Abstract
Transportation infrastructure is one of the mainstays of human modification of terrestrial landscapes. Turtle populations are highly affected by roads through direct mortality, contributing to population declines. However, sub-lethal effects, such as increased physiological stress, may indirectly affect turtle demographic rates, particularly in populations recently exposed to roads. We took advantage of a unique study system in southeast Ohio, where an intact forest was bisected by a four-lane highway in 2013, exposing eastern box turtles (Terrapene carolina carolina) to a new threat. The goal of this study was to evaluate ecological, physiological, and behavioral effects of exposure to a new road by comparing a roadside turtle population to a control population in a nearby roadless area, and guide mitigation on new and existing roadways. We used a unique combination of radio telemetry to assess space use, behavior, and habitat selection of turtle, and bioassay techniques to analyze chronic stress using corticosterone stored in nail keratin. We found no differences in home range sizes and habitat selection between the two sites, but roadside turtles showed strong highway avoidance, despite spending a significant amount of time in its immediate vicinity. All turtles selected for higher woody debris and understory vegetation cover, and males at both sites selected for higher canopy cover. Corticosterone concentrations from nails collected upon initial capture (2017) did not differ between the two sites, but males showed a wider range of variation. Corticosterone concentrations were significantly higher in 2018, with roadside animals showing the highest levels, but they were not correlated with home range size or proximity to highway. As such, further work is needed to evaluate indirect effects of multiple stressors on turtle endocrinology and their demographic implications, as well as the level of demographic compensation resulting from road avoidance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human development has historically created significant encroachment on wildlife populations. Roads are one of the most ubiquitous forms of human impacts on wildlife and the effects of roads influence much of the conterminous USA (Forman 2000). The effects of roads on wildlife include habitat loss, when former habitat is converted to asphalt and artificial edge, and habitat fragmentation, which poses challenges for organisms needing to access habitats or conspecifics in previously intact areas (Coffin 2007; Iglay et al. 2007). Additionally, chemicals, runoff, and the effects of construction alter and degrade wildlife habitat (Collins and Russell 2009). Further, animal populations divided by roads face long-term vulnerability due to genetic barriers and decreased population size, in addition to being prone to direct mortality from traffic (Steen and Gibbs 2004; Shepard et al. 2008; Benítez-López et al. 2010). As road building and improvement continues largely unabated, it is vital to understand animal behavior, ecology, and physiology in relation to roadways to provide mitigation strategies that reduce the effects of roads on wildlife (Coffin 2007).
Deleterious effects of roads, including habitat alteration and related mortality, have been documented for both terrestrial and freshwater turtle populations (Wood and Herlands 1997; Trombulak and Frissell 2000; Crawford et al. 2014). Turtles are among the vertebrate taxa most affected by road mortality because of their low vagility and attraction to roads and right of ways for nesting and basking (Steen and Gibbs 2004; Aresco 2005). These behaviors have been found to bias mortality towards females, potentially skewing populations and increasing population-level effects of exposure to roads (Gibbs and Steen 2005). Road mortality has also been identified as a primary threat to the persistence of eastern box turtles (Terrapene carolina carolina) in Ohio, USA, and throughout their North American range (Forman and Alexander 1998, ODNR 2016, IUCN 2017). Some studies have suggested that eastern box turtles may demonstrate road avoidance, but it is unclear whether this avoidance is a behavioral response that developed over generations, or a rapid adaptation to new conditions (Shepard et al. 2008).
Research on the road ecology of herpetofauna is often performed in landscapes where the road network has been present for many generations, even for long-lived turtles (Marsack and Swanson 2009). As such, little is known about the direct and indirect effects of roads on naïve animal populations, exposed to new roads for short time periods (e.g., < 1 generation). Working with a road-naïve population provides a unique opportunity to assess the immediate effects of proximity to roads on turtles. In addition to effects on space use and direct mortality from traffic, one of the potential effects of proximity to roads is frequent acute stress events or chronically high baseline levels of stress, which could alter behavior and decrease individual fitness (Browne and Hecnar 2007; Baxter-Gilbert et al. 2014). Although quantifying chronic stress is challenging due to the multiple factors that comprise stress responses, corticosterone (CORT) concentrations are often employed as a standard biomarker of physiological stress in ecological studies (Angelier et al. 2010; Baxter-Gilbert et al. 2014). Corticosterone is a glucocorticoid released as part of the response of the hypothalamic-pituitary-adrenal (HPA) axis to stress in turtles. While it is far from being the only physiological response, it is a useful indicator of the amount of stress an animal is challenged with in the environment (Angelier et al. 2010; Baxter-Gilbert et al. 2014).
We aimed to elucidate the effects of new roads on the ecology and behavior of terrestrial turtles by measuring behavioral and physiological responses to a recently built highway in southeastern Ohio. This unique context presents an ideal opportunity to assess the immediate effects of a new highway on the space use and behavior of a population recently exposed to high-traffic roads and evaluate the potential effects of new road construction on terrestrial herpetofauna. We used the eastern box turtle as our study species, as it is a terrestrial turtle species inhabiting much of the eastern USA and is vulnerable throughout its range mainly due to road mortality (ODNR 2016; IUCN 2017). We took a two-pronged approach, combining traditional habitat selection studies via VHF telemetry with recently developed hormone bioassays of baseline CORT and long-term responses to stress from nail keratin (Baxter-Gilbert et al. 2014; Romero and Fairhurst 2016). The unique combination of these methods in the research of terrestrial testudines in a road ecology context is aimed to provide a better understanding of animal-road interactions by inferring potential, subtle, non-lethal effects of exposure to roads. Importantly, we compared a population at the roadside site to a nearby control population in a similar habitat, but completely lacking roads. This control-impact design allowed us to evaluate the magnitude of the direct and indirect road effects of stressors on a road-naïve turtle population.
The goal of this study was to evaluate the effects of a new road on eastern box turtle space use and long-term stress levels compared to those of a population unexposed to roads. Our specific objectives were (1) to quantify differences in home range sizes of turtle populations at roadside and roadless sites, (2) to evaluate differences in habitat selection between the two populations, and (3) to quantify differences in potential long-term stress responses via accumulated CORT concentrations between the two populations. We expected turtles at the roadside site to have larger home ranges, as they would need to seek out resources made unattainable by the highway, and that their home ranges would be bounded by the highway (if animals show road-avoidance behavior; Shepard et al. 2008). In terms of habitat selection, we predicted that roadside animals would select different types of habitat than would turtles from the roadless site, as edge habitat opened by the development of the highway could present appealing, if risk-laden opportunities for thermoregulation and nesting (Compton et al. 2002; Steen et al. 2006). Lastly, we predicted that proximity to roads would result in higher levels of long-term stress in turtles at the roadside site, as indicated by higher CORT levels (i.e., overall HPA activity; Baxter-Gilbert et al. 2014), and that higher CORT levels would be associated with proximity to roadways. Overall, this work is aimed at predicting turtle behavior relative to highways in newly road-exposed populations, identifying sub-lethal effects of roads in long-lived herpetofauna, and informing conservation strategies and policy decisions related to wildlife road ecology and mitigation efforts.
Methods
Study sites and species
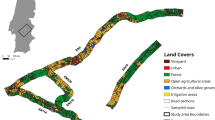
We conducted our study in the Athens Unit of Wayne National Forest in Athens and Hocking Counties in southeastern Ohio at two sites approximately six kilometers apart: a roadside (impact) site (Fig. 1; 39.47654N latitude, − 82.27231W longitude) and a control (control) site devoid of paved roads and closed to off-highway vehicles (39.44445N latitude, − 82.23763W longitude). In 2013, a previously intact, forested portion of Wayne National Forest was bisected by a newly completed 14.5-km section of US Highway 33, a high-speed, high-traffic, four-lane highway. A variety of wildlife mitigation structures was built, including wildlife fencing with jump-outs, snake fencing, large mammal underpasses, and small animal underpasses. The wildlife fence was ~ 3 m tall and made of large wire mesh that allowed turtles to move freely through it. Snake fencing was made of 0.6 m tall wire mesh and overlapped with our roadside study site for a short portion (~ 200 m); this fence was initially buried in the ground, but at the time of our survey, it had numerous gaps resulting from soil erosion or rusting. Neither the large wildlife nor the snake fence was located near the pavement, thus did not impede the turtles from reaching the highway. Some reptile species inhabiting this system, such as the endangered timber rattlesnake (Crotalus horridus), have already been shown to be affected by the new road, including altered movement patterns, habitat selection, and mortality rates (The Ohio Department of Transportation Office of Statewide Planning & Research 2017).
Turtle capture, telemetry, and habitat sampling
We began capturing eastern box turtles in May 2017, with the assistance of detection dogs trained to find turtles in the field (Anderson et al. 2011; Kapfer et al. 2012). We selected 30 animals for the telemetry study, including eight adult males and seven adult females at each site. We recorded morphometrics including carapace length, plastron length, shell width, and dome height using a stainless-steel dial caliper and weight using a Pesola 1000 g (± 10 g) spring scale and determined sex and approximate age (by counting scute annuli). If turtles had more than 10 annuli, we considered them adults (Iglay et al. 2007).
Telemetry
We equipped each of the 30 study turtles with a small VHF transmitter (Advanced Telemetry Systems, Isanti MN) with a lifespan of 18 months (~ 12 g; Forsythe et al. 2004, Iglay et al. 2007). We attached transmitters with epoxy (PC Products, Allentown PA) to single healthy costal scutes near the front of turtle carapaces to minimize interference with mating and daily activities. We housed turtles in the lab overnight to allow epoxy to set; all animals were released at capture site the morning following processing.
We tracked the turtles at least weekly from May to November 2017 and March to July 2018 using a radio receiver and handheld antenna (Advanced Telemetry Systems, Isanti MN; Schubauer 1981, Iglay et al. 2007). This included tracking animals in 2017 until every turtle had buried itself beneath the substrate to overwinter, checking periodically over the winter on warm days to see if animals had emerged or moved, and tracking every day in the spring of 2018 until all turtles were above the soil. While turtles were overwintering, we pinpointed their underground locations within a meter above the surface.
Habitat sampling
We measured habitat characteristics and environmental variables that are ecologically relevant to eastern box turtles at turtle locations and paired random locations (Compton et al. 2002; Kapfer et al. 2012). We used a 1-m2 Daubenmire frame positioned with the turtle at the center to approximate percent ground cover and understory, a densiometer to estimate percent canopy cover, a handheld weather meter to measure temperature and relative humidity (KestrelMeters.com, Minneapolis MN), and a SM150T soil moisture meter to measure the water content of the soil with a 3% accuracy (Delta-T Devices Ltd., Cambridge, UK; Converse et al. 2003). Specifically, we measured volumetric water content for mineral soil, as soils in our study area are primarily clay-rich; under similar precipitation conditions, lower readings suggest lower clay concentrations, and thus a thicker organic layer. Following data collection for each turtle location, we selected a location 50 m away in a random direction (using a compass and random number generator running between 1 and 360), and repeated the data sampling process (Compton et al. 2002; Rossell et al. 2006). This distance is the maximum distance used by Compton et al. (2002), and well within the daily movement distance of box turtles, which is required for the habitat selection analysis of use versus availability using conditional (or paired) logistic regression (data collected at turtle locations represented habitat used) and the paired random locations were habitats available (availability during telemetry surveys).
Stress response bioassays
We evaluated free CORT (i.e., not bound to receptors) concentrations as a proxy for HPA activity presence of accumulated stress in turtles at each of the two sites using CORT bioassays of nail keratin. Corticosterone is one of the hormones released for short periods of time in response to acute stressors or may remain elevated for prolonged periods during exposure to chronic stressors. Therefore, concentrations of CORT in keratin may be interpreted as an integrated measure of HPA activity (i.e., both baseline and stress response CORT levels) for accumulation of stress levels over time (Bortolotti et al. 2008; Baxter-Gilbert et al. 2014). In addition to collecting keratin samples from the turtles tracked via VHF telemetry (N = 30), we opportunistically collected nails from 56 other turtles encountered during the 2017 telemetry surveys (33 at roadside site and 23 at roadless site). We clipped 4–11 toenails per turtle (depending on size and availability) from the hind and front feet of all animals. Nails were clipped below the quick and ranged in length from 0.5 to 4.9 mm (x̄ = 2.48 mm). The total weight of individual nail samples from each turtle ranged from 1.2 to 35.5 mg (x̄ = 15.23 mg). Toenails were clipped using scissor-style, stainless steel nail clippers. Clippers were cleaned with alcohol wipes between uses. Due to a small sample size and a high degree of variation, juveniles were not included in our models.
We collected another set of nail samples in May 2018 from each of our tracked turtles and one incidental recapture sampled the previous year (N = 31). We clipped 4–14 toenails per turtle (depending on size and availability) from the hind and front feet of all animals. Nails ranged in length from 0.9 to 4.9 mm (x̄ = 2.57 mm). The total weight of individual nail samples from each turtle ranged from 5.3 to 25 mg (x̄ = 14.32 mg). The purpose of resampling was to evaluate individual differences in chronic stress across 2 years, acknowledging that handling and tracking likely led to increase CORT keratin deposition.
Following nail clipping, we stored individual keratin samples in 16 × 100-mm vials labeled with individual identification numbers. Samples were stored at − 18 °C until transported to the Tonra Lab of Avian Ecology at The Ohio State University for bio-assaying. We measured the concentration of free CORT in toenail samples using a methanol-based extraction and enzyme immunoassay (EIA), as validated by Baxter-Gilbert et al. (2014), using a commercial CORT ELISA kit (product no. 402810; Neogen Corporation, Ayr, UK). Briefly, crushed samples were incubated in methanol overnight in an oscillating water bath, following which extracts were separated via pipette (including multiple rinses), evaporated under nitrogen gas, and then reconstituted in Neogen extraction buffer before running through kit assay procedures (Angelier et al. 2010; Baxter-Gilbert et al. 2014). Corticosterone concentration values are reported in pg/mg of toenail. Samples were run in four separate bioassays over 8 days (August–September 2017; June 2018). Assay recovery was assessed by adding 20 μL of tritium-labeled CORT in each sample, and mean recovery rate was 0.94. Intra-assay variation based on duplicate samples was 4.1%, and inter-assay variation (N = 4) based on kit standards was 6%.
Data analysis
Home ranges
We calculated 100% minimum convex polygons for individual home ranges for data pooled across the 2 years of study. We evaluated differences between male and female home ranges and between roadless and roadside sites using non-parametric Kruskal-Wallis tests. We also quantified closest proximity to roads for turtles at the roadside site.
Habitat selection
We evaluated habitat selection using conditional logistic regression analyses in program R (package survival; R Core Team 2013; Therneau 2015). Conditional logistic regression models allow for examining microhabitat selection based on used (turtle occurrence) versus available (random) habitat data collected the same time (Compton et al. 2002). As such, conditional logistic regression effectively estimates the probability of turtle occurrence based on the differences in habitat attributes between habitats used and available to the turtle at the same time (i.e., sampled within the turtle daily movement distances) (Compton et al. 2002). Thus, the magnitude of increase or decrease in the odds of the turtles occurring at a given location depends on the difference in measurements between the turtle point and the random point, not the absolute values. Because the estimated coefficients and odds ratios for conditional logistic regression models differ from those of logistic regression models, we analyzed four subsets of data separately: males and females at the roadside and roadless site. Lastly, comparisons of standardized odds ratios for each population subset overcame these issues and provided an adequate method to investigate potential differences in habitat selection between roadside and roadless sites, as well as between sexes. For each model set, we built eight competing models that incorporated combinations of environmental variables that are ecologically relevant to turtles, including ground cover, habitat structure, and weather conditions (Table 1; Kapfer et al. 2013). Variables that were highly correlated (Pearson r > 0.7 or < − 0.7) were removed from the analysis. We used a model selection procedure and identified the best-supported model using Akaike information criteria corrected for small sample size (AICc) in program R (Burnham and Anderson 2002; R Core Team 2013). We then calculated the estimated coefficients for the best-supported model for each subset of turtles and compared habitat selection between sites and sexes (Table 2).
HPA activity
We built and tested nine competing generalized linear models using site, sex, and physical covariates (carapace length, weight, age) as explanatory variables for the 2017 data (N = 86 individuals; Table 3). We employed a model selection procedure and identified the best-supported models using Akaike information criteria corrected for small sample size (AICc) in program R (Burnham and Anderson 2002; R Core Team 2013). We also compared CORT concentrations in N = 31 turtles (30 individuals tracked plus one incidental recapture) between Spring 2017 and Spring 2018 using a paired t test. Based on nail measurement before tracking and a year after initial capture, we estimate that they are indicative of a response to stress levels for the previous several (likely 4–6) months. Lastly, we evaluated whether the changes in CORT levels between 2017 and 2018 for turtles at the roadside site were correlated with space use during the previous year using Spearman’s rank correlations, under the hypothesis that larger home range sizes and turtle proximity to highway would be positively correlated with higher CORT levels.
Results
Home ranges
Based on a total of 1174 turtle locations collected between May–November 2017 and March–July 2018 (x̄Impact = 40.13 points per turtle, x̄Control = 38.13 points per turtle), females at the roadside site had the largest home ranges (100% MCP = 7 ± 2.18 ha), followed by females at the roadless site (5.81 ± 3.47 ha), and followed distantly by males at the roadside site (2.41 ± 0.63 ha) and males at the roadless site (1.82 ± 0.38 ha; Fig. 2). There were no differences in turtle home range sizes between roadside and roadless sites (Kruskal-Wallis χ21 = 1.931, p = 0.165). While females exhibited larger overall home ranges than males (Kruskal-Wallis χ21 = 6.643, p = 0.01), and females at the roadside site exhibited larger home ranges than males at the roadside site (Kruskal-Wallis χ21 = 6.482, p = 0.011), females and males at the roadless site demonstrated no differences in home range size (Kruskal-Wallis χ21 = 1.620, p = 0.203).
Box turtle locations (points) and home ranges as 100% minimum convex polygons. While animals at roadside and control sites demonstrated no significant differences in home range size (a), female turtles (light gray) exhibited larger overall home ranges than male turtles (dark gray) in both roadside (b) and control (c) sites. Background in light gray is forested, showing some of the habitat fragmentation in this portion of the forest
We observed road avoidance behavior for turtles at the roadside site. All seven females spent substantial time (> 6 weeks) within 100 m of the road (Fig. 2), with some found within a few meters from the paved surface, yet we did not record any tracked animals attempting to cross the highway during the two seasons.
Habitat selection
We collected habitat data at 867 turtle points and 867 paired random points between May 2017 and July 2018. The same combination of variables emerged as the best-supported model for three of the four site and sex subsets (Table 1). Based on the best-supported model for females at the roadside site, and both males and females at the roadless site, turtle occurrence was positively associated with % woody debris cover and % understory (Table 2; Fig. 3). Turtles at the roadside site were associated with a higher percentage of herbaceous vegetation (Table 2). For each 1% increase in woody debris between turtle location and random point, there was a 3.5–5.4% increase in the likelihood of turtle occurrence; for each 1% increase in understory, there was a 2.9–4.2% increase in the likelihood of turtle occurrence, depending on population subset (Fig. 3). In addition to these two variables, males at the roadside site also selected for higher leaf litter, herbaceous vegetation, and canopy cover, and showed a significant preference for cooler temperatures (Fig. 3). Males at the roadless site also showed significant selection for canopy cover (Fig. 3).
HPA activity
The two best-supported models explaining initial (2017) CORT concentrations in nail keratin included the null model and site as an explanatory variable (Table 3). While site as an explanatory variable was within two AICc of the best-supported model, contrary to our prediction, there was no difference in HPA activity between the roadside and roadless populations. Although 2017 CORT levels did not differ between sexes (Kruskal-Wallis χ21 = 0.259, p = 0.614), male turtles in both sites exhibited a broader range of variation than female turtles (Fig. 4).
2017 and 2018 corticosterone concentrations for all subsets of turtles. Females in the roadside site (N = 7) exhibited a significant increase in CORT from 2017 to 2018. Males in the roadside site (N = 8) likewise exhibited a significant increase in CORT. Females in the control site (N = 7) demonstrated no significant change in CORT, while males in the control site (N = 8) demonstrated a significant increase
The CORT concentrations in the subset of 31 animals resampled in 2018 were higher compared to 2017 (paired t test, t30 = 3.953, p = 0.0004) (Fig. 4). When considering the roadside site alone (N = 15 individuals), there was no correlation between the change in CORT concentration from 2017 to 2018 and nearest distance turtles were found from the road (rs = − 0.253, p = 0.361) or home range size (rs = − 0.094, p = 0.622).
Discussion
Overall, contrary to our expectations, our study shows a rather limited effect of a new high-traffic four-lane highway on the space use and stress levels in eastern box turtles 4 years post-construction. We considered the roadside population to be road-naïve, and therefore expected that the animals would show higher levels of stress in response to road proximity, as well as different space use and potential direct mortality for turtles crossing the road. However, we did not find differences in home range size between the roadside and roadless populations, although females had larger home range sizes at both sites. All turtles used the same microhabitats at both sites, but space use at the roadside site was bounded by the highway, with turtles showing no attempts to cross the road. In fact, although two different types of eco-passages (underpass bridge and culvert) were present within areas used by turtles, no turtles made use of these passages to reach the other side of the road. The roadside population did not show higher levels of HPA activity (i.e. accumulated stress response), and there was greater variation between males at both sites. However, our second round of stress hormone analyses showed 2018 CORT concentrations higher than 2017 and were not correlated with home range size or proximity to highway. Overall, our study suggests that proximity to high-traffic roads have a limited influence on eastern box turtle habitat selection, space use, and CORT levels, but the high-traffic roadway has the potential to affect local populations by acting as a complete barrier to movements.
Home ranges and activity patterns
We found that females had much larger home ranges than males, which is contrary to other studies that found no difference between sexes (Stickel 1950; Williams and Parker 1987; Doroff and Keith 1990; Bernstein et al. 2007). Although we did not find home range size differences between the two populations, the centers of turtle activity suggest interesting space use patterns. Clusters of activity occurred at both sites and are typically located where canopy openings coincide with proximity to edge habitat. While clearings provide quality basking, mating, and oviposition habitat, the proximity of edge habitat provides for thermoregulatory options, cover, and foraging opportunities. There were differences between the types of clearings accessed in each site: two clearings in the roadless site were naturally vegetated, semi-circular forest openings (0.25–0.5 ha in size); at the roadside site, turtles used the open habitat provided by the new road right-of-way. This open habitat was bounded by the highway and often included steep hillsides that served as natural barriers between turtles and the road, as well as the presence of deer exclusion fencing, which provided no discernible barrier to turtle movement (Claussen et al. 2002). Several females spent the majority of the summer months within 25 m of the highway, but we never encountered individuals attempting to cross the road. Avoidance behavior was previously recorded in box turtles and raises interesting questions about the implications for road mortality and potential population fragmentation (Shepard et al. 2008). Eastern box turtles are often found on smaller roads, and studies have found that box turtles utilize roads for thermoregulation and movement in addition to roadway habitats, leading to higher direct mortality from traffic (Nieuwolt 1996; Converse et al. 2005). These deleterious effects suggest that any viable management strategies for maintaining populations will require broadly forested areas (~ 100 ha) free of roads and rich in microhabitat diversity (Doroff and Keith 1990; Hall et al. 1999). Thus, particular characteristics of roads or traffic levels may pose complete barriers to movement, which could explain the loss of genetic variability following habitat bisection by high-traffic roads (Delaney et al. 2010). These barriers to dispersal have the potential to affect animal ecology, including possible evolutionary effects, as a result of reduced gene flow (Gibbs and Shriver 2002; Steen et al. 2006; Shepard et al. 2008).
Habitat selection
While we did not find evidence for different patterns of habitat selection at the two sites, turtles showed an apparent preference for habitat that provides ample cover and microhabitats that support daily feeding and resting requirements, as well as reproduction and nesting behaviors (Stickel 1950; Reagan 1974; Rossell et al. 2006; McKnight 2011; Kapfer et al. 2013). Overall, turtles demonstrated the strongest selection at the forest floor and understory cover levels. Turtle occurrence was positively associated with percent woody debris, ground cover which includes sticks, logs, and fallen trees on the forest floor, and understory, which includes any cover that obscures at least a foot above the forest floor (e.g., dense vegetation, forbs, and greenbrier and other thickets). Based on the odds ratios for top models, turtles selected understory two to three times more often than it was available, far more than for any other microhabitat variable (Table 2; Fig. 3). Interestingly, both males and females in the roadside site displayed a broader range of apparent preferences. In addition to being found in sites with high incidence of woody debris and understory, females in the roadside site were also often found in areas with high incidence of leaf litter and tended to also be found in areas thick with herbaceous vegetation, with 2% and 1.4% increase in likelihood of occurrence, respectively, per percent increase in difference in cover variable (Table 2). This could suggest a broader range of habitats made available by the presence of the road that roadside turtles are utilizing.
Weather conditions were also included in the top models for males and females at the roadside site. This suggests that the broader variety of microhabitat availability created by the presence of the road may lend itself to more variability in microclimate. Additionally, turtles at the roadside site are likely taking advantage of a broader variety of microhabitats associated with the road construction right-of-way, compared to the relatively homogenous microhabitats at the roadless site. These results corroborate other box turtle habitat studies in demonstrating preference for forested habitats with plentiful cover (Reagan 1974; Williams and Parker 1987; McKnight 2011; Greenspan et al. 2015). However, while box turtle occurrence is often positively associated with increased soil moisture, we found the opposite to be true for two population subsets: males at the roadside site (1% increase in difference in soil moisture content resulted in a 1.3% decrease in likelihood of turtle occupancy) and females at the roadless site (Table 2; Fig. 3; 1% increase in soil moisture resulted in a 1.1% decrease in occupancy). However, our soil moisture readings were the volumetric water content for mineral soils (clay, silt, loam). Box turtles were rarely found nestled into water-rich clays; instead, turtles were most often found using loose organic soils, which had lower soil moisture content compared to the clay soils. These results suggest that maintaining organic soil layers is vital to preserving box turtle habitat; habitat management actions such as high-severity fires may affect these soils, which could then have detrimental effects on population persistence (Russell et al. 1999; Platt et al. 2010; Howey and Roosenburg 2013).
HPA activity
Although 2017 concentrations of CORT did not differ between sites, male turtles in both sites exhibited a broader range of variation in HPA activity than female turtles (Fig. 4). This may seem counter-intuitive because males exhibited smaller home ranges, which were correlated to higher corticosterone levels in other reptiles (e.g., side-blotched lizard, Uta stansburiana, DeNardo and Sinervo 1994). However, higher corticosterone has also been associated with elevated locomotor abilities in the same lizard species (Miles et al. 2007), which could interplay with the fact that males may be more attuned to their smaller home ranges and possibly more sensitive to small environmental alterations therein (Walker et al. 2006; Fairhurst et al. 2011; Owen et al. 2014). While the best model for initial (2017) CORT measurements was the null model, the model including site as an explanatory variable was within two AICc of the top model (slightly higher concentrations at the roadside site, but no statistically significant differences). While the lack of differences could also be a result of turtles returning to metabolic equilibrium post road construction and adapting to the presence of road, it is important to further isolate other indicators of long-term stress (Romero 2004; Baxter-Gilbert et al. 2014).
Corticosterone concentrations increased almost universally for our tracked (N = 30) and recaptured (N = 1) animals between 2017 and 2018 (Fig. 4). Although year effects may have many ecological and research-related (e.g., handling) underpinnings, it seems unlikely that the proximity of the road could be responsible for these increases. Evaluation of corticosterone-binding globulin (CBG) and other relevant hormones might provide a more complete picture of chronic stress in these animals; for example, an animal normalized to chronic stress might have high concentrations of free CORT, but a lower CBG (Romero 2004; Fokidis et al. 2009). We postulate, however, that the use of keratin samples in extracting concentrations of free CORT as an integrated measure of the stress environment is a viable alternative to other extraction methods, including blood and fecal sampling. Keratin samples integrate both baseline and stress-response CORT levels over a greater temporal scale compared to the minute spectrum readable through other sampling techniques. Further, keratin sampling is less invasive and indicates little-to-no stress response due to handling and sampling, due to its longer time scale of accumulation (Bortolotti et al. 2008; Baxter-Gilbert et al. 2014). However, effects of frequent handling could result in long-term accumulation of CORT in nail keratin (Langkilde 2006). This makes it difficult to distinguish between gradual accumulation of CORT in a high-stress environment, and acute CORT accumulation induced by short-term stressors (such as handling) in a low-stress environment. An increased understanding of stress responses will be helpful in assessing the effects that heightened hormone levels might have on long-term fitness and population persistence.
Management and conservation implications
Based on our assessment of space-use, habitat selection, and indicators of HPA activity in a control-impact setting, we are able to provide ecological and behavioral information on turtle road ecology to managers and conservation practitioners. Turtles will access and use the right-of-way habitat of high-traffic roads for extended periods of time, potentially making them subject to direct mortality if they attempt crossing. At the same time, indirect effects of multiple stressors may have a limited influence. Predictors for habitat preference (including edge habitat with ample cover and thermoregulatory options) might influence habitat management strategies (such as preserving forest edges, maintaining clearings, limiting the spatial extent of prescribed burning, and allowing for multiple stages of forest succession). Foremost, we encourage protecting and preserving existing turtle habitat and providing suitable habitat attributes following anthropogenic disturbance. The barrier effect of high-traffic roads identified in this study is a critical finding, as it may affect turtles in the long term through the disruption of gene flow and population isolation (Holderegger and Di Giulio 2010). While eastern box turtles were found to retain high levels of genetic diversity in road-fragmented landscapes despite a past bottleneck (e.g., Michigan’s Lower Peninsula, Marsack and Swanson 2009), the long generation time can mask genetic processes such as genetic drift, slowing population recovery (Kuo and Janzen 2004). Thus, facilitating movement of individuals across highways acting as barriers is likely to be key for maintaining viable turtle populations in the long term. Because our study turtles did not attempt to use existing crossing structures (e.g., circular 1.2 m small animal passages), it is critical to (1) identify attributes of functional eco-passages that allow turtles to access habitat on either side of the road and (2) implement such structures at a density that match the range of movement (for example, at distances equal to home range diameter; in our case, every 200–300 m of highway for roadside males and females with an average home range of 2.4 and 7 ha, respectively).
Maintaining genetic connectivity via eco-passages tailored to the species biology and ecology could be combined with more cost-effective actions, such as protecting and augmenting of existing available habitat. Much of our study area is at a similar stage of forest succession, with thick oak and hickory canopies, and few natural clearings in the forest. As such, strategically placed and seasonally maintained open-canopy and early successional habitat might provide suitable habitat for foraging, gestation, and nesting, a suitable alternative to spending time in proximity to 70 mph heavy traffic. It is important, however, when creating and maintaining clearings and edge habitat to retain organic soils and ground cover and avoid packing soils with heavy machinery. Interestingly, some invasive plants (e.g., multiflora rose, Rosa multiflora) seemed to provide particularly good cover by promoting the accumulation of leaf litter, and soft organic soils, which are heavily favored by box turtles (Nagy et al. 1998; Goodenough 2010). More research should be done into the interactions between box turtles and exotic plants, but management decisions will need to weigh the costs of managing invasive vegetation versus the benefits it could provide to local fauna (Schlaepfer et al. 2011).
Lastly, our assessment of physiological stress suggests the need for further inquiry on the indirect effects of high-traffic roads and combinations of stressors on turtle populations. Although we were unable to draw conclusive connections between the proximity to road and deleterious effects on turtle health, the likelihood of combinations of stressors for sub-lethal effects may predispose turtle populations to further changes in demographic rates. Such issues are likely to exacerbate the substantial threats faced by turtles worldwide from habitat loss, road mortality, invasive species, disease, exploitation, and changing climates. A better understanding of stress ecology (e.g., relative importance of acute and chronic stressors to health) will inform conservation plans and ensure that the anthropogenic march of progress does not rest on the backs of turtles.
References
Anderson E, Zimmerman JW, Fisk E, Handley M (2011) Data report 2010-2011 ornate box turtle (Terrapene ornata) telemetry project Ayers sand prairie and Thomson-Fulton sand prairie nature preserves
Angelier F, Tonra CM, Holberton RL, Marra PP (2010) How to capture wild passerine species to study baseline corticosterone levels. J Ornithol 151:415–422. https://doi.org/10.1007/s10336-009-0471-6
Aresco MJ (2005) The effect of sex-specific terrestrial movements and roads on the sex ratio of freshwater turtles. Biol Conserv 123:37–44. https://doi.org/10.1016/j.biocon.2004.10.006
Baxter-Gilbert JH, Riley JL, Mastromonaco GF, Litzgus JD, Lesbarreres D (2014) A novel technique to measure chronic levels of corticosterone in turtles living around a major roadway. Conserv Physiol 2
Benítez-López A, Alkemade R, Verweij PA (2010) The impacts of roads and other infrastructure on mammal and bird populations: a meta-analysis. Biol Conserv 143:1307–1316. https://doi.org/10.1016/j.biocon.2010.02.009
Bernstein NP, Richtsmeier RJ, Black RW, Montgomery BR (2007) Home range and philopatry in the ornate box turtle, Terrapene ornata ornata, in Iowa. Am Midl Nat 157:162–174. https://doi.org/10.1674/0003-0031(2007)157[162:HRAPIT]2.0.CO;2
Bortolotti GR, Marchant TA, Blas J, German T (2008) Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct Ecol 22:494–500. https://doi.org/10.1111/j.1365-2435.2008.01387.x
Browne CL, Hecnar SJ (2007) Species loss and shifting population structure of freshwater turtles despite habitat protection. Biol Conserv 138:421–429. https://doi.org/10.1016/j.biocon.2007.05.008
Burnham KP, Anderson DR (2002) Model selection and multi-model inference, 2nd edn. Springer, Berlin
Claussen DL, Lim R, Kurz M, Wren K (2002) Effects of slope , substrate , and temperature on the locomotion of the ornate box turtle, Terrapene ornata. Copeia 2:411–418
Coffin AW (2007) From roadkill to road ecology: a review of the ecological effects of roads. J Transp Geogr 15:396–406. https://doi.org/10.1016/j.jtrangeo.2006.11.006
Collins SJ, Russell RW (2009) Toxicity of road salt to Nova Scotia amphibians. Environ Pollut 157:320–324. https://doi.org/10.1016/j.envpol.2008.06.032
Compton BW, Rhymer JM, McCollough M (2002) Habitat selection by wood turtles (Clemmys insculpta): an application of paired logistic regression. Ecology 83:833–843. https://doi.org/10.1890/0012-9658(2002)083[0833:HSBWTC]2.0.CO;2
Converse SJ, Iverson JB, Savidge JA (2005) Demographics of an ornate box turtle population experiencing minimal human-induced disturbances. Ecol Appl 15:2171–2179. https://doi.org/10.1890/04-0431
Converse SJ, Savidge JA, Whitford WG, Hutchinson VH (2003) Ambient temperature, activity, and microhabitat use by ornate box turtles (Terrapene ornata ornata). J Herpetol 37:665–670
Crawford BA, Maerz JC, Nibbelink NP, Buhlmann KA, Norton TM, Albeke SE (2014) Hot spots and hot moments of diamondback terrapin road-crossing activity. J Appl Ecol 51:367–375. https://doi.org/10.1111/1365-2664.12195
Delaney K, Riley S, Fisher R (2010) A rapid, strong, and convergent genetic response to urban habitat fragmentation in four divergent and widespread vertebrates. PLoS One 5:e12767
DeNardo DF, Sinervo B (1994) Effects of corticosterone on activity and home-range size of free-ranging male lizards. Horm Behav 28:53–65. https://doi.org/10.1006/hbeh.1994.1005
Doroff AM, Keith LB (1990) Demography and ecology of an ornate box turtle (Terrapene ornata) population in south- central Wisconsin. Copeia 2:387–399
Fairhurst GD, Frey MD, Reichert JF, et al (2011) Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective PLoS One 6:.https://doi.org/10.1371/journal.pone.0017663
Fokidis HB, Orchinik M, Deviche P (2009) Corticosterone and corticosteroid binding globulin in birds: relation to urbanization in a desert city. Gen Comp Endocrinol 160:259–270. https://doi.org/10.1016/j.ygcen.2008.12.005
Forman RTT (2000) Estimate of the area affected ecologically by the road system in the United States. Conserv Biol 14:31–35. https://doi.org/10.1046/j.1523-1739.2000.99299.x
Forman RTT, Alexander LE (1998) Roads and their major ecological effects. Annu Rev Ecol Syst 29:207–2C2
Forsythe P, Flitz B, Mullin SJ (2004) Radio telemetry and post-emergent habitat selection of neonate box turtles (Emydidae: Terrapene carolina) in Central Illinois. Herpetol Rev 35:333–335
Gibbs JP, Shriver WG (2002) Estimating the effects of road mortality on turtle populations estimating the effects of road mortality on turtle populations. Conserv Biol 16:1647–1652
Gibbs JP, Steen DA (2005) Trends in sex ratios of turtles in the United States: implications of road mortality. Conserv Biol 19:552–556. https://doi.org/10.1111/j.1523-1739.2005.00155.x
Goodenough AE (2010) Are the ecological impacts of alien species misrepresented? A review of the “native good, alien bad” philosophy. Community Ecol 11:13–21. https://doi.org/10.1556/ComEc.11.2010.1.3
Greenspan SE, Condon EP, Smith LL (2015) Home range and habitat selection in the eastern box turtle (Terrapene carolina carolina) in a longleaf pine (Pinus palustris) reserve. Herpetol Conserv Biol 10:99–111
Hall RJ, Henry PFP, Bunck CM (1999) Fifty-year trends in a box turtle population in Maryland. Biol Conserv 88:165–172. https://doi.org/10.1016/S0006-3207(98)00107-4
Holderegger R, Di Giulio M (2010) The genetic effects of roads: a review of empirical evidence. Basic Appl Ecol 11:522–531. https://doi.org/10.1016/j.baae.2010.06.006
Howey CAF, Roosenburg WM (2013) Effects of prescribed fire on the eastern box turtle (Terrapene carolina carolina). Northeast Nat 20:493–497
Iglay RB, Bowman JL, Nazdrowicz NH (2007) Eastern box turtle (Terrapene carolina carolina) movements in a fragmented landscape. J Herpetol:102. https://doi.org/10.2307/4498557
IUCN (2017) Terrapene carolina. IUCN Red List Threat. Species, In
Kapfer JM, Muñoz DJ, Groves JD, Kirk RW (2013) Home range and habitat preferences of eastern box turtles (Terrapene carolina Linnaeus, 1758) in the Piedmont Ecological Province of North Carolina (USA). Herpetol Notes 6:251–260
Kapfer JM, Muñoz DJ, Tomasek T (2012) Use of wildlife detector dogs to study eastern box turtle (Terrapene carolina carolina) populations. Herpetol Conserv Biol 7:169–175
Kuo C-H, Janzen FJ (2004) Genetic effects of a persistent bottleneck on a natural population of ornate box turtles (Terrapene ornata). Conserv Genet 5:425–437. https://doi.org/10.1023/B:COGE.0000041020.54140.45
Langkilde T (2006) How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei. J Exp Biol 209:1035–1043. https://doi.org/10.1242/jeb.02112
Marsack K, Swanson BJ (2009) A genetic analysis of the impact of generation time and road-based habitat fragmentation on eastern box turtles (Terrapene c. carolina). Copeia 2009:647–652. https://doi.org/10.1643/CE-08-233
McKnight DT (2011) Observed plant preferences of eastern box turtles (Terrapene carolina carolina) in a Maryland forest. Herpetol Notes 4:97–102
Miles DB, Calsbeek R, Sinervo B (2007) Corticosterone, locomotor performance, and metabolism in side-blotched lizards (Uta stansburiana). Horm Behav 51:548–554. https://doi.org/10.1016/j.yhbeh.2007.02.005
Nagy KA, Henen BT, Vyas DB (1998) Nutritional quality of native and introduced food plants of wild desert tortoises. J Herpetol 32:260–267
Nieuwolt PM (1996) Movement, activity, and microhabitat selection in the Western box turtle, Terrapene ornata luteola, in New Mexico. Herpetologica 52:487–495
ODNR (2016) Ohio’s listed species
Owen DAS, Carter ET, Holding ML, Islam K, Moore IT (2014) Roads are associated with a blunted stress response in a North American pit viper. Gen Comp Endocrinol 202:87–92. https://doi.org/10.1016/j.ygcen.2014.04.020
Platt SG, Liu H, Borg CK (2010) Fire ecology of the Florida box turtle (Terrapene Carolina Bauri Taylor) in pine Rockland forests of the lower Florida keys. Nat Areas J 30:254–260. https://doi.org/10.3375/043.030.0301
R Core Team (2013) R: A language and environment for statistical computing
Reagan DP (1974) Habitat selection in the three-toed box turtle, Terrapene carolina triunguis. Copeia 1974:512–527
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255. https://doi.org/10.1016/j.tree.2004.03.008
Romero LM, Fairhurst GD (2016) Measuring corticosterone in feathers: strengths, limitations, and suggestions for the future. Comp Biochem Physiol Part A Mol Integr Physiol 202:112–122
Rossell CR, Rossell IM, Patch S (2006) Microhabitat selection by eastern box turtles (Terrapene c. carolina) in a North Carolina Mountain wetland. J Herpetol 40:280–284. https://doi.org/10.1670/236-05N.1
Russell KR, Van Lear DH, Guynn DC (1999) Prescribed fire effects on herptofauna: review and management implications. Wildl Soc Bull 27:374–384
Schlaepfer MA, Sax DF, Olden JD (2011) The potential conservation value of non-native species. Conserv Biol 25:428–437. https://doi.org/10.1111/j.1523-1739.2010.01646.x
Schubauer JP (1981) A reliable radio-telemetry tracking system suitable for studies of chelonians. J Herpetol 15:117–120
Shepard DB, Kuhns AR, Dreslik MJ, Phillips CA (2008) Roads as barriers to animal movement in fragmented landscapes. Anim Conserv 11:288–296. https://doi.org/10.1111/j.1469-1795.2008.00183.x
Steen DA, Aresco MJ, Beilke SG, Compton BW, Condon EP, Kenneth Dodd C, Forrester H, Gibbons JW, Greene JL, Johnson G, Langen TA, Oldham MJ, Oxier DN, Saumure RA, Schueler FW, Sleeman JM, Smith LL, Tucker JK, Gibbs JP (2006) Relative vulnerability of female turtles to road mortality. Anim Conserv 9:269–273. https://doi.org/10.1111/j.1469-1795.2006.00032.x
Steen DA, Gibbs JP (2004) Effects of roads on the structure of freshwater turtle populations. Conserv Biol 18:1143–1148. https://doi.org/10.1111/j.1523-1739.2004.00240.x
Stickel LF (1950) Populations and home range relationships of the box turtle, Terrapene c. carolina. Ecol Monogr 20:351–378
The Ohio Department of Transportation Office of Statewide Planning & Research (2017) Timber rattlesnakes’ (Crotalus horridus) use of man-made rocky features constructed in roadway right-of-ways
Therneau T (2015) A package for survival analysis in R version 2.38
Trombulak SC, Frissell CA (2000) Review of ecological effects of roads on terrestrial and aquatic communities. Conserv Biol 14:18–30. https://doi.org/10.1046/j.1523-1739.2000.99084.x
Walker BG, Boersma PD, Wingfield JC (2006) Habituation of adult Magellanic penguins to human visitation as expressed through behavior and corticosterone secretion. Conserv Biol 20:146–154
Williams EC, Parker WS (1987) A long-term study of a box turtle (Terrapene carolina) population at Allee memorial woods, Indiana, with emphasis on survivorship. Herpetologica 43:328–335. https://doi.org/10.2307/3892499
Wood RC, Herlands R (1997) Turtles and tires: the impact of roadkills on northern diamondback terrapin, Malaclemys terrapin terrapin, populations on the Cape May peninsula, southern New Jersey, USA. In: Conservation, Restoration, and Management of Tortoises and Turtles-An International Conference. pp 46–53
Acknowledgments
We thank Lynda Andrews (Wayne National Forest), the Ohio Department of Transportation, and Hocking College for facilitating access to the two sites. John Rucker and his team of Boykin spaniel dogs were instrumental in collecting animals for this study. We also thank the dozens of volunteers that contributed hundreds of hours in the field tracking turtles daily for many months, in particular Vince Schlauch, Jonna Curtiss, Christine Hanson, and Eva Garcia. David Swanson (Hocking College) connected us to an endless source of Hocking College student volunteers and interns. Marcello D’Amico and two anonymous reviewers provided helpful comments on previous manuscript drafts.
Funding
This research was funded by the Ohio University Department of Biological Sciences, Ohio University Graduate Student Senate, Society for Integrative and Comparative Biology, Ohio Biological Survey, and the Society for Conservation Biology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection Road Ecology
Guest Editor: Marcello D’Amico
Rights and permissions
About this article
Cite this article
Weigand, N.M., Wagner, R.B., Tonra, C.M. et al. Proximity to highways has limited influence on space use and physiology of terrestrial testudines. Eur J Wildl Res 65, 80 (2019). https://doi.org/10.1007/s10344-019-1315-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-019-1315-1