Abstract
Anthelmintic resistance (AR) and gastrointestinal (GI) parasitism are well-known phenomena in domestic ruminants. Wild ruminants, however, pose a potential threat for the spread of AR to sheep and goat farms. We infected three species of wild ruminants, European mouflon (Ovis musimon), roe deer (Capreolus capreolus) and fallow deer (Dama dama), with 8000 third-stage larvae (L3) of the susceptible MHco3 strain and the resistant MHco4 strain of Haemonchus contortus. Infection intensity was highest in mouflons (26,500 ± 150.00 eggs per gramme (EPG) of faeces) on day (D) 58. The roe and fallow deer had low egg counts (50 ± 23.57 to 150 ± 80.64 EPG) to D58, after which no eggs were detected. In vitro egg hatch tests (EHTs) and larval development tests (LDTs) indicated the same level of benzimidazole resistance between mouflons and domestic sheep. Two domestic lambs were then infected with 2500 MHco4 L3 larvae and moved to a clean pasture. Two parasite-free roe deer were introduced to the same pasture 6 weeks later. Infection intensity in the two roe deer was highest on D35, at 800 and 4400 EPG. ED50 from the EHTs, LD50 from the LDTs, and the presence of H. contortus in faecal samples confirmed the transmission of the resistant strain from the wild to the domestic ruminants in the same pasture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Farming on pastures is still the most widely used method for the breeding of small ruminants. The frequent occurrence of wild and domestic ruminants together on common pastures raises the question about the role of wild species in the transmission of gastrointestinal (GI) nematodes between farms of small ruminants. The similar feeding habits of wild and domestic ruminants may represent a high potential risk for the transmission of GI nematodes from wild to domestic sheep and goats. Roe deer (Capreolus capreolus) occur from lowland pastures to montane forests. The European mouflon (Ovis musimon) usually selects areas with open woods and broad pastures at altitudes between 1000 and 1500 m a.s.l. (Pfeffer 1967). Fallow deer (Dama dama) prefer old deciduous broadleaf forests interspersed with grassy areas (Feldhamer et al. 1988). The habitats are well known for these species, but less information is available about the composition of parasitic fauna and the status of anthelmintic resistance (AR) of these animals. Pato et al. (2013) identified 20 species of GI nematodes in 218 roe deer in northwestern Iberian Peninsula, including species common in small domestic ruminants such as Haemonchus contortus, Trichostrongylus colubriformis, T. capricola, T. vitrinus and Teladorsagia circumcincta. A survey of the parasitic fauna of 92 roe deer in nine regions of the Ukraine found a high prevalence of H. contortus (57.6%) (Kuzmina et al. 2010). H. contortus was also found in > 50% of the abomasa of 15 roe deer in the central region of Turkey near the Black Sea (Bolukbas et al. 2012). Cerutti et al. (2010) confirmed the low host specificity of H. contortus by finding it in common populations of roe deer, chamois, alpine ibex, and domestic goats and sheep in various alpine areas.
Anthelmintic treatment is performed in feeding stations as pellets coated with active drugs. Hunters are required to estimate the total number of animals and their total weights in hunting districts. The distribution of drugs in the feed may thus easily be inconsistent, so the drugs may be under-dosed. Under-dosing is one of the most common managerial flaws contributing to the development of AR (Torres-Acosta and Hoste 2008). If this approach is repeated too often, parasites with resistant alleles begin to appear on pastures. European surveys of the occurrence of AR on small ruminant farms have confirmed both low and high levels of AR (Domke et al. 2012; Gallidis et al. 2012; Rose et al. 2015; Babják et al. 2018). Higher levels of AR have been reported for farms that share common pastures with wild ruminants (Babják et al. 2018). This finding raised the question of the role of wild ruminants in the transmission of resistant parasites to small domestic ruminants. Wild ruminants are usually infected with a wide range of GI nematodes. The cross-transmission of H. contortus under experimental conditions between white tailed deer, cattle and sheep was described three decades ago (McGhee et al. 1981). A few studies have since monitored the course of parasitic infection in wild ruminants and the cross-transmission between wild and domestic ruminants under field conditions (Santin-Duran et al. 2004; Pato et al. 2009; Chintoan-Uta et al. 2014). All these studies hypothesised that wild deer could serve as cross-transmission vectors and reservoirs of GI nematodes.
We selected H. contortus as a model parasite for this study because it is the most pathogenic nematode of small ruminants causing financial and production losses and represents the largest AR problem worldwide. Our study was divided into two phases. The first phase monitored the course of an experimental parasitic infection in three species of wild ruminants (roe deer, fallow deer and European mouflon) with resistant and susceptible strains of H. contortus. The main goal of the second phase was to confirm the possible transmission of H. contortus between sheep and roe deer under field conditions. Confirmation of the transmission of resistant parasites under field conditions is important, because wild ruminants pose a potential threat for the spread of AR parasites between small ruminant farms.
Materials and methods
Phase 1
Experimental design
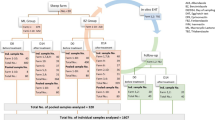
Three species of wild ruminants were selected for phase 1: roe deer, fallow deer and European mouflon. All animals were approximately 2 years old. The study was conducted at the Facility for Breeding and Diseases of Game, Fish and Bees of the University of Veterinary Medicine and Pharmacy in Košice, Slovakia. Three animals from each species were infected with 8000 third-stage larvae (L3) of a resistant H. contortus strain (MHco4), and three animals from each species were infected with 8000 L3 larvae of a susceptible strain (MHco3). Six lambs were similarly infected with the susceptible and resistant strains for comparing the courses of infection between the domestic and wild ruminants. Faecal samples were collected rectally on day (D) 0, D16, D37, D58 and D77 after infection.
Faecal egg counts
The numbers of eggs per gramme (EPG) of faeces were quantified using a modified McMaster technique (Coles et al. 1992). Three grammes of faeces from each animal were mixed with 42 ml of water and passed through a sieve. The filtrate was centrifuged at 605g for 2 min. The sediment was mixed with a saturated sugar solution and centrifuged under the same conditions. One millilitre of the mixture was then transferred to McMaster chambers. Egg counts with sensitivities > 50 EPG were determined for each sampling day.
In vitro egg hatch tests
Egg hatch tests (EHTs) were performed as described by Coles et al. (2006). Eggs isolated from the faecal samples were incubated in concentrations of thiabendazole (TBZ) ranging from 0.05 to1.00 μg/ml. The number of hatched eggs was counted after 48 h of incubation at 27 °C. ED50 (the concentration of TBZ required to kill 50% of the eggs) was determined for each species using a statistical logistic regression model (Dobson et al. 1987). The test was performed three times with two replicates for each drug concentration and ED50 values were calculated.
In vitro larval development tests
Larval development tests (LDTs) are based on the ability of larvae from resistant strains to survive and develop in various concentrations of an anthelmintic (Várady et al. 1996). Isolated eggs were cultivated at 27 °C for a week in 96-well microtitre plates with increasing concentrations of TBZ, ranging from 0.0006 to 1.28 μg/ml. The numbers of eggs and first-stage larvae (L1), second-stage larvae (L2) and L3 larvae were counted in each well. LD50 (the concentration of TBZ where development to the L3 stage is inhibited by 50%) was determined for each species.
Phase 2
Experimental design
Two lambs were infected with 2500 L3 larvae of the resistant H. contortus strain (MHco4) at the beginning of phase 2. A pasture was selected in cooperation with the Research Institute for Animal Production where animals had never grazed. Infected lambs were moved to this clean pasture, and egg counts were determined 28 days after infection. Two parasite-free roe deer were subsequently introduced to the pasture 6 weeks later. Faecal samples were collected on D21, D28, D35, D42 and D49.
Faecal egg counts and in vitro EHTs and LDTs
Egg counts and in vitro EHTs and LDTs were performed in phase 1 and phase 2. LD50 for the LDTs was evaluated for TBZ and ivermectin (IVM). Tests were performed on 96-well microtitre plates and the final TBZ concentrations ranged from 0.0006 to 1.28 μg/ml. A stock solution of ivermectin aglycone was serially diluted with DMSO 1:2 to produce 12 final concentrations ranging from 0.084 to 173.6 μg/ml (Dolinská et al. 2012).
Morphological identification of L3 larvae
Faecal samples from both parasite-free roe deer were used to prepare coprocultures. L3 larvae harvested after 1 week of incubation at 27 °C were identified by morphological features described by Van Wyk and Mayhew (2013).
Results
Phase 1
Faecal egg counts
Mean egg counts for all groups of wild ruminants are presented in Table 1. The fallow deer infected with MHco4 and the roe deer infected with MHco3 were the first animals with acquired infections, both with a mean of 100 EPG on D16. Infection was observed in all experimental animals on D37, with the highest egg count of 13,250 EPG in the mouflons infected with MHco4. Egg counts for the roe and fallow deer varied between 50 and 150 EPG in all groups. Helminth eggs were not observed the in roe and fallow deer samples after D58. Egg counts for the mouflons ranged between 13,250 and 26,500 EPG in the group infected with MHco4 and from 2600 to 3800 EPG in the MHco3 group (Table 1). Egg counts for the simultaneously infected lambs were highest on D58 after infection, at 28000 EPG for the MHco4 group and 4000 EPG for the MHco3 group. Infection in the mouflons and lambs persisted throughout the study.
In vitro EHT and LDT
The in vitro tests were evaluated using an EHT ED50 threshold of 0.1 μg/ml and an LDT LD50 threshold of 0.02 μg/ml (Coles et al. 2006). The in vitro tests could not be performed for the roe and fallow deer due to low egg counts. A comparison of the results for the mouflon and sheep groups indicated the same level of benzimidazole (BZ) resistance in both species. ED50 in the susceptible groups of both species varied from 0.043 ± 0.005 to 0.064 ± 0.008 μg/ml TBZ, which correlated with the LDT levels (LD50 0.007 ± 0.002 to 0.009 ± 0.001 μg /ml TBZ). ED50 in the resistant groups ranged from 0.091 ± 0.001 to 0.111 ± 0.004 μg/ml TBZ), and LD50 ranged from 0.024 ± 0.003 to 0.035 ± 0.002 μg/ml TBZ (Table 2).
Phase 2
Faecal egg counts
The presence of parasitic eggs in the lambs was confirmed on D28 after infection, with a mean of 5000 EPG. Infection in the roe deer was observed on D21 after their introduction to the clean pasture, with 150 EPG for roe deer no. 1 and 450 EPG for roe deer no. 2. Egg counts were highest for roe deer nos. 1 and 2 on D35, at 800 and 4400 EPG, respectively. Infection persisted in both animals throughout phase 2. The egg counts from all sampling days are presented in Table 3.
In vitro EHT and LDT
EHT ED50 ranged between 0.111 ± 0.004 and 0.115 ± 0.008 μg/ml for TBZ. LDT LD50 also confirmed the transmission of the resistant strain from sheep to roe deer, with values from 0.021 ± 0.002 to 0.031 ± 0.001 μg/ml for TBZ and from 39.450 ± 2.510 to 46.670 ± 5.508 ng/ml for IVM aglycone (Table 4).
Morphological identification of L3 larvae
A total of 100 harvested L3 larvae were identified following Van Wyk and Mayhew (2013). Morphological identification also confirmed the presence of only H. contortus in all faecal samples from the roe deer in phase 2.
Discussion
Many species of GI parasites are common to domestic and wild ruminants, including some of the most pathogenic species with the highest incidences of AR. We thus need more information about the transmission of parasitic infections between domestic and wild animals. Transmission of nematodes between hosts is a complex process influenced by several factors. Studying the possibility of host switching is necessary to obtain information about the historical contexts, relationships, and patterns of contact between host species (Walker and Morgan 2014). The course of infection in the first phase of our study was similar between the mouflons and sheep, and the in vitro tests confirmed similar levels of BZ resistance, which was expected because the mouflon is considered to be the ancestor of domestic sheep. Zaffaroni et al. (2000) compared the contents of 641 abomasa from five species of wild ruminants (O. musimon, C. capreolus, Capra ibex, Rupicapra rupicapra and Cervus elaphus) to those of 19 domestic sheep. The five wild hosts were infected with nine species of helminths common in domestic sheep, and a discriminant analysis indicated that species composition differed significantly between the species except between the mouflon and domestic sheep.
Our results confirmed that mouflons can easily obtain persistent infections with the susceptible and resistant H. contortus strains and that mouflons can serve as hosts under field conditions. Most populations of free-ranging European mouflons have the potential to transfer parasitic infections to small domestic ruminants on unfenced pastures. GI nematodes can be divided into a specialist group (parasites specific for each host species) and a generalist group where host specificity is lower (Bush and Holmes 1986). Zaffaroni et al. (2000) reported that the generalist group contains species such as H. contortus, Trichostrongylus axei, T. capricola, Teladorsagia circumcincta and many other GI nematodes common on small ruminant farms, which is important from agricultural and veterinary points of view, because these species are most frequently associated with the occurrence of AR. Chintoan-Uta et al. (2014) identified alleles for BZ resistance in H. contortus in wild roe deer that had never been treated. Our study (phase 2) clearly demonstrated the transmission of resistant H. contortus parasites between domestic and wild animals in a shared pasture. The roe deer infection in phase 2 persisted throughout the study; in contrast, the egg counts for the roe and fallow deer infections acquired in phase 1 were low, and no eggs were found after D58.
The transmission of nematodes between two generalist hosts is thus not always guaranteed. Factors such as climate and anthropogenic changes in land use can provide the conditions needed for nematode host switching (Altizer et al. 2013). The possibility of transmission also depends on the size of host herds and the level of contact between hosts on shared pastures (Morgan et al. 2004). The loss of infection in the roe and fallow deer may have been due to the phenomenon of self-cure, which usually occurs in domestic and wild ruminants. High stocking rates lead to a higher susceptibility to GI infections (Maublanc et al. 2009; Pato et al. 2013). Roe deer can also change their habitat selection (Morellet et al. 2011), and these changes can lead to the modification of feeding habits (Serrano Ferron 2012). The transformation of roe deer from browser to grazer increases the risk of GI infection, mainly when they share pastures with domestic ruminants.
We selected roe deer as a model species for the second phase of our study for several reasons. Roe deer can occupy a wider variety of habitats and altitudes than mouflon, from groups on isolated farms to free-ranging flocks in mountains and rocky foothills. Ferté et al. (2000) summarised and compared the prevalence of H. contortus in three species of wild ruminants (red, roe and fallow deer) from surveys carried out across Europe. Thirty-six surveys in 17 European countries recorded the incidence of H. contortus in roe deer, with prevalences ranging from 0.3 to 85% (63% in Slovakia) (Farkas 1989). For comparison, H. contortus was found in red deer in nine countries, with prevalences between 5 and 25%, and in fallow deer in four countries (4–7%). Chintoan-Uta et al. (2014) compared abomasal and faecal samples from three species of wild ruminants from areas of extensive or intensive livestock farming in the United Kingdom. Roe deer had the highest parasite burdens and diversity of abomasal nematode species. Nematodes associated with livestock, such as Ostertagia ostertagi, T. axei, T. colubriformis and H. contortus were also found. The susceptibility of roe deer to naturally acquired infection with H. contortus was also confirmed in our study.
These findings indicate that roe deer potentially represent the main threat to the spread of AR parasites between small ruminant farms. Detecting the level of BZ/IVM resistance in free-ranging roe deer in the vicinity of farms would be necessary to determine the actual extent of this potential threat to small ruminants. Determining GI nematode burdens and identifying species diversity in samples collected from different species of wild ruminants would also be needed. Determination of the efficacy of drugs after treatment should be amongst the prophylactic measures used in every hunting district. If the occurrence of AR in wild ruminants is not confirmed, however, these animals could serve as refugia for susceptible populations of GI nematodes, which would extend the efficacy of anthelmintic drugs.
In conclusion, knowledge about the AR status of wild ruminants is important from therapeutic and prophylactic points of view. The issue of the transmission of GI nematodes is not a unilateral problem, because domestic species can also pose a threat to wild ruminants. Such information can form an important part of measures for the successful management of small ruminant farms, hunting areas and areas with farmed wild ruminants.
References
Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD (2013) Climate change and infectious diseases: from evidence to a predictive framework. Science 341:514–519. https://doi.org/10.1126/science.1239401
Babják M, Königová A, Urda Dolinská M, Vadlejch J, Várady M (2018) Anthelmintic resistance in goat herds—in vivo versus in vitro detection methods. Vet Parasitol 254:10–14. https://doi.org/10.1016/j.vetpar.2018.02.036
Bolukbas CS, Gurler T, Beyhan EY, Acici M, Umur S (2012) Helmints of roe deer (Capreolus capreolus) in middle Black Sea region of Turkey. Parasitol Int 61:729–730. https://doi.org/10.1016/j.parint.2012.06.008
Bush AO, Holmes JC (1986) Intestinal helminths of lesser scaup ducks: an interactive community. Canad J Zool 64:142–152. https://doi.org/10.1139/z86-022
Cerutti MC, Citterio CV, Bazzocchi C, Epis S, D’Amelio S, Ferrari N, Lanfranchi P (2010) Genetic variability of Haemonchus contortus (Nematoda: Trichostrongyloidea) in alpine ruminant host species. J Helminthol 84:276–283. https://doi.org/10.1017/S0022149X09990587
Chintoan-Uta C, Morgan E, Skuce PJ, Coles GC (2014) Wild deer as potential vectors of anthelmintic-resistant abomasal nematodes between cattle and sheep farms. Proc R Soc B 281:20132985. https://doi.org/10.1098/rspb.2013.2985
Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ (1992) World association for the advancement of veterinary parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 44:35–44. https://doi.org/10.1016/0304-4017(92)90141-U
Coles GC, Jackson F, Pomroy WE, Prichard RK, Von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J (2006) The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 136:167–185. https://doi.org/10.1016/j.vetpar.2005.11.019
Dobson RJ, Griffiths DA, Donald AD, Waller PJ (1987) A genetic model describing the evolution of levamisole resistance in Trichostrongylus colubriformis, a nematode parasite of sheep. J Math Appl Med Biol 4:279–293. https://doi.org/10.1093/imammb/4.4.279
Dolinská M, Königová A, Várady M (2012) Is the micro-agar larval development test reliable enough to detect ivermectin resistance? Parasitol Res 111:2201–2204. https://doi.org/10.1007/s00436-012-2944-4
Domke AVM, Chartier C, Gjerde B, Höglund J, Leine N, Vatn S, Stuen S (2012) Prevalence of anthelmintic resistance in gastrointestinal nematodes of sheep and goats in Norway. Parasitol Res 111:185–193. https://doi.org/10.1007/s00436-012-2817-x
Farkas J (1989) Invasion cycle of the most frequent pneumo- and gastrointestinal helminthiases of roe deer and red deer in Central Slovakia. Lesnictvi 35:51–56
Feldhamer GA, Farris-Renner KC, Barker CM (1988) Dama dama. Mamm Species 317:1–8. https://doi.org/10.2307/3504141
Ferté H, Cléva D, Depaquit J, Gobert S, Léger N (2000) Status and origin of Haemonchinae (Nematoda: Trichostrongylidae) in deer: a survey conducted in France from 1985 to 1998. Parasitol Res 86:582–587. https://doi.org/10.1007/PL00008534
Gallidis E, Angelopolou K, Papadopulos E (2012) First identification of benzimidazole resistant Haemonchus contortus in sheep in Greece. Small Rumin Res 106:27–29. https://doi.org/10.1016/j.smallrumres.2012.04.016
Kuzmina T, Kharchenko VA, Malega AM (2010) Helminth fauna of roe deer (Capreolus Capreolus) in Ukraine: biodiversity and parasite community. Vestn Zool 44:15–22. https://doi.org/10.2478/v10058-010-0002-1
Maublanc M, Bideau E, Picot D, Rames J, Dubois M, Ferte H, Gerard J (2009) Demographic crash associated with high parasite load in an experimental roe deer (Capreolus capreolus) population. Eur J Wildl Res 55:621–625. https://doi.org/10.1007/s10344-009-0298-8
McGhee MB, Nettles VF, Rollor EA, Prestwood AK, Davidson WR (1981) Studies on cross-transmission and pathogenicity of Haemonchus contortus in white-tailed deer, domestic cattle and sheep. J Wildl Dis 17:353–364. https://doi.org/10.7589/0090-3558-17.3.353
Morellet N, Van Moorter B, Cargnelutti B, Angibault J-M, Lourtet B, Merlet J, Ladet S, Hewison AJM (2011) Landscape composition influences roe deer habitat selection at both home range and landscape scales. Landsc Ecol 26:999–1010. https://doi.org/10.1007/s10980-011-9624-0
Morgan ER, Milner-Gulland EJ, Torgerson PR, Medley GF (2004) Ruminating on complexity: macroparasites of wildlife and livestock. Trends Ecol Evol 19:181–188. https://doi.org/10.1016/j.tree.2004.01.011
Pato FJ, Vázquez L, Painceira A, Díaz P, Uriarte J, Díez Baños N, Dacal V, López C, Panadero R, Díez Baños P, Morrondo P (2009) Gastrointestinal nematode species shared by roe deer (Capreolus capreolus) and grazing cattle from Galicia. Asoc Interprof Desarro Agrar 1:176–178 http://agris.fao.org/agrissearch/search.do?recordID=ES2010001088
Pato FJ, Vázquez L, Díez-Baños N, López C, Sánchez-Andrade R, Fernández G, Díez-Baños P, Panadero R, Díaz P, Morrondo P (2013) Gastrointestinal nematode infections in roe deer (Capreolus capreolus) from the NW of the Iberian Peninsula: assessment of some risk factors. Vet Parasitol 196:136–142. https://doi.org/10.1016/j.vetpar.2013.01.027
Pfeffer P (1967) The Corsican mouflon (Ovis ammon musimon Schreber, 1782); systematic position and comparative ecology and ethology. (Le mouflon de Corse (Ovis ammon musimon Schreber, 1782); position systematique, ecologie et ethologie comparees.). Mammalia 31(Suppl):1–262
Rose H, Rinaldi L, Bosco A, Mavrot F, De Waal T, Skuce P, Charlier J, Torgerson PR, Hertzberg H, Hendrickx G, Vercruysse J, Morgan ER (2015) Widespread anthelmintic resistance in European farmed ruminants: a systematic review. Vet Rec 176:546. https://doi.org/10.1136/vr.102982
Santin-Duran M, Alunda JM, Hoberg EP (2004) Abomasal parasites in wild sympatric cervids, red deer (Cervus elaphus) and fallow deer (Dama dama) from three localities across central and western Spain: relationship to host density and park management. J Parasitol 90:1378–1386. https://doi.org/10.1645/GE-3376
Serrano Ferron E (2012) Digestive plasticity as a response to woodland fragmentation in roe deer. Ecol Res 1:77–82. https://doi.org/10.1007/s11284-011-0872-x
Torres-Acosta JFJ, Hoste H (2008) Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Rum Res 77:159–173. https://doi.org/10.1016/j.smallrumres.2008.03.009
Van Wyk JA, Mayhew E (2013) Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: a practical lab guide. Onderstepoort J Vet Res 80:539. https://doi.org/10.4102/ojvr.v80i1.539
Várady M, Bjorn H, Nansen P (1996) In vitro characterization of anthelmintic susceptibility of field isolates of the pig nodular worm Oesophagostomum spp., susceptible or resistant to various anthelmintics. Int J Parasitol 26:733–740. https://doi.org/10.1016/0020-7519(96)00051-3
Walker JG, Morgan ER (2014) Generalists at the interface: nematode transmission between wild and domestic ungulates. Int J Parasitol Parasites Wildl 3:242–250. https://doi.org/10.1016/j.ijppaw.2014.08.001
Zaffaroni E, Manfredi MT, Citterio C, Sala M, Piccolo G, Lanfranchi P (2000) Host specificity of abomasal nematodes in free ranging alpine ruminants. Vet Parasitol 90:221–230. https://doi.org/10.1016/S0304-4017(00)00240-5
Acknowledgements
The authors thank S. Spišáková and M. Krčmárik for their technical assistance.
Funding
This study was supported by funds from the Scientific Grant Agency VEGA 2/0120/16, VEGA 2/0099/19 and Slovak Research and Development Agency APVV-18-0131.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the ethics committee on 20 April 2015, meet the requirements of the ethics Committee of the Institute of parasitology of the Slovak Academy of Sciences in accordance with the national legislation in Slovakia - animal welfare act no. 23/2009 and was approved on 1 January 2016.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laca Megyesi, Š., Königová, A., Babják, M. et al. Wild ruminants as a potential risk factor for transmission of drug resistance in the abomasal nematode Haemonchus contortus. Eur J Wildl Res 66, 9 (2020). https://doi.org/10.1007/s10344-019-1351-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-019-1351-x