Abstract
Habitat loss leads to habitat fragmentation. Habitat connectivity, however, could mitigate effects of habitat fragmentation on wildlife populations. This study was carried out to assess habitat suitability and connectivity of a brown bear population located along the Iran-Iraq border in the Zagros Mountains, at the southernmost extreme of the species range. A total of 34 presences of brown bear and seven environmental variables were used for habitat modeling using MaxEnt, and connectivity among habitat patches was assessed by electrical-circuit methods using Circuitscape. Distance from villages, elevation, slope, and distance from roads were respectively the most important variables in habitat modeling of the brown bear in the study area. In total, 33 habitat patches were identified for the brown bear, which covered about 12% of the study area. Results of connectivity revealed high connectivity among habitat patches in the Iran section, whereas in the Iraq section, only low connectivity was observed in areas close to the Iran-Iraq border. Systematic monitoring is recommended to assess potential habitat patches and habitat connectivity of the brown bear in future research as a first step towards cooperative management efforts between wildlife managers of Iran and Iraq. Moreover, establishing a transboundary protected area is highly recommended along the Iran-Iraq border to provide safety and connectivity for the brown bear in this region and reduce the effect of the country border as a separating factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid growth of human population and occupation of natural habitats by humans have generated habitat loss and habitat fragmentation (Bennett 2003; Berger et al. 2008), i.e., the conversion of a large natural habitat into several smaller and spatially separated habitat patches (Baskent and Jordan 1995; Bennett 2003; Ewers and Didham 2006). Although a few studies have discussed the advantages of habitat fragmentation (e.g., Fahrig 2003; Fahrig 2017), many have highlighted the negative effects on wildlife (e.g., Fletcher Jr et al. 2018).
Habitat fragmentation usually has a number of major effects including (1) decrease in ratio between patch circumference and patch size leading to a reduction in core area and major border effects (Collinge 1996; Hamazaki 1996; Collinge and Palmer 2002), (2) restriction of connectivity of living organisms and a reduction in gene flow within metapopulations (Crooks and Sanjayan 2006) resulting in decreased genetic diversity (Frankham 1996) and consequently increased inbreeding depression (Ebert et al. 2002), (3) major effects on the viability and dynamics of species populations (Soulé 1986; Dixon et al. 2007; Bruggeman et al. 2010), (4) decreased population adaptation to climate change (Opdam and Wascher 2004), (5) population decline (Donovan and Flather 2002; Revilla and Wiegand 2008; Bruggeman et al. 2010), and even (6) extinction of species (Soulé et al. 1992; Fulgione et al. 2009).
Connectivity is any movement of organisms among habitat patches and in general, better habitat connectivity enhances the maintenance of populations, generating a lesser risk of extinction of species/population, especially in fragmented habitats (Beier et al. 2007; Crooks and Sanjayan 2006). As a matter of fact, lack of connectivity not only decreases available large patches but also leads to further isolation of the remaining smaller habitat patches (Crooks and Sanjayan 2006). Conservation of connectivity is essential in order to access food and breeding sites and to facilitate dispersal to neighboring habitat patches, particularly for immature individuals (Beier and Noss 1998; Hilty et al. 2006; Crooks et al. 2011).
Large carnivores such as the brown bear (Ursus arctos, Linnaeus 1758) are extremely sensitive to habitat loss and fragmentation due to their vast distribution and often low population density, particularly populations located at the extreme of their ranges (Calvignac et al. 2009; Noss et al. 1996). Reduction in population sizes of large carnivores and extinction of these members of higher trophic levels in natural food chains will lead to structural and ecological changes in natural ecosystems and their biotic communities (Crooks 2002; Estes et al. 2011). Large carnivores such as the brown bear are in priority for conservation, as covering the needs of these umbrella species in their vast home ranges and establishing viable populations could also protect other mammal species, vertebrates, plants, and insects (Beier et al. 2008; Sampson 2013).
In the west of Asia, brown bears have lost much of their habitats and only limited isolated populations of this species remain in Iran, Iraq, and Turkey (Can and Togan 2004). These populations located in the most southern distribution of the brown bear in the world are prone to extinction (Calvignac et al. 2009). In Iran, brown bears belong to a unique clade with restricted connectivity due to unsuitable habitats and human pressure (Calvignac et al. 2009; Ashrafzadeh et al. 2018). Brown bear populations with an estimated number of 1200–1800(Ashrafzadeh et al. 2016) inhabit north (i.e., Alborz Mountains and coast of the Caspian Sea), northwest (Iranian Caucasus area), and west (Zagros Mountains) of Iran, covering an area of about 277,000 km2 (i.e., about 17% of Iran’s total area) (Gutleb and Ziaie 1999; Ashrafzadeh et al. 2016). In Iran, the brown bear, a relatively rare species, is exposed to local extinction particularly in the Zagros Mountains (Karami et al. 2015) and is classified as a protected species by the Department of Environment (DoE) in Iran due to the loss of most of its historical range caused by habitat loss and exploitation (Ashrafzadeh et al. 2016). Brown bear hunting is forbidden in Iran (DoE 2018) and poaching occurs in Iran mainly due to human–brown bear conflict (Karami et al. 2015). In Iraq, brown bear distribution, with an area of about 22,700 km2 (i.e., about 5% of Iraq’s total area), is limited to the Zagros Mountains in the northeast of the country (i.e., Kurdistan region) (Hadi 2008). As an endangered species in Iraq, the brown bear has lost 50% of its population as compared to the past due to human activities such as military operations (Hadi 2008).
The Zagros Mountains are a less known habitat for the brown bear compared with other areas such as Europe in terms of different characteristics including land cover and climate change. In addition, not many habitat studies have been carried out on the brown bear in areas with low vegetation cover. Therefore, the present study was carried out to assess habitat requirements of the brown bear, predict potential suitable habitat patches, and to map out the connectivity among those patches, using electrical-circuit modeling with the aim of boosting conservation efforts of the brown bear in the Zagros Mountains, a population located at the southern extreme of the brown bear range. Indeed, conservation of this population can be achieved through the study of potential habitat patches of the brown bear and its connectivity to locate new populations, to protect new areas, etc.
Materials and methods
Study area
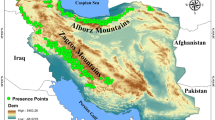
The study area is located in the Zagros Mountains, covering an area of about 34,800 km2, which comprises four protected areas (i.e., Shaho-Kosalan [Sh-Ko], Buzin-Marakhil [Bu-Ma], Piramagroon [Pi], and Qara-Dagh [Qa-Da] protected areas) with a total area of about 1230 km2 (i.e., about 4% of the study area) (Fig. 1). The Zagros Mountains are a long range of mountains (i.e., total length of 1600 km) across the west of Iran, extending from the Persian Gulf to the northeast of Iraq and southeast of Turkey (Sagheb Talebi et al. 2014). The study area provided a suitable natural habitat for a number of major large mammals such as the brown bear, Persian leopard (Panthera pardus saxicolor), Eurasian lynx (Lynx lynx), roe deer (Capreolus capreolus), and wild goat (Capra aegagrus) (Hadi 2008; Karami et al. 2015; Nature Iraq 2017; DoE 2018). Based on the information of areas close to our study area, the food diet of the brown bear often consists of plant species (Nezami and Farhadinia 2011; Soofi et al. 2017). According to direct observations and reports of the locals, fruits of Quercus brantii, Crataegus azarolus, and Pyrus communis are the most popular food items for the brown bear in the study area.
The elevation in the study area ranges between 99 and 3334 m, and there are two main topographic features: mountains located in all parts of the study area except the southwest, with a relatively cold climate (i.e., cold winter averaging 4 °C, mild summer averaging 22 °C, and annual precipitation of about 450 mm), and plains located in the southwest of the study area (i.e., mild winter averaging 10 °C, relatively warm summer averaging 28 °C, and annual precipitation of about 350 mm) (IRIMO 2017). The land cover of the study area included mosaic croplands/vegetation (35% of the study area), mosaic vegetation/croplands (27.3%), sparse vegetation (16.6%), bare areas (7.9%), rainfed croplands (5%), mosaic forest-shrubland/grassland (4.1%), mosaic grassland/forest-shrubland (2.2%), closed to open shrubland (1.6%), water bodies (0.28%), and four cover types including closed needleleaved evergreen forest, closed broadleaved deciduous forest, open broadleaved deciduous forest, and closed to open mixed broadleaved and needleleaved forest (0.02%).
Data collection
Presence points were collected from 2015 to 2018 based on official reports of the DoE (i.e., 12 points based on direct observation and signs) and informed locals (i.e., 4 points), as well as observations of brown bear signs during the field survey (i.e., 10 scat points and 8-ft-sign points). Field survey was carried out randomly in areas with a higher probability of bear presence in order to search for the signs (i.e., scats and foot signs). Some information on bear presence in the study area were obtained from the DoE. In addition, we questioned informed locals to gather data on areas with bear reports in recent years. In certain cases, we asked informed locals to survey their surrounding area which yielded 4 presence points. In general, we had 18 sampling efforts in different seasons (i.e., 5 spring, 6 summer, 4 fall, and 3 winter). To minimize spatial autocorrelation among bear presences, we used the Spatially Rarify Occurrence Data tool in SDMtoolbox (Brown 2014) to eliminate any bear presence with a distance less than 2 km from another bear presence, with respect to the mean daily movements of the brown bear (about 4 km) (Ćirović et al. 2015). We considered 2 km as we assumed a circle with a diameter of 4 km with a brown bear standing in its center. The remaining 34 independent presence points of the brown bear were used for habitat modeling. Thirteen out of 34 points were located in two protected areas of Sh-Ko and Bu-Ma. In addition, 21 points were obtained from the other areas of the study area.
Environmental variables
Seven environmental variables describing topographic variables (i.e., elevation above sea level [elevation] and slope steepness [slope]), land cover variables (i.e., distance from mixed forest, shrubland, and grassland [dis_frst_shrb_grs] and distance from mixed cropland and vegetation [dis_crp_vg]), water resources (i.e., distance from river [dis_rv]), and human disturbance (i.e., distance from roads [dis_rd] and distance from villages [dis_vl]) were selected for habitat suitability modeling; all in cell sizes of 100 m (Table 1). Digital elevation model (DEM) as the elevation variable generated by 30-m Shuttle Radar Topography Mission (SRTM) was resampled to the cell size of 100 m and used to create slope map in percentage.
We used GlobCover version 2.3 as a global land cover map with 23 cover types including 13 cover types in the study area for habitat modeling. Bearing in mind that the brown bear is a generalist species and usually prefers varied cover types (Gula et al. 1998), we first re-categorized the land cover from 13 to 4 cover types based on the ecology of the species (Falcucci et al. 2013)(Table S1). Then, we applied the re-categorized map separately to the habitat modeling in order to detect important cover types. Mixed forest, shrubland and grassland, and mixed cropland and vegetation were the most important cover types in the habitat modeling of the brown bear. Finally, the most important cover types were developed using the Euclidean distance tool in ArcGIS 10.2. We used the distance method instead of the density method within a moving window around the focal pixel as the value of each pixel changed with different sizes of the moving window (Beier et al. 2007). Therefore, Dis_frst_shrb_grs and Dis_crp_vg variables were considered for habitat modeling.
Given the importance of water for animals, Dis_rv was considered for habitat suitability modeling. Dis_rd was selected as a human disturbance variable because bears tend to avoid roads (Brody and Pelton 1989). Dis_vl was also created as another human disturbance variable. Collinearity among variables was checked and all pairwise variables were with correlation coefficient of less than 70% (Table S2) (Zuur et al. 2010).
Habitat modeling
Habitat suitability map of the brown bear was obtained to (1) show areas with a high probability of bear presence and (2) be used in connectivity modeling. Habitat modeling was done using MaxEnt software version 3.3.3.k (Phillips et al. 2006). MaxEnt software uses a comparison of presence points with pseudo-absence points to predict habitat suitability. This method was frequently used for animal populations with unknown densities and distributions (Fois et al. 2018) with a small number of presence points (Elith et al. 2006; van Proosdij et al. 2016). A total of 10,000 pseudo-absence points as the default setting of MaxEnt software and also known as the high predictive accuracy (Phillips and Dudik 2008) were considered to predict the distribution. Seventy-five percent of the presence points was considered as training data and the remaining 25% as test data.
The area under the receiver operating characteristic curve (AUC) was used to evaluate model performance (0–1, in which 1 indicates perfect discrimination of presence points from pseudo-absence points). As suggested by Merow et al. (2013) and van Proosdij et al. (2016), linear/quadratic features, instead of all features, were considered due to the small data set of presence points in this study. In the linear feature, the continuous variable should be close to the observed values, and in the quadratic feature, the variance of continuous variables should be close to the observed values (Phillips et al. 2006). One linear and quadratic feature was created for each variable (Merow et al. 2013). In addition, according to the method used by Fois et al. (2018), four numbers were used for regularization multiplier (i.e., 0.5, 1, 2, and 5) and the highest AUC was selected as the final model. Regularization multiplier is a user-specified coefficient that makes the constraints less strict and produces a less localized prediction. Therefore, higher regularization multiplier decreases constraints and consequently more generality is achieved (i.e., more spread out distribution) (Phillips and Dudik 2008; Fois et al. 2018). To delimit the background area to an informative set of pseudo-absence points, a minimum convex polygon was created around the buffer of each presence point (Anderson and Raza 2010; Barbet-Massin et al. 2012; Almasieh et al. 2016). According to the method presented by Grilo et al. (2018), the buffer around each presence point was obtained based on the circular home range of the male brown bear in Turkey (i.e., 5.15-km radius to create 83-km2 home range; Ambarli et al. 2016) as the nearest area to the study area. Home range of males was larger than of females and we aimed to delimit the background area based on the larger home range. First, habitat modeling was fitted for the background area and then extrapolated to the entire study area using modeling projection in MaxEnt.
A Jackknife test within MaxEnt was used to evaluate the relative contribution of each variable. Response curves of brown bear presence to each environmental variable were used to show the probability of species presence to the gradient of each variable. MaxEnt created two types of response curves (i.e., response curves of each variable considering correlation with other variables and without considering it). We selected those curves in which the correlation between each variable and the other variables was considered.
Habitat prediction
Habitat patches were obtained in order to (1) detect polygons of potential distribution of the brown bear and to (2) apply these patches as start/stop points for habitat connectivity modeling. Using the fitted model of MaxEnt and the threshold value of 0.281 (ranging from 0 to 1), habitat suitability map was converted to a binary map (i.e., suitable and non-suitable map) based on maximum training sensitivity plus specificity in MaxEnt model (Jimenez-Valverde and Lobo 2007). This threshold is the point where sensitivity (i.e., model classifies presence points correctly) and specificity (i.e., model classifies absence points correctly) are maximized (Manel et al. 2001; Liu et al. 2005). The accuracy of the binary map was obtained using sensitivity, specificity, and true skill statistic (TSS). The sensitivity was obtained in MaxEnt results (i.e., 1 minus omission rate). A total of 10,000 pseudo-absence points were generated randomly out of presence point cells and the percentage of these random points in non-suitable polygons was calculated for specificity. Finally, TSS was calculated according to the formula calculation presented by Allouche et al. (2006) (i.e., TSS = sensitivity plus specificity minus 1). Among habitat suitability patches, we removed patches with an area of < 14 km2 according to the mean home range of the female brown bear in Turkey (i.e., 14.07 km2; Ambarli et al. 2016). We considered home range of females based on the importance of this sex for reproduction and population dynamics (Maynard Smith 1978; Stearns 1987), and because we intended to remove the least number of habitat patches.
Connectivity
Electrical circuit theory (McRae et al. 2008), as a method in designing habitat connectivity, is based on a random walk and uses the principles of electrical circuit to design habitat connectivity. In this approach, the current (living organisms) moves among focal nodes (habitat patches) in relation to voltage (probability of organisms’ movement) and resistance (habitat permeability) (McRae et al. 2008; Roever et al. 2013). Electrical circuit method identifies different probable movements among habitat patches and is therefore considered as superior to the least-cost method as it only identifies one possible movement (Urban and Keitt 2001; Urban et al. 2009). Electrical circuit theory could be particularly useful for modeling of gene flow in the landscape (McRae and Beier 2007).
Electrical circuit connectivity was done using Circuitscape version 4 (McRae and Shah 2009) using the pairwise method. One minus habitat suitability map obtained by the MaxEnt model was used as a raster resistance map and habitat patches of the brown bear were used as focal nodes. For each cell, connection to its 8 neighboring cells was determined. Areas with lower resistance show a higher density of species movements and vice versa.
Results
Habitat modeling
MaxEnt model showed an AUC value of 0.894 (Table S3), which represents high accuracy of the model. The logistic threshold of maximum training sensitivity plus specificity had a sensitivity of 0.846, specificity of 0.841, and TSS of 0.687, indicating good accuracy of the model for the binary map.
The jackknife test revealed that Dis_vl, elevation, slope, and Dis_rd were, respectively, the most important variables in habitat modeling of the brown bear in the study area (Fig. 2, top). According to the response curve of brown bear presence to the Dis_vl variable, a positive quadratic relationship was observed between presence points of the brown bear and Dis_vl variable; therefore, the probability of brown bear presence increased as the distance from villages increased. Also, there was a positive quadratic relationship between presence points and the elevation variable as brown bears inhabit areas above an elevation of 2000 m. A similar relationship was detected between presence points and the slope variable, such that when slope increased, the probability of brown bear presence increased as well. Finally, there was a positive linear relationship between presence points and Dis_rd variable; the probability of brown bear presence increased as the distance from roads increased (Fig. 2, bottom).
Top: Jackknife test within MaxEnt to investigate the importance of each habitat variable in habitat suitability modeling of the brown bear in the study area in the Zagros Mountains. Bottom: Response curves of habitat variables in habitat modeling of the brown bear in the study area in the Zagros Mountains (full names of variables are presented in Table 1)
Habitat prediction
The potential suitability habitat of the brown bear in the study area showed an area of 4185 km2 (i.e., 12% of the study area). There were 33 habitat patches (i.e., H1–H33) in the study area with a mean size of 127 km2 indicating fragmented habitat, among which 8 habitat patches were occupied by the brown bear (i.e., H1, H2, H4, H5, H14, H16, H20, and H29) with an area of 3272 km2 (i.e., 78% of habitat patches) (Fig. 3, Fig. S1). The largest occupied habitat patch (H1) with an area of 1984 km2 was located outside of the protected areas. The second largest occupied habitat patch (H2) with an area of 980 km2 covered 64% of Sh-Ko. Also, there were two occupied habitat patches (H5 and H29) that covered 11% of Bu-Ma. A relatively large patch (H6) was located in the north of Pi (Fig. 3).
Connectivity
High density of currents among habitat patches was observed among 11 patches (i.e., H1, H2, H7, H10, H15, H16, H22, H23, H27, H30, and H33). Lower density of currents was observed among the remaining patches and their neighboring patches. Patch H6 was relatively isolated and an insignificant density of currents occurred between this patch and the other patches (Fig. 4).
Discussion
This research was carried out to identify suitable habitat patches and connectivity of the brown bear as a top predator in the border of the species’ southern range along the Iran-Iraq border. Our results can help wildlife experts manage the brown bear population through conserving the important habitat patches and areas with high connectivity, detecting potential habitat patches and consequently new protected areas, etc. Habitat prediction determined 33 fragmented patches mainly in the east of the study area and the main connectivity was observed between the two largest occupied habitat patches (i.e., H1 and H2), which encompassed the other 9 patches situated between these two patches.
Regarding important environmental variables, villagers are in constant conflict with brown bears; thus, bears try to keep a safe flushing radius from human settlements (Worthy and Foggin 2008). Elevation determines temperature and precipitation patterns (i.e., rain or snow), as well as the distribution of species (Beier et al. 2007). Brown bears prefer an elevation higher than 2000 m and avoid lower elevations as most villages are located in lower elevations. Moreover, it seems the brown bear prefers areas with elevations above 2000 m as the ecotone between forest and rangeland occurs approximately at this elevation in the study area (i.e., 2400 m) (Sagheb Talebi et al. 2014). The brown bear, particularly females with cubs, prefer rugged and steep areas (Zarzo-Arias et al. 2019). Finally, roads are related to human disturbance (Almasieh et al. 2016; Beier et al. 2008; Mohammadi et al. 2018) and threaten animals via road mortality or isolation of populations (Holderegger and Giulio 2010). Roads also allow humans to more easily access natural areas and therefore heighten poaching pressure on the species (Haines et al. 2012; Shaffer and Bishop 2016).
Patch analysis revealed that the main modeled patches were located in the Iran section. The largest patch (H1) included 47% of all modeled patches and extended to the vicinity of patch H2. We received unconfirmed reports of brown bear presence from locals in two villages (i.e., Barda-sefid and Kora-dareh) near south of this patch (Fig. 4). Patch H1 covered about two-thirds of a formerly no-hunting area (i.e., Chehel Cheshmeh-Saral). It seemed that this area had the highest capacity for protection of brown bear populations in the study area and had the potential to be upgraded to a protected area. However, it has been recently downgraded to a free area. We strongly recommend that the DoE consider this conservation gap as a new protected area. Patch H2 encompassed 23% of all modeled patches and covered two-thirds of Sh-Ko. This protected area was the most important area for the brown bear among the available protected areas in the study area. Two closely located patches (H5 and H29) included less than 3% of all modeled patches, covering more than one-tenth of Bu-Ma. This protected area was located along the Iran-Iraq border and could enhance the movement of individuals of the brown bear from Iran to Iraq and vice versa (Fig. 3). Only 8 out of 33 habitat patches were occupied by the brown bear (Fig. 3). Considering the low number of presence points and the non-systematic field survey, the brown bear might occur in some other potential patches. We received unconfirmed reports of brown bear presence from locals of villages near patch H7 (i.e., Palangan, Goaz, Paygalan, Sar-rez, Tangisar, Shian, and Zhan) (Fig. 4). This patch could be surveyed in the future.
With the exception of the 11 mentioned patches with relatively high connectivity, the remaining patches had lower connectivity including patches H12 and H29 that connected the brown bear of Iran and Iraq in the study area. We received unconfirmed reports of brown bear occurrence from locals of villages near patches H1 and H12 (i.e., Garmalah and Hawraman) (Fig. 4). This area could be surveyed to identify its connectivity or perhaps new habitat patches. The non-occupied patch H6, located near Pi, as the only patch in the northwest of the study area, was considered as the most isolated patch due to its long distance from the other patches and insignificant connectivity with the other patches.
Local people can be regarded as valuable sources of information concerning wildlife of their region (White et al. 2005; Zeller et al. 2011). Well-established interviews could serve as an effective alternative to extensive field surveys, particularly regarding nocturnal and cryptic carnivores with unknown ecology such as bears (Sargeant et al. 1998; Pike et al. 1999; Van der Hoeven et al. 2004; Zeller et al. 2011). We gained incomplete information from interviews with locals which was limited to few villages. In the future, systematic interview with informed locals is recommended to gather more extensive data on brown bear presence in the study area.
Iranian protected areas are close to the VI category of the IUCN, whereas wildlife refuges and national parks resemble the IV and II categories of the IUCN, respectively (Lausche and Burhenne-Guilmin2011). Based on the modeled connectivity, connectivity currently occurs among some habitat patches of the brown bear in the study area. To ensure the safety of movement for individuals of the brown bear, laws of the DoE for protected areas could be implemented in areas with a high density of currents. Initially, the range of connectivity areas could be determined using warning signs. Patrolling and monitoring could be carried out in a way similar to protected areas. Limitation of industrial and mining activities and domestic grazing is highly recommended and could only be allowed with the prior permission of the DoE (DoE 2018). Without doubt, local education on values of the brown bear in the region could also be effective along with appropriate law enforcement to reduce bear–human conflicts and consequently enhance brown bear conservation in both habitat patches and areas of connectivity (Baruch-Mordo et al. 2011).
This study aimed to detect the potential connectivity among identified habitat patches of the brown bear in the Zagros Mountains, a poorly known area between the two countries of Iran and Iraq. As the first step of cooperative efforts between wildlife managers of Iran and Iraq, systematic monitoring is recommended to assess potential habitat patches and habitat connectivity of the brown bear in future research (Williams et al. 2009). In the Iran section, parts of two protected areas were tangent to the Iran-Iraq border, whereas, in the Iraq section, there were no protected areas tangent to the border. Therefore, wildlife managers of Iraq should introduce some border areas as protected areas to maintain the safety of animal movement, particularly for large carnivores such as the brown bear, between Iran and Iraq. It would be ideal to establish a transboundary protected area along the Iran-Iraq border according to the IUCN criteria (Sandwith et al. 2001; Vasilijević et al. 2015) in cooperation between the two countries to help maintain safety and connectivity for animals in this area and reduce the effect of country border as a separating factor.
References
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232
Almasieh K, Kaboli M, Beier P (2016) Identifying habitat cores and corridors for the Iranian black bear in Iran. Ursus 27(1):18–30
Ambarli H, Erturk A, Soyumert A (2016) Current status, distribution, and conservation of brown bear (Ursidae) and wild canids (gray wolf, golden jackal, and red fox; Canidae) in Turkey. Turk J Zool 40:944–956
Anderson RP, Raza A (2010) The effect of the extent of the study region on GIS models of species geographic distributions and estimates of niche evolution: preliminary tests with montane rodents (genus Nephelomys) in Venezuela. J Biogeogr 37:1378–1393
Ashrafzadeh MR, Kaboli M, Naghavi MR (2016) Mitochondrial DNA analysis of Iranian brown bears (Ursus arctos) reveals new phylogeographic lineage. Mamm Biol 81:1–9
Ashrafzadeh MR, Khosravi R, Ahmadi M, Kaboli M (2018) Landscape heterogeneity and ecological niche isolation shape the distribution of spatial genetic variations in Iranian brown bears, Ursus arctos (Carnivora: Ursidae). Mamm Biol 93:64–75
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3:327–338
Baruch-Mordo S, Breck SW, Wilson KR, Broderick J (2011) The carrot or the stick? Evaluation of education and enforcement as management tools for human-wildlife conflicts. PLoS One 6(1):e15681
Baskent EZ, Jordan GA (1995) Characterizing spatial structure of forest landscapes. Can J For Res 25:1830–1849
Beier P, Noss RF (1998) Do habitat corridors provide connectivity? Conserv Biol 12:1241–1252
Beier P, Majka D, Jenness J (2007) Conceptual steps for designing wildlife corridors. https://www.corridordesign.org/. Accessed 20 June 2017
Beier P, Majka D, Spencer WD (2008) Forks in the road: choices in procedures for designing wildland linkages. Conserv Biol 22(4):836–851
Bennett AF (2003) Linkages in the landscape: the role of corridors and connectivity in wildlife conservation. IUCN, Gland, Switzerland and Cambridge, UK
Berger J, Young JK, Berger KM (2008) Protecting migration corridors: challenges and optimism for Mongolian saiga. PLoS Biol 6:1365–1367
Brody AJ, Pelton MR (1989) Effects of roads on black bear movements in western North Carolina. Wildlife Soc B 17:5–10
Brown JL (2014) SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic, and species distribution model analyses. Methods Ecol Evol 5(7):694–700
Bruggeman DJ, Wiegand T, Fernández N (2010) The relative effects of habitat loss and fragmentation on population genetic variation in the red-cockaded woodpecker (Picoides borealis). Mol Ecol 19(17):3679–3691
Calvignac S, Hughes S, Hanni C (2009) Genetic diversity of endangered brown bear (Ursus arctos) populations at the crossroads of Europe, Asia and Africa. Divers Distrib 15:742–750
Can ÖE, Togan I (2004) Status and management of brown bears in Turkey. Ursus 15:48–53
Ćirović D, Hernando MG, Paunović M, Karamanlidis AA (2015) Home range, movements, and activity patterns of a brown bear in Serbia. Ursus 26(2):1–7
Collinge SK (1996) Ecological consequences of habitat fragmentation: implications for landscape architecture and planning. Landsc Urban Plan 36:59–77
Collinge SK, Palmer TM (2002) The influences of patch shape and boundary contrast on insect response to fragmentation in California grasslands. Landsc Ecol 17:647–656
Crooks KR (2002) Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv Biol 16:488–502
Crooks KR, Sanjayan M (2006) Connectivity conservation. Cambridge University Press, Cambridge, UK
Crooks KR, Christopher LB, Theobald DM, Rondinini C, Boitani L (2011) Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Philos Trans R Soc B 366:2642–2651
Dixon JD, Oli MK, Wooten MC, Eason TH, McCown JW, Cunningham MW (2007) Genetic consequences of habitat fragmentation and loss: the case of the Florida black bear (Ursus americanus floridanus). Conserv Genet 8:455–464
DoE (Department of the Environment of Iran) (2018) Department of the Environment of Iran. www.doe.ir. Accessed 1 October 2018
Donovan TM, Flather CH (2002) Relationships among north American songbird trends, habitat fragmentation and landscape occupancy. Ecol Appl 12:364–374
Ebert D, Haag C, Kirkpatrick M, Riek M, Hottinger JW, Pajunen VI (2002) A selective advantage to immigrant genes in a Daphnia metapopulation. Science 295:485–488
Elith J, Graham CH, Anderson RP et al (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
ESA (2009) GlobCover land-cover (global land cover map). http://due.esrin.esa.int/page_globcover.php. Accessed 20 May 2018
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet earth. Science 333:301–307
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol S 34:487–515
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol S 48:1–23
Falcucci A, Maiorano L, Tempio G, Boitani L, Ciucci P (2013) Modeling the potential distribution for a range-expanding species: wolf recolonization of the alpine range. Biol Conserv 158:63–72
Fletcher RJ Jr, Didham RK, Banks-Leite C, Barlow J, Ewers RM, Rosindell J, Holt RD, Gonzalez A, Pardini R, Damschen EI, Melo FPL, Ries L, Prevedello JA, Tscharntke T, Laurance WF, Lovejoy T, Haddad NM (2018) Is habitat fragmentation good for biodiversity? Biol Conserv 226:9–15
Fois M, Cuena-Lombraña A, Fenu G, Bacchetta G (2018) Using species distribution models at local scale to guide the search of poorly known species: review, methodological issues and future directions. Ecol Model 385:124–132
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Fulgione D, Maselli V, Pavarese G, Rippa D, Rastogi RK (2009) Landscape fragmentation and habitat suitability in endangered Italian hare (Lepus corsicanus) and European hare (Lepus europaeus) populations. Eur J Wildl Res 55:385–396
Grilo C, Lucas PM, Fernandez-Gil A, Seara M, Costa G, Roque S, Rio-Maior H, Nakamura M, Alvares F, Petrucci-Fonseca F, Revilla E (2018) Refuge as major habitat driver for wolf presence in human-modified landscapes. Anim Conserv 22(1):59–71
Gula R, Rackowiakd W, Erzanowskid K (1998) Current status and conservation needs of the brown bear in the polish Carpathians. Ursus 10:81–86
Gutleb B, Ziaie H (1999) On the distribution and status of the brown bear, Ursus arctos, and the Asiatic black bear, U. thibetanus, in Iran. Zool Middle East 18(1):5–8
Hadi AM (2008) A survey and recording the regions of Brown bear distribution in the mountains of Kurdistan-Iraq. Second international environment forum: new environmental horizons of sustainable development. Tanta University, Egypt 27–29 /11/2008
Haines AM, Elledge D, Wilsing LK, Grabe M, Barske MD, Burke N, Webb SL (2012) Spatially explicit analysis of poaching activity as a conservation management tool. Wildlife Soc B 36(4):685–692
Hamazaki T (1996) Effects of patch shape on the number of organisms. Landsc Ecol 11:299–306
Hilty JA, Lidicker WZ Jr, Merenlender AM (2006) Corridor ecology. The science and practice of linking landscapes for biodiversity conservation. Island Press, Washington
Holderegger R, Giulio MD (2010) The genetic effects of roads: a review of empirical evidence. Basic Appl Ecol 11:522–531
IRIMO (Islamic Republic of Iran Meteorological Organization) (2017) Climate data-base, Iranian cities, from 1993 to 2017. https://www.irimo.ir. Accessed 1 October 2018
Jimenez-Valverde A, Lobo JM (2007) Threshold criteria for conversion of probability of species presence to either-or presence-absence. Acta Oecol 31(3):361–369
Karami M, Ghadirian T, Faizolahi K (2015) The atlas of the mammals of Iran. In: Department of the Environment of Iran. Iran, Tehran
Lausche BJ, Burhenne-Guilmin F (2011) Guidelines for protected areas legislation. IUCN
Liu C, Berry PM, Dawson TP, Pearson RG (2005) Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28:385–393
Manel S, Williams HC, Ormerod SJ (2001) Evaluating presence-absence models in ecology: the need to account for prevalence. J Appl Ecol 38:921–931
Maynard Smith J (1978) The evolution of sex. Cambridge University Press
McRae BH, Beier P (2007) Circuit theory predicts gene flow in plant and animal populations. Proc Natl Acad Sci U S A 104:19885–19890
McRae BH, Shah VB (2009) Circuitscape user’s guide. The university of California, Santa http://www.circuitscape.org. Accesses 10 Jan 2018
McRae BH, Dickson BG, Keitt TH, Shah VB (2008) Using circuit theory to model connectivity in ecology, evolution and conservation. Ecology 89(10):2712–2724
Merow C, Smith MJ, Silander JA Jr (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36:1058–1069
Mohammadi A, Almasieh K, Clevenger AP, Fatemizadeh F, Rezaei A, Jowkar H, Kaboli M (2018) Road expansion: a challenge to conservation of mammals, with particular emphasis on the endangered Asiatic cheetah in Iran. J Nat Conserv 43:8–18
Nature Iraq (2017) Key biodiversity areas of Iraq. Tablet House Publishing
NCC (National Cartographic Center of Iran) (2012) Integrated report of rail, road and river studies. National Cartographic Center of Iran
Nezami B, Farhadinia MS (2011) Litter sizes of brown bears in the central Alborz protected area, Iran. Ursus 22(2):167–171
Noss RF, Quigley HB, Hornocker MG, Merrill T, Paquet PC (1996) Conservation biology and carnivore biology in the Rocky Mountain. Conserv Biol 10(4):949–963
OCHA (2018a) Iraq - Roads network. https://data.humdata.org/organization/ocha-iraq. Accessed 10 May 2018
OCHA (2018b) Iraq - water courses (Rivers and Streams). https://data.humdata.org/organization/ocha-iraq. Accessed 10 May 2018
OCHA (UN Office for the Coordination of Humanitarian Affairs) (2017) Iraq - settlements (villages, towns, cities). https://data.humdata.org/organization/ocha-iraq. Accessed 10 May 2018
Opdam P, Wascher D (2004) Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biol Conserv 117:285–297
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Pike JR, Shaw JH, Leslie DM Jr, Shaw MG (1999) A geographic analysis of the status of mountain lions in Oklahoma. Wildlife Soc B 27:4–11
Revilla E, Wiegand T (2008) Individual movement behavior, matrix heterogeneity, and the dynamics of spatially structured populations. Proc Natl Acad Sci U S A 105:19120–19125
Roever CL, van Aarde RJ, Leggett K (2013) Functional connectivity within conservation networks: delineating corridors for African elephants. Biol Conserv 157:128–135
Sagheb Talebi K, Sajedi T, Pourhashemi M (2014) Forests of Iran a treasure from the past, a hope for the future. Springer Dordrecht Heidelberg New York London
Sampson AM (2013). A habitat suitability analysis for cougar (Puma concolor) in Minnesota. MSc Thesis, University of Minnesota, USA
Sandwith T, Shine C, Hamilton L, Sheppard D (2001) Transboundary protected areas for peace and co-operation. IUCN, Gland, Switzerland and Cambridge, UK
Sargeant GA, Johnson DH, Berg WE (1998) Interpreting carnivore scent-station surveys. J Wildl Manag 62:1235–1245
Shaffer MJ, Bishop JA (2016) Predicting and preventing elephant poaching incidents through statistical analysis, GIS-based risk analysis, and aerial surveillance flight path modeling. Trop Conserv Sci 9(1):525–548
Soofi M, Turk Qashqaei A, Aryal A, Coogan SCP (2017) Autumn food habits of the brown bear Ursus arctos in the Golestan National Park: a pilot study in Iran. Mammalia 82(4):338–342
Soulé ME (1986) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, MA.
Soulé ME, Alberts AC, Bolger DT (1992) The effects of habitat fragmentation on chaparral plants and vertebrates. Oikos 63:39–47
Stearns SC (1987) The evolution of sex and its consequences. Birkhauser Verlag, Basel
Urban D, Keitt T (2001) Landscape connectivity: a graph-theoretic perspective. Ecology 82:1205–1218
Urban DL, Minor ES, Treml EA, Schick RS (2009) Graph models of habitat mosaics. Ecol Lett 12:260–273
Van der Hoeven CA, De Boer WF, Prins HHT (2004) Pooling local expert opinions for estimating mammal densities in tropical rainforests. J Nat Conserv 12:193–204
van Proosdij ASJ, Sosef MSM, Wieringa JJ, Raes N (2016) Minimum required number of specimen records to develop accurate species distribution models. Ecography 39:542–552
Vasilijević M, Zunckel K, McKinney M, Erg B, Schoon M, Rosen Michel T (2015) Transboundary conservation: a systematic and integrated approach. Best practice protected area guidelines series no. 23, gland, Switzerland: IUCN
White PC, Jennings NV, Renwick AR, Barker NHL (2005) Questionnaires in ecology: a review of past use and recommendations for best practice. J Appl Ecol 42:421–430
Williams JN, Seo C, Thorne J, Nelson JK, Erwin S, O’Brien JM, Schwartz MW (2009) Using species distribution models to predict new occurrences for rare plants. Divers Distrib 15:565–576
Worthy FR, Foggin JM (2008) Conflicts between local villagers and Tibetan brown bears threaten conservation of bears in a remote region of the Tibetan plateau. Hum–Wildl Interact 2(2):200–205
Zarzo-Arias A, Penteriani V, Delgado MM, Peon Torre P, Garcia-Gonzalez R, Mateo- Sanchez MC, Garcia PV, Dalerum F (2019) Identifying potential areas of expansion for the endangered brown bear (Ursus arctos) population in the Cantabrian Mountains (NW Spain). PLoS One 14(1):e0209972
Zeller KA, Nijhawan S, Salom-Pérez R, Potosme SH, Hines JE (2011) Integrating occupancy modeling and interview data for corridor identification: a case study for jaguars in Nicaragua. Biol Conserv 144:892–901
Zuur AF, Leno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgments
We thank the Department of Environments in Iran and kindly locals who provided information about the brown bear. We would like to show our gratitude to anonymous reviewers for their helpful comments on the earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Department of Environment of Iran.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Road Ecology
Guest Editor: Marcello D’Amico
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Almasieh, K., Rouhi, H. & Kaboodvandpour, S. Habitat suitability and connectivity for the brown bear (Ursus arctos) along the Iran-Iraq border. Eur J Wildl Res 65, 57 (2019). https://doi.org/10.1007/s10344-019-1295-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-019-1295-1