Abstract
The European mink is a critically endangered mustelid species of conservation concern throughout Europe. Several conservation interventions have been implemented in recent years, supported by both national and European governments. However, knowledge about the natural history of the European mink is scarce and localized to a few specific areas. From 2007 to 2009, we studied mink activity patterns, home range sizes, and macrohabitats of mink home ranges based on 28 radio-tracked European mink (10 adult females, 11 adult males, 3 young females, and 4 young males) in the Foral Community of Navarre (northern Spain), in the Arga and Aragón rivers. We also provide insights on the spatial organization of the species. European mink presented a stable, mainly nocturnal and crepuscular activity pattern and required between 15 and 75 ha of fluvial habitats to establish their home ranges, which were also quite stable throughout the year. There were great differences between adult females and adult males, the latter having home ranges five times larger. In addition, whereas adult females mainly settled in lagoons and small tributaries, males also used to a large extent the main river sections. European mink presented a polygynous mating system, where males were territorial and encompassed several female home ranges within their home ranges. Lagoons and similar structures should be preserved and favored in management strategies, and tributaries maintained in good condition, as female requirements should be prioritized in plans to improve the general habitat quality for the species. Any conservation plan aimed at the improvement or recovery of European mink populations through habitat management should consider management blocks of at least 15 ha per each potential breeding female.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European mink (Mustela lutreola) is an endemic and critically endangered European mustelid (Maran et al. 2016), with few populations located in Eastern Europe and another located in Western Europe (Maran et al. 2016). The mink population in Spain is believed to be recently established as it was first discovered in the 1950s, and no previous records for the species existed in the area (Palomares 1991; Clavero 2014). This Spanish population may have come from the French population, where the first record dates back to the first half of the nineteenth century (de Bellefroid 1999; Michaux et al. 2005).
The history of the species in Western Europe, in addition to genetic data, has generated discussion about if this European mink population has naturally colonized the area or come from putative human introductions (Palomares 1991; Michaux et al. 2005; Clavero 2014; Cabria et al. 2015; Maran et al. 2016). Anyway, the European mink is listed in the Annex II of the EU Habitats Directive and is a species of conservation concern throughout Europe, where both the Eastern and Western populations have declined in the last few decades (Maran and Henttonen 1995; Lodé et al. 2001; Maran et al. 2016). Several conservation interventions have been implemented by both national and European governments, such as population reinforcements and reintroductions, control of non-native competitors (American mink Neovison vison), and habitat restoration (Maran et al. 2016; Palazón 2010), despite a lack of scientific knowledge on these topics in most areas with the presence of the species. Competition with feral American mink, changes in land use, overexploitation, infectious diseases, and the negative effects of pesticides are the main causes invoked to explain the decline of mink populations (Maran and Henttonen 1995; Lodé et al. 2001; Maran et al. 2016; Fournier-Chambrillon et al. in press).
Knowledge about the natural history of European mink is scarce or inexistent in many parts of its distribution area. However, an evidence-based conservation approach to the recovery of the species requires scientific information on its biology and ecology in order to inform proper decision-making processes and to delineate effective conservation actions. Under this framework, the present study aims to expand our knowledge of some basic aspects of the temporal and spatial ecology of the Spanish population of European mink (Foral Community of Navarre), where several management plans have been implemented in recent years (LIFE09/NAT/ES/53, LIFE05 NAT/E/000073), and scientific support is needed to validate or improve conservation measures. Specifically, we studied activity patterns, home range size, and macrohabitats within mink home ranges, and to a lesser extent, we provide some insights on the spatial organization of the species. Some information exists on spatial ecology for wild European mink, and there are a few studies on classical habitat use (Arambarri et al. 1997; Maizaret et al. 1998; Sidorovich and Macdonald 2001; Zabala and Zuberogoitia 2003a; Zabala et al. 2003; Fournier et al. 2007), and a few more are on the topics studied in this paper (activity, home range size, and spatial organization; Palazón and Ruíz-Olmo 1998; Garin et al. 2002a, b; Ceña et al. 2003a; Fournier et al. 2008). The abovementioned studies indicated that European mink are nocturnal and crepuscular, that the home ranges of males are much larger than those of females, and that intrasexual overlap among adult males and females is minimal. Based on this knowledge, we expected that European mink in our study area present a mainly nocturnal and crepuscular activity pattern, with males having larger home ranges than females, and a male spatial structure that overlaps with the territory of several females, but with little or no home range overlap between individuals of the same sex. We tested these predictions by radio-tracking 28 European mink over a 3-year period (2007–2009).
Materials and methods
Study area
The study area is located in the Foral Community of Navarre, Spain, on sections of the Arga (42° 29′ N–42° 44′ N, 1° 81′ W–1° 78′ W) and Aragón (42° 29′ N–42° 39′ N, 1° 78′ W–1° 47′ W) rivers, which include the riverbeds and wide networks of small lagoons (mostly formed from abandoned meanders), channels, and irrigation ditches at their margins. The lower reaches of the Aragón and Arga rivers are included in the Natura 2000 network due to a strong representation of Mediterranean river forests (poplars, Populus sp., and willow, Salix sp., forests), with patches of brambles, Rubus sp., and small lakes with abundant reedbeds, Phragmites australis. However, most of the floodplains have been occupied by agricultural lands or poplar plantations in recent decades. Dikes and breakwater defenses were built in the past to defend agricultural and forestry plantations from flooding. The Arga River was canalized to protect downstream towns from floods, which are common in both rivers (Ollero-Ojeda 2000). These defense infrastructures have diminished the natural dynamics of these two rivers, leading to a decrease in available undisturbed habitats.
Capture and radio-tracking
Mink were captured in two sections of 25 and 35 km of the Arga and Aragón rivers, respectively, with box traps baited with oil sardines and trout (Fournier-Chambrillon et al. in press). A total of six 10-day trapping sessions were carried out during March and October–November (pre-breeding and post-breeding periods, respectively; Palazón 2010) of the years 2007 and 2008 in the Arga River and 2007 in the Aragón River. In total, an effort of 455 and 422 traps/day was set in each river, respectively.
Once captured, animals were anesthetized intramuscularly with a combination of ketamine (Imalgene©; 7.5 mg/kg) and medetomidine (Domtor©; 150 mg/kg; Fournier-Chambrillon et al. 2003) to implant an intraperitoneal radio-transmitter (Fournier et al. 2001). After the handling of the animals, an equivalent dose of atipamezol (Antisedans©) was administered to revert the effects of anesthesia. Radio-transmitters were designed for mink and were provided with movement and mortality sensors (Biotrack©, ATS©, TELONICS©, models 150-STP and 130-HP; Fournier et al. 2001; Fournier-Chambrillon et al. 2003). The size and the weight of the transmitters were 66 × 17 mm and 18 g (1.4–2.6% of body mass) for males and 59 × 11 mm and 9 g (1.3–2.5% of body mass) for females. All captured animals were sexed and aged as adults (nearly 1 or > 1 years old) or young. Since the parturition period of European mink begins in April (Fournier-Chambrillon et al. 2010), all individuals captured in March were considered adults. In autumn, distinction between adults and 6–7-month-old young was done by field researchers with long experience with the species (captures and necropsies) using the combination of different parameters. Young were these individuals simultaneously showing (1) white new teeth, without abrasion or tartar; (2) fur in perfect condition, not molting; (3) in males, very small size of the testicles (which remains much lower in young males than in adult males, even during sexual rest); and (4) in females, tiny nipples, without signs of previous lactation (undrawn). All mink were radio-located one or two times daily between 2007 and 2009, or during blocks of 6 h with locations every 2 h, 4 days every week during the breeding seasons of 2007 and 2008. The locations of animals were obtained by triangulation with a maximum difference between bearings of 10 min, by one bearing and estimating distance to animal position when we estimated (by radio signal intensity) that it was less than 50 m from observer, or by homing when minks were resting.
Estimating range size
Home range size estimation in semiaquatic mammals, such as otters and mink, is a difficult task as individuals spent most, if not all, of their time in or close to aquatic habitats, which are very often linear structures. One accepted alternative is to express the space used by animals in linear distances of the fluvial courses (e.g., see Garin et al. 2002a; Ceña et al. 2003a; Melero et al. 2008; Zschille et al. 2012). However, this method is susceptible to inaccuracies mainly when animals use both rivers and lagoons, only lagoons, or wide rivers and tributaries. To address this issue, some studies have combined minimum convex polygons (MCPs) or density kernel estimations with linear distances of the fluvial courses within these polygons (Ceña et al. 2003a; Melero et al. 2008; Zschille et al. 2012). Although this approach improves range size estimates, it cannot estimate the actual area used by animals.
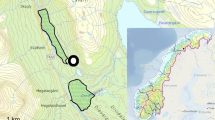
European mink rarely use areas outside of fluvial courses, marshy systems, and their associated vegetation (Zuberogoitia and Zabala 2003, authors unpubl.). Therefore, in order to estimate range sizes, following a similar multi-approach to that used by Melero et al. (2008), we first estimated seasonal home range sizes by the 100% MCP method using the extension home range (Rodgers et al. 2007) for ArcGIS 10© (ESRI, Inc., Redlands, CA). Instead of measuring the linear distance of fluvial systems inside these polygons, we then delineated the surface occupied by rivers, tributaries, irrigation ditches, lagoons, and their associated riverside vegetation, inside the MCP seasonal home ranges (Fig. 1a), using high-resolution ortho-images of the study area, and then estimated an adjusted home range (AHR) (Fig. 1b). AHRs were estimated seasonally according to the annual cycle of European mink. Thus, we estimated AHRs for three seasons between March and April, May and August, and September and February, periods corresponding to mating, breeding, and independence of young, respectively (Palazón 2010). On the other hand, we also calculated the length of the fluvial courses within these polygons (Ceña et al. 2003a; Melero et al. 2008; Zschille et al. 2012) for comparative purposes.
a Method proposed in this study to estimate the adjusted home range (AHR) of a given European mink in comparison to the 100% minimum convex polygon (MCP). b Section of the AHR where it is possible to appreciate how delimitation of the potential area used by mink was undertaken by delineating the surface occupied by rivers, tributaries, irrigation ditches, lagoons, and their associated riverside vegetation on high-resolution ortho-images. Red polygon denotes the AHR within the 100% MCP home range estimate
Data analysis
We first studied general mink activity patterns by representing the probability of finding active mink on an hourly basis during the 24-h cycle (i.e., the percentage of active locations considering the total number of mink locations available for a given hour). We then tested for differences in the probability of a mink being active (each mink location was binary coded: 1—active, 0—inactive) in different periods of the day, and among sex-age classes and seasons. To do this, we classified all mink locations according to four time blocks: night (from 1 h after sunset to 1 h before sunrise), day (from 1 h after sunrise to 1 h before sunset), dusk (1 h before and after sunset), and dawn (1 h before and after sunrise). In this way, the time blocks accounted for fluctuations in the length of the photoperiod throughout the year. On the other hand, we considered three sex-age classes: adult females, adult males, and young (both young males and females pooled) as well as three seasons (March–April, May–August, and September–February, periods corresponding to mating, breeding, and independence of young, respectively, after Palazón 2010). We then built a GLMM with binomial error distribution and logit link to test for differences in the probability of a mink being active in relation to the period of the day, sex-age classes, and seasons. Individual identity was treated as a random effect in the model.
Secondly, we explored the variation in seasonal AHR sizes (Fig. 1) in relation to sex-age classes (same three levels), seasons (same three levels), and main type of macrohabitat within AHRs. Individuals radio-located less than 10 times in a season were excluded for these analyses. Nevertheless, we included the number of locations as a covariate in the model in order to control for its possible effect on home range estimation. Three main types of macrohabitats were found in the study area: rivers (more than 50 m wide and with running water year-round), tributaries (smaller rivers, which may dry out during summer), and lagoons. We pooled rivers and tributaries into the category “linear landscape attributes,” and we calculated the percentage of the estimated AHRs by linear elements to be considered as a predictor in subsequent analyses. We built a GLMM with Gaussian error distribution and identity link to evaluate the influence of the abovementioned predictors on the logarithm of the seasonal AHRs. Individual identity and year were treated as random effects in the model.
We tested for significant differences in the proportion of the main macrohabitats considered (rivers, tributaries, and lagoons) within individual home ranges across seasons. To do this, we built three GLMMs with beta error distribution, considering the proportion of every macrohabitat within AHRs as response variable and sex-age classes and seasons as predictors in these models. Individual identity and year were treated as random effects in the model. We used the “glmmADMB” package for R (Fournier et al. 2012) to run the GLMMs and the “car” package for R to calculate Wald χ 2 to evaluate the significance levels for model parameters (Fox and Weisberg 2011).
Finally, some insights on the spatial organization of mink in the study area were gained after the representation of locations of adult individuals of both sexes simultaneously radio-tracked.
Ethics
This study was carried out in strict compliance with the European (Directives 92/43/CEE) and Spanish (Act 42/2007) legislation on the protection of threatened wildlife. Exceptional permits for trapping, movement, and equipping the target species with transmitters were obtained from the Service of Biodiversity Conservation of Navarre Government. The protocols used were consistent with best practices and technical and scientific recommendations related to animal welfare according to guidelines of the Animal Behaviour Society (2006).
Results
Sampled animals
Twenty-eight individuals (10 adult females, 11 adult males, 3 young females, and 4 young males; Table 1) were captured and radio-tagged between 2007 and 2009 (23 and 5 mink in the Arga and Aragón rivers, respectively). Males were significantly heavier (mean = 830 g, SE = 14.9) than females (mean = 522 g, SE = 11.5; Gaussian GLM considering the body mass of mink as a response variable and sex (F = 251.59, d.f. = 1, P < 0.0001) and age (F = 0.09, d.f. = 1, P = 0.765), as covariates; the sex × age interaction term could not be examined due to lost degrees of freedom).
A total of 2581 locations were obtained during the study period, with an average of 92 locations per individual (range = 1–312; Table 1). For 21 individuals, the radio-tracking monitoring ended due to radio signal loss or because the study ended, whereas seven mink were found dead: four killed by other carnivores, one drowned, one road-killed, and one for which the cause of death was undetermined (Table 1).
Activity patterns
There was at least one activity record for 26 mink (10 adult females, 10 adult males, and 6 young individuals). Overall, European mink were mainly active at night and at twilight, with a peak in activity around dawn; during daylight, the probability of finding a mink active was generally lower than 30% (Fig. 2).
Activity of mink significantly varied along the circadian period, being higher at dawn and night compared to dusk, and the least activity was observed during the day (χ 2 = 11.97, d.f. = 3, P = 0.007). We did not detect different activity patterns across seasons (χ 2 = 4.85, d.f. = 2, P = 0.088) or sex-age classes (χ 2 = 0.03, d.f. = 2, P = 0.987). No significant interaction between sex-age classes and seasons was found (χ 2 = 2.57, d.f. = 4, P = 0.631), or between sex-age classes and the period of the day (χ 2 = 0.74, d.f. = 6, P = 0.994). These results indicate that all mink showed similar activity patterns across seasons and daily periods regardless of individual attributes. However, we detected a significant interaction between the period of the day and the season (χ 2 = 20.19, d.f. = 6, P = 0.002; Fig. 3). It is worth noting that the probability of mink being active at night, dusk, and during the day was higher in the independence period compared to other seasons. This was probably related to the activity of young, as most of the animals radio-tracked in this season were young.
Home range size
We obtained information on seasonal home range size from 26 European mink, which represented a total of 48 mink-season data points. An average of 47 locations per individual (range = 10–125) were used to estimate seasonal home range sizes. Adult males showed larger seasonal home ranges compared to young and adult females regardless of the approach used to estimate home ranges (Table 2). Based on MCPs or AHRs, the size of adult female home ranges was between 16 and 22% of adult male home ranges, respectively (Table 2). Young individuals showed intermediate range size values (Table 2). The AHR estimates were highly correlated to MCP estimates (r = 0.864, P < 0.001; n = 48; Pearson’s product-moment correlation) and linear home range sizes (r = 0.705, P < 0.001; n = 48). However, the mean seasonal home range size using the MCP method was on average three times higher than the same estimate using the AHR method (range 0.7–7.9 times). Such differences between sex-age classes in seasonal home range size were statistically significant according to AHR estimates (χ 2 = 52.67, d.f. = 2, P < 0.0001). No significant effects of seasons and macrohabitats included within home ranges were detected on home range sizes (χ 2 = 3.74, d.f. = 2, P = 0.154 and χ 2 = 3.27, d.f. = 1, P = 0.070, respectively). Sampling effort did not affect results (P = 0.341).
Spatial organization
Although data are limited, there were in both study years and rivers some cases where adult mink of same or different sex were simultaneously radio-tracked, which may provide insights about the spatial organization of the species. Males and females overlapped their territories (Figs. 4 and 5), and at least six males included within their home ranges more than one adult female (Figs. 4a, b and 5b). On the other hand, in the four cases where there was information for more than an adult female, little overlap was observed between their home ranges (Figs. 4 and 5). In the cases where two or more adult males were radio-tracked in the same river and year, in three cases, they seemed to overlap a small portion of their home ranges (Figs. 4a, b and 5a), although in two cases did not (Figs. 4c and 5b). As a rule, adult males seemed to overlap more their home ranges than adult females did.
Macrohabitat use
European mink settled their seasonal home ranges mainly in areas with lagoons, followed by rivers and to a lesser extent in tributaries (Table 3; Friedman test = 6.12, d.f. = 2, P = 0.047). Adult females and young mainly settled their home ranges in lagoons, and males in river sections (Table 3). Rivers were used in a significantly different proportion among sex-age classes (focusing on AHR estimates: χ 2 = 8.38, d.f. = 2, P = 0.015), with adult males and young using rivers more frequently than adult females (Table 3). On the contrary, the proportions of tributaries and lagoons within home ranges did not statistically differ among sex-age classes (focusing on AHR estimates: both P > 0.121; Table 3). We did not detect seasonal differences in the proportion of rivers, tributaries, and lagoons in individual seasonal home ranges (all P > 0.701). European mink settled seasonal home ranges exclusively in the macrohabitat lagoons on 14 occasions (mainly adult females; Table 3), but only on four occasions in tributaries (always adult females) and one in rivers. Whereas adult males only once (5%) settled seasonal home ranges in only one type of macrohabitat, adult females did so in lagoons or tributaries on 64% of occasions (Table 3).
Discussion
European mink presented a stable, mainly nocturnal and crepuscular activity pattern, although some animals were sometimes active during the day. Information available for other areas indicated a similar activity pattern (Palazón and Ruíz-Olmo 1998; Garin et al. 2002a), including for reintroduced individuals (Peters et al. 2009). Other similar and potential competing species sometimes present similar circadian activity patterns with higher activity around twilight and night, although when studied in detail, marked differences have been found between sexes and individuals (e.g., see Marcelli et al. (2003) for differences between individuals in polecats, Mustela putorius; Zschille et al. (2010) for differences between sexes and individuals in the American mink). More detailed studies may provide evidence of sexual or individual differences in European mink, which could not be detected with the data obtained in this study. It is worth noting that seasonal differences in activity patterns have also been found in American mink, with both sexes increasing activity during mating, and males decreasing activity during the breeding season while females maintaining higher activity levels (Zschille et al. 2010). No such differences were detected in European mink.
General circadian activity patterns in small mammals may be an adaptation to the activity rhythm of main prey, to prevent potential predation when moving during daylight, or both (Halle and Stenseth 2000), in addition to the endogenous circadian rhythm modulated by the light–dark cycle (Daan and Aschoff 1982). In fact, some European mink were killed by other carnivore species (Table 1), which might be influencing their activity pattern. There is little information on diet of European mink in the study area, but the available data suggests that they are feeding on a wide prey spectrum with crustaceans (Procambarus clarkii), small mammals, amphibians and fishes being important in the diet, and to a lesser extent reptiles and birds (Urra and Román 2013; also see Palazón et al. 2004, 2008 to confirm the generalist character of species in other areas of the European mink western population). Such a wide prey spectrum reflects the opportunistic nature of European mink, but with the available data, we can not speculate if main prey types consumed in the area could suppose a strong ecological pressure on European mink to be mainly nocturnal.
European mink required between 15 and 75 ha (corresponding to 2.5 and 12.7 km) of fluvial habitats to establish their home ranges, which were quite stable throughout the year. The ranges observed in our study area are within the available information for other areas in Spain (Garin et al. 2002a found males using between 11 and 17 km and females between 0.6 and 3.6 km of riverbanks; Ceña et al. 2003a found an average of 9.7 km in males and 4.9 km in females, and Palazón and Ruíz-Olmo 1998 found one female using 5 km and males using between 3 and 11 km), but somewhat smaller than those reported in southwestern France (9–16 km males and 2–10 km females; Fournier et al. 2008). These last differences could be in relation to the much smaller densities of mink in southwestern France than in Spain, including our study area, where the highest densities of the western European mink population have been detected (Ceña et al. 2003b; Fournier-Chambrillon et al. in press). One adult male and five adult females reintroduced in Germany moved by 7.2 and 0.2–4.3 km of river length, respectively (Peters et al. 2009), which, mainly in females, is much lesser than what European mink moved in our study area.
The most remarkable result was the great difference in the home range size between adult females and males, the latter having home ranges five times larger. In addition, it was interesting to note that adult males and females use different types of macrohabitats. Whereas adult females mainly settled their home ranges in lagoons and small tributaries, males also used, to a large extent, the main river sections. Similar findings were reported by Zabala et al. (2007) for a similar competing species, the American mink, and data available for the European mink also suggest that this may be the case (Garin et al. 2002a; Ceña et al. 2003a).
The scarce information from this and other available studies (Palazón and Ruíz-Olmo 1998; Garin et al. 2002a; Ceña et al. 2003a; Zabala and Zuberogoitia 2003b; Fournier et al. 2008) suggests that European mink present a polygynous mating system, where males encompass several female home ranges within their home ranges, and both males and females are territorial (i.e., they defense an exclusive area from other adult individuals of the same sex). This pattern, in addition to clear differences in home range size and types of fluvial systems used by each sex, fit well with what is expected for solitary carnivores with sexual size dimorphism. Female home ranges are regulated by habitat quality, while female presence and density are also important for males (Erlinge and Sandell 1986; Sandell 1989). Thus, female European mink settled their home ranges in areas with helophytic vegetation, which is expected to hold a higher diversity and density of prey, while males also used other river sections connecting the areas where female are settled.
Conservation implications
This study contributes information crucial to the conservation of the European mink in the study area and other areas with similar characteristics, mostly in the Western distribution area of the species. The most relevant information with conservation implications is related to the different spatial strategies of each sex. Whereas females settled small home ranges mainly in lagoons and small tributaries, males also included other fluvial habitats within their home ranges, such as river sections. Therefore, lagoons and similar structures must be preserved and favored, and tributaries maintained in good condition, since female requirements should be prioritized in plans to improve general habitat quality for the species. Small tributaries are much more susceptible to deterioration by the surrounding use of the landscape. For instance, margins could easily be removed for agricultural activities.
Another important result of this study is that for the first time, we know the exact area of fluvial habitats in which European mink live. Previous estimations were imprecise as lineal home range estimates or MCP areas do not provide the actual area that animals use or require. Using the approach proposed in this study to estimate home range sizes (the AHR to habitat), we obtained more realistic estimates about the space requirements of males and females of European mink. This method could easily be implemented to study the spatial behavior of other semiaquatic mammals. Taking into consideration that females are breeding alone and have more specific habitat requirements, female home range sizes should be taken as a reference to delineate conservation actions and landscape planning integrating the needs of European mink. Thus, any conservation plan aimed at the improvement or recovery of European mink populations through habitat management in areas similar to our study area should consider management blocks which recover natural vegetation typical of small lagoons and tributaries of at least 15 ha per each potential breeding female. Nevertheless, it should not be forgotten that adult males move by, on average, 73 ha (or 13 km considering the lineal distance) in small lagoon, small tributaries, and main rives, which should be also maintained in optimal vegetation condition for the species.
References
Animal Behaviour Society (2006) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 71:245–253
Arambarri R, Rodríguez AF, Belamendia G (1997) Selección de hábitat, mortalidad y nueva aportación a la distribución del visón europeo (Muestela lutreola) en Alava. Est Mus Cienc Alava 12:217–225
de Bellefroid MN (1999) Etude biogéographique de l’evolution de la population de vison européen, Mustela lutreola, en France. PhD thesis, Université de Rennes
Cabria MT, Gonzalez EG, Gomez-Moliner BJ, Michaux JR, Skumatov D, Kranz A, Fournier P, Palazon S, Zardoya R (2015) Patterns of genetic variation in the endangered European mink (Mustela lutreola L., 1761). BMC Evol Biol 15:141. https://doi.org/10.1186/s12862-015-0427-9
Ceña JC, Ceña A, Gómez MA, López de Luzuriaga J (2003a) Aspectos de ecología y composición de la población de visón europeo Mustela lutreola (Linnaeus, 1761) en la cuenca alta del río Ebro (España). International Conference on the Conservation of European mink (Mustela lutreola). 5-8 November, Logroño, Spain
Ceña JC, Ceña A, Gómez MA, López de Luzuriaga J, Rafart E (2003b) The European mink (Muestela lutreola) populations’s situation at the central and southeastern area of the iberian nucleus (2002-2003). International Conference on the Conservation of European mink (Mustela lutreola). 5-8 November, Logroño, Spain
Clavero M (2014) Shifting baselines and the conservation of non-native species. Conserv Biol 28:1434–1436
Daan S, Aschoff J (1982) Circadian contributions to survival. In: Aschoff J, Daan S, Groos GA (eds) Vertebrate circadian Systems: Structure and Physiology. Springer-Verlag, Berlin-Heidelberg, pp 305–321
Erlinge S, Sandell M (1986) Seasonal changes in the social organization of male stoats, Mustela erminea: an effect of shifts between two decisive resources. Oikos 47:57–62
Fournier P, Chusseau J-P, Dupuch J, Fournier-Chambrillon C, Maizeret C (2001) Radio-tracking del visón europeo y del turón: Radioemisores intraperitoneales pueden constituir una alternativa a las heridas causadas por los collares. In: V Jornadas de la Sociedad Española de Conservación y Estudio de Mamíferos, 5–8 de Diciembre de 2001, Vitoria-Gasteiz, Spain, pp 72–72
Fournier P, Maizeret C, Jimenez D, Chusseau JP, Aulagnier S, Spitz F (2007) Habitat utilization by sympatric European mink Mustela lutreola and polecat Mustela putorius in south-western France. Acta Theriol 52:1–12
Fournier P, Maizeret C, Fournier-Chambrillon C, Ilbert N, Aulagnier S, Spitz F (2008) Spatial behavior of European mink Mustela lutreola and polecat Mustela putorius in southwestern France. Acta Theriol 53:343–354
Fournier DA, Skaug HJ, Ancheta J et al (2012) AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Method Softw 27:233–249
Fournier-Chambrillon C, Chusseau JP, Dupuch J, Maizaret C, Fournier P (2003) Immobilization of free-ranging European mink (Mustela lutreola) and polecat (Mustela putorius) with medetomidine-ketamine and reversal by atipamezole. J Wildl Dis 39:393–399
Fournier-Chambrillon C, Bifolchi A, Mazzola-Rossi E, Sourice S, Albaret M, Bray Y, Ceña JC, Urra Maya F, Agraffel T, Fournier P (2010) Reliability of stained placental scar counts in farmed American mink and application to free-ranging mustelids. J Mammal 91:818–826
Fournier-Chambrillon C, Ceña JC, Urra F et al (In press) A 9-year demographic and health survey of an European mink population in Navarre (Spain): role of the canine distemper virus. In: Do Linh San E, Sato JJ, Belant JL, Somers MJ (eds) Small Carnivores in Space and Time: Evolution, Ecology, Behaviour and Conservation Wisley
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage, Thousand Oaks CA Available: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion
Garin I, Aihartza J, Zuberogoitia I, Zabala J (2002a) Activity pattern of European mink (Mustela lutreola) in southwestern Europe. Z Jagdwiss 48:102–106
Garin I, Zuberogoitia I, Zabala J, Aihartza J, Clevenger AP, Rallo A (2002b) Home ranges of European mink Mustela lutreola in southwestern Europe. Acta Theriol 47:55–62
Halle S, Stenseth NC (2000) Activity patterns in small mammals—an ecological approach. Springer, Berlin
Lodé T, Cormier JP, Le Jacques D (2001) Decline in endangered species as an indication of anthropic pressures: the case of European mink Mustela lutreola western population. Environ Manag. https://doi.org/10.1007/s002670010257
Maizaret, C, Migot, P, Galineau, H, Grisser, P, Lode T (1998) Répartition et habitats du Vison d’Europe (Mustela lutreola) en France. Arvicola, Actes “Amiens” 97:67–72
Maran T, Henttonen H (1995) Why is the European mink, Mustela lutreola, disappearing? – A review of the process and hypotheses. Ann Zool Fenn 32:47–54
Maran T, Skumatov D, Gómez A et al (2016) Mustela lutreola, European mink. The IUCN Red List of Threatened Species 2016. http://www.iucnredlist.org
Marcelli M, Fusillo R, Boitani L (2003) Sexual segregation in the activity patterns of European polecats (Mustela putorius). J Zool 261:249–255
Melero Y, Palazón S, Revilla E, Martelo J, Gosálbez J (2008) Space use and habitat preferences of the invasive American mink (Mustela vison) in a Mediterranean area. Eur J Wildl Res 54:609–617
Michaux JR, Libois R, Hardy OJ et al (2005) Conservation genetics and population history of the threatened European mink Mustela lutreola, with an emphasis on the west European population. Mol Ecol 14:2373–2388
Ollero-Ojeda A (2000) Crecidas fluviales en la cuenca del Ebro desde 1980: Estado de la cuestión, principales eventos y sistemas de prevención. Serie Geográfica 9:151–162
Palazón S (2010) Visón europeo. Mustela lutreola. In: Salvador A, Cassinello J (eds) Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales, Madrid http://www.vertebradosibericos.org/
Palazón S, Ruíz-Olmo J (1998) A preliminary study of behaviour of the European mink (Mustela lutreola), by means of radio-tracking. In: Dunstone N, Gorman ML (eds) Behaviour and ecology of riparian mammals. Cambridge University Press, Cambridge, pp 93–105
Palazón S, Ruiz-Olmo J, Gosálbez J (2004) Diet of European mink (Mustela lutreola) in the Iberian Peninsula. Mammalia 68:159–165
Palazón S, Ruiz-Olmo J, Gosálbez J (2008) Autumn-winter diet of three carnivores, European mink (Mustela lutreola), Eurasian otter (Lutra lutra) and small-spotted genet (Genetta genetta), in northern Spain. Anim Biodivers Conserv 31:37–43
Palomares F (1991) Situation of the European and American mink populations in the Iberian peninsula. Mustelid Viverrid Conserv 4:16
Peters E, Brinkmann I, Krüger F, Zwirlein S (2009) Reintroduction of the European mink Mustela lutreola in Saarland, Germany. Preliminary data on the use of space and activity as revealed by radio-tracking and live-trapping. Endanger Species Res 10:205–320
Rodgers AR, Carr AP, Beyer HL, Smith L, Kie JK (2007) HRT: home range tools for ArcGIS. Version 2.04. Ontario Ministry of Natural Resources, Centre for Northern Forest Ecosystem Research, Ontario
Sandell M (1989) Ecological energetics, optimal body size and sexual size dimorphismo: a modell applied to stoad, Mustela erminea L. Funct Ecol 3:315–324
Sidorovich V, Macdonald DW (2001) Density dynamics and changes in hábitat use by the European mink and other native mustelids in connection with the American mink expansion in Belarus. Neth J Zoology 51:107–126
Urra F, Román J (2013) Uso del alimento por el visón europeo en Navarra. In: II taller para la conservación del visón europeo en Navarra: dossier de trabajo, conclusiones y recomendaciones de gestión. Gestión Ambiental de Navarra, S.A., Navarra
Zabala J, Zuberogoitia I (2003a) Habitat use of male European mink (Mustela lutreola) during the activity period in south western Europe. Z Jagdwiss 49:77–81
Zabala J, Zuberogoitia I (2003b) Implications of territoriality in the spatial ecology of European mink Mustela lutreola. Biota 4:121–127
Zabala J, Zuberogoitia I, Garin I, Aihartza J (2003) Landscape features in the habitat selection of European mink (Mustela lutreola) in south-western Europe. J Zool Lond 260:415–421
Zabala J, Zuberogoitia I, Martínez-Climent J (2007) Spacing pattern, intersexual competition and niche segregation in American mink. Ann Zool Fennici 44:249–258
Zschille J, Stier N, Roth M (2010) Gender differences in activity patterns of American mink Neovison vison in Germany. Eur J Wildl Res 56:187–194
Zschille J, Stier N, Roth M (2012) Dynamic in space use of American mink (Neovison vison) in a fishpond area in Northern Germany. Eur J Wildl Res 58:955–968
Zuberogoitia I, Zabala J (2003) Does European mink use only rivers or do they also use other habitats? Small Carniv Conserv 28:7–8
Acknowledgements
This study was funded by the European Union projects LIFE TERRITORIO VISÓN (LIFE09/NAT/ES/53), GIRE Interreg II A program, and LIFE GERVE (LIFE05 NAT/E/000073), as well as by the Navarre Government. JVLB was supported by a “Juan de la Cierva” research contract (JCI-2012-13066) from the Spanish Ministry of Economy and Competitiveness. We thank M. Delibes for his advice with the study design and discussion and A. Ceña, U. Itoiz, I. Bidegain, G. Berasategui, and I. Alfaro for their help during the fieldwork and assistance with data management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palomares, F., López-Bao, J.V., Telletxea, G. et al. Activity and home range in a recently widespread European mink population in Western Europe. Eur J Wildl Res 63, 78 (2017). https://doi.org/10.1007/s10344-017-1135-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-017-1135-0