doi:10.1038/415947a
Défarge C, Trichet J, Maurin A, Hucher M (1994) Kopara in Polynesian atolls: early stages of formation of calcareous stromatolites. Sediment Geol 89:9–23. doi:10.1016/0037-0738(94)90080-9
de Villiers S, Nelson BK, Chivas AR (1995) Biological controls on coral Sr/Ca and δ18O reconstructions of sea surface temperature. Science 269:1247–1249. doi:10.1126/science.269.5228.1247
Diaz MC, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Domart-Coulon I, Tambutté S, Tambutté E, Allemand D (2004) Short term viability of soft tissue detached from the skeleton of reef-building corals. J Exp Mar Biol Ecol 309:199–217. doi:10.1016/j.jembe.2004.03.021
Ehrlich HL (1990) Geomicrobiology, 2nd edn. Marcel Dekker, New York
Enmar R, Stein M, Bar-Matthews M, Sass E, Katz A, Lazar B (2000) Diagenesis in live corals from the Gulf of Aqaba. I. The effect on paleo-oceanography tracers. Geochim Cosmochim Acta 64:3123–3132. doi:10.1016/S0016-7037(00)00417-8
Fallon SJ, McCulloch MT, Alibert C (2003) Examining water temperature proxies on Porites corals from the Great Barrier Reef: a cross-shelf comparison. Coral Reefs 22:389–404. doi:10.1007/s00338-003-0322-5
Fallon SJ, McCulloch MT, van Woesik R, Sinclair DJ (1999) Corals at their latitudinal limits: laser ablation trace element systematics in Porites from Shirigai Bay, Japan. Earth Planet Sci Lett 172:221–238. doi:10.1016/S0012-821X(99)00200-9
Fine M, Loya Y (2002) Endolithic algae: an alternative source of photoassimilates during coral bleaching. Proc R Soc Lond B Biol Sci 269:1205–1210. doi:10.1098/rspb.2002.1983
Folk R (1974) The natural history of crystalline calcium carbonate; effect of magnesium content and salinity. J Sediment Petrol 44:40–53
Frost R, Kloprogge T, Schmidt J (1999) Non-destructive identification of minerals by Raman microscopy. Int J Vibr Spectrosc 3(4):2
Gaetani GA, Cohen AL (2006) Element partitioning during precipitation of aragonite from seawater: a framework for understanding paleoproxies. Geochim Cosmochim Acta 70:4617–4634. doi:10.1016/j.gca.2006.07.008
Gaffey SJ, Bronnimann CE (1993) Effects of bleaching on organic and mineral phases in biogenic carbonates. J Sediment Petrol 63:752–754
Gautret P (2000) Matrices organiques intrasquelettiques des sclératiniaires récifaux: évolution diagénétique précoce de leurs caractéristiques biochimiques et conséquences pour les processus de cementation. Geobios 33:73–78. doi:10.1016/S0016-6995(00)80150-3
Gautret P, Marin F (1993) Evaluation of diagenesis in scleractinian corals and calcified demosponges by substitution index measurement and intraskeletal organic matrix analysis. Courier Forschungsinstitut Senckenberg 164:317–327
Gebelein CD, Hoffman P (1973) Algal origin of dolomite laminations in stromatolitic limestone. J Sediment Petrol 43:603–613
Gektidis M, Dubinsky Z, Goffredo S (2006) Microendoliths of the shallow euphotic zone in open and shaded habitats at 30°N-Eilat, Israel–paleoecological implications. Facies 53:43–55. doi:10.1007/s10347-006-0091-z
Ginsburg RN, James NP (1976) Submarine botryoidal aragonite in Holocene reef limestones, Belize. Geology 4:431–436. doi:10.1130/0091-7613(1976)4<431:SBAIHR>2.0.CO;2
Ginsburg RN, Marszalek DS, Schneidermann N (1971) Ultrastructure of carbonate cements in a Holocene algal reef of Bermuda. J Sediment Petrol 41:472–482
Gischler E, Lomando AJ (1997) Holocene cemented beach deposits in Belize. Sediment Geol 110:277–297. doi:10.1016/S0037-0738(96)00088-7
Given RK, Wilkinson BH (1985) Kinetic control of morphology, composition, and mineralogy of aboitic sedimentary carbonates. J Sediment Petrol 55:109–119
Golubic S, Brent G, Le Campion T (1970) Scanning electron microscopy of endolithic algae and fungi using a multipurpose casting embedding technique. Lethaia 3:203–209. doi:10.1111/j.1502-3931.1970.tb01858.x
Golubic S, Radtke G, Le Campion-Alsumard T (2005) Endolithic fungi in marine ecosystems. Trends Microbiol 13:229–235. doi:10.1016/j.tim.2005.03.007
Grammer GM, Crescini CM, McNeill DF, Taylor LH (1999) Quantifying rates of syndepositional marine cementation in deeper platform environments - new insight into a fundamental process. J Sediment Res 69:202–209
Grammer GM, Ginsburg RN, Swart PK, McNeill DF, Jull AJ, Prezbindowski DR (1993) Rapid growth of syndepositional marine aragonite cements in steep marginal slope deposits, Bahamas and Belize. J Sediment Petrol 63:983–989
Grottoli AG, Eakin CM (2007) A review of modern coral δ18O and δ14C proxy records. Earth Sci Rev 81:67–91. doi:10.1016/j.earscirev.2006.10.001
Grottoli AG, Rodrigues LJ, Matthews KA, Palardy JE, Gibb OT (2005) Pre-treatment effects on coral skeletal δ13C and δ18O. Chem Geol 221:225–242. doi:10.1016/j.chemgeo.2005.05.004
Gvirtzman G, Friedman GM (1976) Sequence of progressive diagenesis in coral reefs. In. Frost SH, Weiss MP, Saunders JB (eds) Reef and related carbonates-ecology and sedimentology. American association of petroleum geologists studies in geology, vol 4, pp 357–380
Hanor JS (1978) Precipitation of beachrock cements: mixing of marine and meteoric waters vs. CO2-degassing. J Sediment Petrol 48:489–501
Hendy EJ, Gagen MK, Lough JM, McCulloch M, deMenocal PB (2007) Impact of skeletal dissolution and secondary aragonite on trace element and isotopic climate proxies in Porites corals. Palaeoceanography 22:PA4101. doi:10.1029/2007PA001462
Hendy EJ, Lough JM, Gagen MK (2003) Historical mortality in massive Porites from the central Great Barrier Reef, Australia: evidence for past environmental stress? Coral Reefs 22:207–215. doi:10.1007/s00338-003-0304-7
Highsmith RG (1980) Geographic patterns of coral bioerosion: a productivity hypothesis. J Exp Mar Biol Ecol 46:177–196. doi:10.1016/0022-0981(80)90030-1
Hill MS (1996) Symbiotic zooxanthellae enhance boring and growth rates of the tropical sponge Anthosigmella varians forma varians. Mar Biol (Berl) 125:649–654. doi:10.1007/BF00349246
Holmes KE, Edinger EN, Hariyadi, Limmon GV, Risk MJ (2000) Bioerosion of live massive corals and branching coral rubble on Indonesian coral reefs. Mar Pollut Bull 40:606–617. doi:10.1016/S0025-326X(00)00067-9
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866. doi:10.1071/MF99078
Hoegh-Guldberg O, Fine M, Skirving W, Johnstone R, Dove S, Strong A (2005) Coral bleaching following wintry weather. Limnol Oceanogr 50:265–271
Hubbard JAEB (1972) Cavity formation in living scleractinian reef corals and fossil analogues. Geol Rundsch 61:551–564. doi:10.1007/BF01896333
Hubbard JAEB (1975) Life and the afterlife of reef corals: a timed study of incipient diagenesis. IX Int Sedimentol Congr 7:75–80
Hubbard JAEB, Swart PK (1982) Sequence and style in scleractinian coral preservation in reefs and associated facies. Palaeogeogr Palaeoclimatol Palaeoecol 37:165–219. doi:10.1016/0031-0182(82)90038-4
James NP (1972) Holocene and Pleistocene calcareous crust (caliche) profiles: critera for subaerial exposure. J Sediment Petrol 42:817–836
Jordan G, Rammensee W (1996) Dissolution rates and activation of brucite (001): a new method based on the microtopography of crystal surfaces. Geochim Cosmochim Acta 60:5055–5062. doi:10.1016/S0016-7037(96)00309-2
Knox GJ (1977) Caliche profile formation, Saldanha Bay (South Africa). Sedimentology 24:657–674. doi:10.1111/j.1365-3091.1977.tb00263.x
Lafuste J (1970) Lames ultra-minces à faces polies. Procédé et application à la microstructure des Madréporaires fossils. CR Acad Sci Paris 270:679–681
Lahann RD (1978) A chemical model for calcite growth morphology control. J Sediment Petrol 48:337–344
Land LS, Moore LS (1980) Lithification, micritization and syndepositional diagenesis of biolithites on the Jamaican Island slope. J Sediment Petrol 50:357–370
Lazar B, Enmar R, Schossberger M, Bar-Matthews M, Halicz L, Stein M (2004) Diagenetic effects on the distribution of uranium in live and Holocene corals from the Gulf of Aqaba. Geochim Cosmochim Acta 68:4583–4593. doi:10.1016/j.gca.2004.03.029
Le Campion-Alsumard T, Golubic S, Hutchings P (1995a) Microbial endoliths in skeletons of live and dead corals: Porites lobata (Moorea, French Polynesia). Mar Ecol Prog Ser 117:149–157. doi:10.3354/meps117149
Le Campion-Alsumard T, Golubic S, Priess K (1995b) Fungi in corals: symbiosis or disease? Interaction between polyps and fungi causes pearl-like skeleton biomineralization. Mar Ecol Prog Ser 117:137–147. doi:10.3354/meps117137
Lewis SE, Shields GA, Kamber BS, Lough JM (2007) A multi-trace element coral record of land-use changes in the Burdekin River catchment, NE Australia. Palaeogeogr Palaeoclimatol Palaeoecol 246:471–487. doi:10.1016/j.palaeo.2006.10.021
Lighty RG (1985) Preservation of internal reef porosity and diagenetic sealing of submerged early Holocene Barrier Reef, Southeast Florida Shelf. In: Schneidermann N, Harris PM (eds) Carbonate cements. SEPM Spec Publ 36:123–154
Longman MW (1980) Carbonate diagenetic textures from nearsurface diagenetic environments. AAPG Bull 64:461–487
Lukas HJ (1974) Two species of the chlorophyte genus Osreobium from skeletons of Atlantic and Caribbean reef corals. J Phycol 10:331–335
Macintyre IG (1977) Distribution of submarine cements in a modern Caribbean fringing reef, Galeta Point, Panama. J Sediment Petrol 47:503–516
Macintyre IG (1984) Preburial and shallow-subsurface alteration of modern scleractinian corals. Palaeontogr Am 54:229–244
Macintyre IG (1985) Submarine cements—the peloidal question. In: Schneidermann N, Harris PM (eds) Carbonate cements. SEPM Spec Publ 36:109–116
Macintyre IG, Marshall JF (1988) Submarine lithification in coral reefs: some facts and misconceptions. Proceedings of 6th international coral reef symposium 1:263–271
Macintyre IG, Prufert-Bebout L, Reid RP (2000) The role of endolithic cyanobacteria in the formation of lithified laminae in Bahamian stromatolites. Sedimentology 47:915–921. doi:10.1046/j.1365-3091.2000.00327.x
Mackenzie FT, Bischoff WB, Bishop FC, Loijens M, Schoonmaker J, Wollast R (1983) Magnesian calcites: low-temperature occurrence, solubility and solid-solution behaviour. In: Reeder RJ (ed) Carbonate: mineralogy and chemistry. Mineral Soc Am, Chelsea, pp 97–144
Marshall AT (2002) Occurrence, distribution, and localisation of metals in cnidarians. Microsc Res Tech 56:341–357. doi:10.1002/jemt.10035
Marshall JF (1983) Submarine cementation in a high-energy platform reef: one tree reef, Southern Great Barrier Reef. J Sediment Petrol 53:1133–1149
Marshall JF (1986) Regional distribution of submarine cements within an epicontinental reef system: central Great Barrier Reef, Australia. In: Shroeder JH, Purser BH (eds) Reef diagenesis. Springer, Berlin, pp 8–26
Marshall JF, McCulloch MT (2002) An assessment of the Sr/Ca ratio in shallow water hermatypic corals as a proxy for sea surface temperature. Geochim Cosmochim Acta 66:3263–3280. doi:10.1016/S0016-7037(02)00926-2
May JA, Macintyre IG, Perkins RD (1982) Distribution of microborers within planted substrates along a barrier reef transect, Carrie Bow Cay, Belize. In: Rützler K, Macintyre IG (eds) The Atlantic Barrier Reef ecosystem at Carrie Bow Cay, Belize I: structure and communities. Smithsonian Contrib Mar Sci 12: 93–107
McCulloch M, Fallon S, Wyndham T, Hendy E, Lough J, Barnes D (2003) Coral record of increased sediment flux to the inner Great Barrier Reef since European settlement. Nature 421:727–730. doi:10.1038/nature01361
McGregor HV, Gagan MK (2003) Diagenesis and geochemistry of Porites corals from Papua New Guinea: Implications for paleoclimate reconstruction. Geochim Cosmochim Acta 67:2147–2156. doi:10.1016/S0016-7037(02)01050-5
McKenna SA (1997) Interactions between the boring sponge, Cliona lampa, and two hermatypic corals from Bermuda. In: Proceedings of 8th international coral reef symposium 2:1369–1374
Meibom A, Cuif J-P, Hillion F, Constanz B, Juillet-Leclerc A, Dauphin Y, Watanabe T, Dunbar R (2004) Distribution of magnesium in coral skeleton. Geophys Res Lett 31:L23306. doi:10.1029/2004GL021313
Meibom A, Cuif J-P, Houlbreque F, Mostefaoui S, Dauphin Y, Meibom KL, Dunbar R (2008) Compositional variations at ultra-structure length scales in coral skeleton. Geochim Cosmochim Acta 72:1555–1569. doi:10.1016/j.gca.2008.01.009
Meibom A, Stage M, Wooden J, Constantz BR, Dunbar RB, Owen A, Grumet N, Bacon CR, Chamberlain CP (2003) Monthly strontium/calcium oscillations in symbiotic coral aragonite: Biological effects limiting the precision of the paleotemperature proxy. Geophys Res Lett 30:1418–1421. doi:10.1029/2002GL016864
Meibom A, Yurimoto H, Cuif J-P, Domart-Coulon I, Houlbreque F, Constanz B, Dauphin Y, Tambutté E, Tambutté S, Allemand D, Wooden J, Dunbar R (2006) Vital effects in coral skeletal composition display strict three-dimensional control. Geophys Res Lett 33:L11608. doi:10.1029/2006GL025968
Meldrum FC, Hyde ST (2001) Morphological influence of magnesium and organic additives on the precipitation of calcite. J Cryst Growth 231:544–558. doi:10.1016/S0022-0248(01)01519-6
Mitsuguchi T, Matsumoto E, Abe O, Uchida T, Isdale PJ (1996) Mg/Ca thermometry in coral skeletons. Science 274:961–963. doi:10.1126/science.274.5289.961
Mitsuguchi T, Uchida T, Matsumoto E, Isdale PJ, Kawana T (2001) Variations in Mg/Ca, Na/Ca, and Sr/Ca ratios of coral skeletons with chemical treatments: implications for carbonate geochemistry. Geochim Cosmochim Acta 65:2865–2874. doi:10.1016/S0016-7037(01)00626-3
Moberly RJ (1970) Microprobe study of diagenesis in calcareous algae. Sedimentology 14:113–123. doi:10.1111/j.1365-3091.1970.tb00185.x
Moberly RJ (1973) Rapid chamber-filling growth of marine aragonite and Mg–calcite. J Sediment Petrol 43:634–635
Morimoto M, Kayanne H, Abe O, McCulloch MT (2007) Intensified mid-Holocene Asian monsoon records in corals from Kikai Island, subtropical northwestern Pacific. Quat Res 67:204–214. doi:10.1016/j.yqres.2006.12.005
Morse JW, Arvidson RS (2002) The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci Rev 58:51–84. doi:10.1016/S0012-8252(01)00083-6
Morse JW, Mackenzie FT (1990) Geochemistry of sedimentary carbonates. Elsevier, Amsterdam
Müller A, Gagan MK, Lough JM (2004) Effect of early marine diagenesis on coral reconstructions of surface-ocean 13C/12C and carbonate saturation state. Global Biogeochem Cycles 18(GB1033). doi:10.1029/2003GB002112
Müller A, Gagan MK, McCulloch M (2001) Early marine diagenesis in corals and geochemical consequences. Geophys Res Lett 28:4471–4474. doi:10.1029/2001GL013577
Müller A, McGregor HV, Gagan MK, Lough JM (2006) The effects of early marine aragonite, Mg–calcite and vadose-zone calcite diagenesis on reconstructions of coral calcification rate and the oceanic Suess effect. In: Proceedings of 10th international coral reef symposium, pp 607–614
Neumann AC (1966) Observations on coastal erosion in Bermuda and measurements of the boring rate of the sponge Cliona lampa. Limnol Oceanogr 11:92–108
Nothdurft LD, Webb GE (2007) Microstructure of common reef-building coral genera Acropora, Pocillopora, Goniastrea, and Porites: constraints on spatial resolution in geochemical sampling. Facies 53:1–26. doi:10.1007/s10347-006-0090-0
Nothdurft LD, Webb GE, Bostrom T, Rintoul L (2007) Calcite-filled borings in the most recently deposited skeleton in live-collected Porites (Scleractinia): implications for trace element archives. Geochim Cosmochim Acta 71:5423–5438. doi:10.1016/j.gca.2007.09.025
Nothdurft LD, Webb GE, Buster NA, Holmes CW, Sorauf JE, Kloprogge JT (2005) Brucite microbialites in living coral skeletons: indicators of extreme microenvironments in shallow-marine settings. Geology 33:169–172. doi:10.1130/G20932.1
Pandolfi JM, Greenstein BJ (1997) Taphonomic alteration of reef corals: effects of reef environment and coral growth form. I. The Great Barrier Reef. Palaios 12:27–42. doi:10.2307/3515292
Pang RK (1973) The ecology of some Jamaican excavating sponges. Bull Mar Sci 23:227–243
Perrin C (2004) Diagenese precoce des biocristaux carbonayes: tranformations isominerales de l’aragonite corallienne. Bull Soc Geol Fr 175:95–106. doi:10.2113/175.2.95
Perrin C, Cuif J-P (2001) Ultrastructural controls on diagenetic patterns of scleractinian skeletons: evidence at the scale of colony lifetime. Bull Tohoku Univ Mus 1:210–218
Perrin C, Smith DC (2002) A preliminary Raman spectroscopic study of the earliest steps of diagenesis of mineral and organic material in living scleractinian corals. Acta Univ Carol Geogr 46:66–68
Perrin C, Smith DC (2007) Earliest steps of diagenesis in living scleractinian corals: evidence from ultrastructural pattern and Raman spectroscopy. J Sediment Res 77:495–507. doi:10.2110/jsr.2007.051
Perry CT, Macdonald IA (2002) Impacts of light penetration on the bathymetry of reef microboring communities: implications for the development of microendolithic trace assemblages. Palaeogeogr Palaeoclimatol Palaeoecol 186:101–113. doi:10.1016/S0031-0182(02)00446-7
Pomponi SA (1980) Cytological mechanisms of calcium carbonate excavation by boring sponges. Int Rev Cytol 65:301–319. doi:10.1016/S0074-7696(08)61963-4
Potthast I (1992) Short-term progressive early diagenesis in density bands of recent corals: Porites colonies, Mauritius Island, Indian Ocean. Facies 27:105–112. doi:10.1007/BF02536806
Potts DC, Swart PK (1984) Water temperature as an indicator of environmental variability on a coral reef. Limnol Oceanogr 20:504–516
Priess K, Le Campion-Alsumard T, Golubic S, Gadel F, Thomassin BA (2000) Fungi in corals: black bands and density-banding of Porites lutea and P. lobata skeleton. Mar Biol (Berl) 136:19–27. doi:10.1007/s002270050003
Przenioslo R, Stolarski J, Mazur M, Brunelli M (2008) Hierarchically structured scleractinian coral biocrystals. J Struct Biol 161:74–82. doi:10.1016/j.jsb.2007.09.020
Quinn TM, Taylor FW (2006) SST artifacts in coral proxy records produced by early marine diagenesis in a modern coral from Rabaul, Papua New Guinea. Geophys Res Lett 33:L04601. doi:10.1029/2005GL024972
Rehman J, Jones B, Hagan TH, Coniglio M (1994) The influence of sponge borings on aragonite-to-calcite inversion in Late Pleistocene Stombus gigas from Grand Cayman, British West Indies. J Sediment Res A64:174–179
Reid RP, Macintyre IG (2000) Microboring versus recrystallization: further insight into the micritization process. J Sediment Res 70:24–28. doi:10.1306/2DC408FA-0E47-11D7-8643000102C1865D
Reitner J (1993) Modern cryptic microbialite/metazoan facies from Lizard Island (Great Barrier Reef, Australia): formation and concepts. Facies 29:3–40. doi:10.1007/BF02536915
Reitner J, Gautret P, Marin F, Neuweiler F (1995) Automicrites in a modern marine microbialite. Formation model via organic matrices (Lizard Island, Great Barrier Reef, Australia). Bull Inst Océanogr Monaco Spec 14(2):237–263
Reuter M, Brachert TC, Kroeger KF (2005) Diagenesis of growth bands in fossil scleractinian corals: identification and modes of preservation. Facies 51:146–159. doi:10.1007/s10347-005-0064-7
Revelle R, Emery KO (1957) Chemical erosion of beach rock and exposed reef rock. In: US Geol Surv Prof Paper, Reston, pp 699–709
Riding R (2000) Microbial carbonate: the geological record of calcified bacterial–algal mats and biofilms. Sedimentology 47:179–214. doi:10.1046/j.1365-3091.2000.00003.x
Risk MJ, MacGeachy JK (1978) Aspects of erosion of modern Caribbean reefs. Rev Biol Trop 26:85–105
Risk MJ, Pagani SE, Elias RJ (1987) Another internal clock: preliminary estimates of growth rates based on cycles of algae boring activity. Palaios 2:323–331. doi:10.2307/3514757
Rollion-Bard C, Blamart D, Cuif J-P, Juillet-Leclerc A (2003) Microanalysis of C and O isotopes of azooxanthellate and zooxanthellate corals by ion microprobe. Coral Reefs 22:405–415. doi:10.1007/s00338-003-0347-9
Runnalls LA, Coleman ML (2003) Record of natural and anthropogenic changes in reef environments (Barbados West Indies) using laser ablation ICP-MS and sclerochronolgy on coral cores. Coral Reefs 22:416–426. doi:10.1007/s00338-003-0349-7
Rützler K (1975) The role of burrowing sponges in bioerosion. Oceologia 19:203–216. doi:10.1007/BF00345306
Rützler K, Rieger G (1973) Sponge burrowing: fine structure of Cliona lampa penetrating calcareous substrata. Mar Biol (Berl) 21:144–162. doi:10.1007/BF00354611
Scherer M (1974) The influence of two endolithic micro-organisms on the diagenesis of recent coral skeletons. N Jahrb Geol PaläontMh 9:557–566
Schönberg CH (2000) Bioeroding sponges common to the central Australian Great Barrier Reef: description of three new species, two new records, and additions to two previously described species. Senckenb Marit 30:161–221
Schönberg CH (2002) Substrate effects on the bioeroding demosponge Cliona orientalis. 1. Bioerosion rates. Mar Ecol (Berl) 23:313–326. doi:10.1046/j.1439-0485.2002.02811.x
Schönberg CHL (2008) A history of sponge erosion: from past myths and hypotheses to recent approaches. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Heidelberg, pp 165–202
Schönberg CH, de Beer D, Lawton A (2005) Oxygen microsensor studies on zooxanthellate clionaid sponges from the Costa Brava, Mediterranean Sea. J Phycol 41:774–779. doi:10.1111/j.0022-3646.2005.04226.x
Schroeder JH (1972) Calcified filaments of an endolithic alga in recent Bermuda reefs. N Jahrb Geol PaläontMh 1:16–33
Scoffin TP, Bradshaw C (2000) The taphonomic significance of endoliths in dead- versus live-coral skeletons. Palaios 15:248–254
Shinn EA (1969) Submarine lithification of Holocene carbonate sediments in the Persian Gulf. Sedimentology 12:109–144. doi:10.1111/j.1365-3091.1969.tb00166.x
Shirai K, Kusakabe M, Nakai S, Ishii T, Watanabe T, Hiyagon H, Sano Y (2005) Deep-sea coral geochemistry: implication for the vital effect. Chem Geol 224:212–222. doi:10.1016/j.chemgeo.2005.08.009
Sinclair DJ (2005) Correlated trace element “vital effects” in tropical corals: a new geochemical tool for probing biomineralization. Geochim Cosmochim Acta 69:3265–3284. doi:10.1016/j.gca.2005.02.030
Sinclair DJ, Kinsley LPJ, McCulloch MT (1998) High resolution analysis of trace elements in corals by laser ablation ICP-MS. Geochim Cosmochim Acta 62:1889–1901. doi:10.1016/S0016-7037(98)00112-4
Sinclair DJ, McCulloch MT (2004) Corals record low mobile barium concentrations in the Burdekin River during the 1974 flood: evidence for limited Ba supply to rivers? Palaeogeogr Palaeoclimatol Palaeoecol 214:155–174
Sinclair DJ, Risk MJ (2006) A numerical model of trace-element coprecipitation in a physicochemical calcification system: application to coral biomineralization and trace element ‘vital effects’. Geochim Cosmochim Acta 70:3855–3868. doi:10.1016/j.gca.2006.05.019
Smith PL, De Long RC (1978) Brucite in modern corals, Abs with programs. Geol Soc Am 10(7):494
Sorauf JE (1972) Skeletal microstructure and microarchitecture in Scleractinia (Coelenterata). Palaeontology 15:88–107
Sorauf JE, Cuif J-P (2001) Biomineralization and diagenesis in the Scleractinia: part 2, diagenesis. Bull Tohoku Univ Mus 1:152–163
Stolarski J, Mazur M (2005) Nanostructure of biogenic versus abiogenic calcium carbonate crystals. Acta Palaeontol Pol 50:847–865
Supko PR (1971) “Whisker” crystal cement in a Bahamian rock. In: Bricker OP (ed) Carbonate cements. Studies in Geology, vol 19. John Hopkins University, Baltimore, pp 143–146
Vénec-Peyré M-T, Boyer H (1978) Application de la microsonde moléculaire à laser MOLE à l’étude de test de quelques foraminiferes calcaires. CR Acad Sci D 287:607
Verrecchia EP, Loisy C, Braissant O, Gorbushina AA (2003) The role of fungal biofilms and networks in the terrestrial calcium carbonate cycle. In: Krumbein WE, Paterson DM, Zavarzin GA (eds) Fossil and recent biofilms—a natural history of life on Earth. Kluwer, Dordrecht, pp 363–369
Verrecchia EP, Verrecchia KE (1994) Needles-fiber calcite: a critical review and a proposed classification. J Sediment Res A64:650–664
Vieira MM, De Ros LF (2006) Cementation patterns and genetic implications of Holocene beachrocks from northern Brazil. Sediment Geol 192:207–230. doi:10.1016/j.sedgeo.2006.04.011
Vogel K, Gektidis M, Golubic S, Kiene WE, Radtke G (2000) Experimental studies on microbial bioerosion at Lee Stocking Island, Bahamas and One Tree Island, Great Barrier Reef, Australia: implications for paleoecological reconstructions. Lethaia 33:190–204. doi:10.1080/00241160025100053
Ward WC (1975) Petrology and diagenesis of carbonate eolianites of NE Yucatan Peninsula, Mexico. In: Belize Shelf carbonate sediment and ecology. AAPG Stud Geol 2:500–571
Ward P, Risk MJ (1977) Boring pattern of the sponge Cliona vermifera in the coral Montastrea annularis. J Paleontol 51:520–526
Webb GE (2001) Biologically induced carbonate precipitation in reefs through time. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems. Kluwer Academic/Plenum Publishers, New York, pp 159–203
Webb GE, Jell JS, Baker JC (1999) Cryptic intertidal microbialites in beachrock, Heron Island, Great Barrier Reef: Implications for the origin of microcrystalline beachrock cement. Sediment Geol 126:317–334. doi:10.1016/S0037-0738(99)00047-0
Webb GE, Baker JC, Jell JS (1998) Inferred syngenetic textural evolution in Holocene cryptic reefal microbialites, Heron Reef, Great Barrier Reef, Australia. Geology 26:355–358. doi:10.1130/0091-7613(1998)026<0355:ISTEIH>2.3.CO;2
Weiss CP, Wilkinson BH (1988) Holocene cementaion along the central Texas coast. J Sediment Petrol 58:468–478
Whittle GL, Kendall CG, Dill RF, Rouch L (1993) Carbonate cement fabrics displayed: a traverse across the margin of the Bahamas Platform near Lee Stocking Island in Exuma Cays. Mar Geol 110:213–243. doi:10.1016/0025-3227(93)90086-B
Wyndham T, McCulloch M, Fallon S, Alibert C (2004) High-resolution coral records of rare earth elements in coastal seawater: biogeochemical cycling and a new environmental proxy. Geochim Cosmochim Acta 68:2067–2080. doi:10.1016/j.gca.2003.11.004
Yu K-E, Zhao J-X, Wei G-J, Cheng X-R, Chen T-E, Felis T, Wang P-X, Lui T-S (2005) δ18O, Sr/Ca and Mg/Ca records of Porites lutea corals from Leizhou Peninsula, northern South China Sea, and their applicability as paleoclimatic indicators. Palaeogeogr Palaeoclimatol Palaeoecol 218:57–73. doi:10.1016/j.palaeo.2004.12.003
Zundelevich A, Lazar B, Ilan M (2007) Chemical versus mechanical bioerosion of coral reefs by boring sponges–lessons from Pione cf. vastifica. J Exp Biol 210:91–96. doi:10.1242/jeb.02627
Acknowledgments
The authors wish to thank the staff of the Analytical Electron Microscopy Facility at the Queensland University of Technology (QUT) where all the scanning electron microscopy was carried out. We also thank J. T. Kloprogge and M. Hales from QUT for their assistance with Raman spectroscopy analyses and J. Pandolfi of the University of Queensland for initial aid in identifying coral species. We are grateful to reviewers J. Stolarski and M. Reuter and Editor A. Freiwald for comments that improved the manuscript. The research was funded primarily by QUT and by a QUT Australian Tertiary Network Small Grant to Webb and an Australian Postgraduate Award to Nothdurft. Corals were collected under Marine Parks Permit G03/9787.1 from Great Barrier Reef Marine Parks Authority.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nothdurft, L.D., Webb, G.E. Earliest diagenesis in scleractinian coral skeletons: implications for palaeoclimate-sensitive geochemical archives. Facies 55, 161–201 (2009). https://doi.org/10.1007/s10347-008-0167-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10347-008-0167-z