Abstract
This study evaluates the trophic position of adult Yellow-legged Gulls Larus michahellis atlantis resident in the Azores archipelago in the past (1921–1928) and in the present (2009–2010), and analyses the decadal variation in the diet of breeding birds from the 1990s to the 2000s for three main colonies (Topo Islet, Baixo Islet and Mistério da Prainha). Using mixing models, we compared stable isotope signatures of nitrogen and carbon in adult breast feathers between birds from 1921 to 1928 (held in museum collections) and 2009 to 2010, jointly with both isotopic signatures of their main prey groups (fish, goose barnacles (Lepas anatifera), seabirds, mammals and refuse). The diet of breeding birds was analysed using pellets collected in 1989, 1995, 1996, 2004, 2009 and 2010. Stable isotopes analysis (SIA) results were in accordance with the results provided from the analysis of pellets, showing a relatively recent and significant change in the diet of adult gulls. In particular, SIA revealed a significant decrease in the trophic position of Yellow-legged Gulls in the Azores, over the last 89 years in response to the decrease in the consumption of seabirds and fish and, an increase in the consumption of marine invertebrates (goose barnacles) and refuse. The analysis of pellets confirmed the significant decrease in the fish ingested, whereas the ingestion of lower trophic level prey (i.e. goose barnacles, mammals and refuse) increased. Both methods reflect the feeding plasticity and opportunistic foraging behavior of this species, and are in accordance with patterns described for continental Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The availability of food resources has been described as a major factor influencing reproductive output, survival, recruitment and, ultimately, population growth rates in most species (e.g. Sinclair and Krebs 2003; Rutz and Bijlsma 2006). Gulls are highly flexible and opportunistic feeders, and are also widespread seabird predators and competitors (Vidal et al. 1998; Finney et al. 2003; Ramos et al. 2011a). Refuse tips and fishing discards are important food resources for generalist gull species (Larus spp.), providing locally abundant and daily renewed food, which minimizes energy and foraging time (Garthe et al. 1996; Arcos et al. 2001). Therefore, the sharp increase in availability of food derived from human activities has been pointed out as an important cause for the rapid increase of gull populations (Spaans 1971; Oro et al. 1995; Duhem et al. 2008). A demographic explosion of several large gull species has occurred in Europe (Spaans et al. 1991), North America (Blokpoel and Scharf 1991) and Australia (Smith and Carlile 1993) over the past few decades. Owing to this strong demographic expansion, gulls became problematic to human populations due to the damage caused in airports, cities, reservoirs, arable lands and fisheries, to the transmission of diseases (Monaghan et al. 1985; Dolbeer et al. 1997; Ferns and Mudge 2000) and, also because gulls have been held responsible for affecting other bird species, usually under protection (Vidal et al. 1998; Oro et al. 2005; Matias and Catry 2010). In order to reduce the number of gulls, control programs have been implemented in several areas, especially in the largest gull colonies (e.g. Kress 1983; Blokpoel and Tessier 1992; Morais et al. 1998; Bosch et al. 2000).
The Yellow-legged Gull (Larus michahellis) has a breeding range that extends from the Azores archipelago (Neves et al. 2006) to the Aral Sea (Pérennou et al. 1996), and a wide trophic niche (Witt et al. 1981; Bosch et al. 1994). These opportunistic birds can broaden or narrow their trophic niche according to changes in the availability of their main food sources (Dolbeer 1990; Richards and Wilson 2000). In the Mediterranean region this gull species became superabundant from the 1960’s to the 1990’s (Yésou and Beaubrun 1995; Thibault et al. 1996) reaching ca. 120, 000 nesting pairs in the 1990’s (Pérennou et al. 1996), with an estimate rate of population increase by up to 10 % per year (Thibault et al. 1996; Vidal et al. 1998). Presently, the overall European population appears to be increasing, although the trend for some populations is unknown (Birdlife Internacional 2013). Several studies reported that the Yellow-legged Gull has changed its feeding habits and tends to feed today mostly on terrestrial prey and on human refuse, particularly in the areas where its populations have increased dramatically (Sol et al. 1995; Duhem et al. 2003; Soldatini et al. 2005). In the Azores archipelago, subtropical north-eastern Atlantic, the resident Yellow-legged Gull subspecies Larus michahellis atlantis has no direct competitors and relies more on fish prey than its counterparts in mainland Europe (Ramos et al. 1998; Neves et al. 2006). The population size of this species in the Azores increased almost 60 % between 1984 and 2004, corresponding to an average annual rate of 2.3 %. Apparently, this increase was mainly due to higher refuse availability (Neves et al. 2006), in line with the increase throughout Europe and despite the reduced human population of the archipelago (250,000).
The combination of conventional dietary analysis (e.g. direct sampling method) with stable isotope analysis is a powerful approach to infer diet and habitat selection (Inger and Bearhop 2008), providing an integrated overview of the assimilated diet (Ramos et al. 2009c). Measurements of the stable isotope ratios of carbon and nitrogen can provide trophic-level and space-use information of consumers in marine food webs (Hobson and Welch 1992; Forero et al. 2002; Hobson et al. 2002). The use of δ13C analyses can additionally provide information about feeding on inshore or benthic prey vs. offshore or pelagic prey (Hobson and Welch 1992; France 1995). In the present study, we aimed to: (1) evaluate the trophic position of the Yellow-legged Gull breeding in the central Island group of the Azores archipelago between 1921–1928 and 2009–2010, and investigate the relative contribution of the main prey groups to their diet in the past and in the present; (2) evaluate the decadal variation in the diet of breeding birds resident in three main colonies of the archipelago (Topo Islet – São Jorge Island, Baixo islet – Graciosa Island and Mistério da Prainha – Pico Island), according to the foraging habitat used; and (3) analyze the variation in the presence of garbage materials (digestible and indigestible) among colonies and years. To accomplish the first objective, nitrogen and carbon stable isotope signatures were obtained using adult breast feathers stored in museum collections (captured between 1921 and 1928, Monteiro 1996), from birds trapped in 2009 and 2010 at the same colonies of Museum specimens and from tissues of their main prey items. To answer objectives (2) and (3), the diet presented in previous studies for those three colonies (Hamer et al. 1994, Ramos et al. 1998 and Neves et al. 2006) was assembled with information obtained by the analyses of pellets collected during this study (in 2009 and 2010) in the same areas. In particular, we addressed the following questions: (1) Did the trophic position of the Yellow-legged Gull changed between 1921–1928 and 2009-10? (2) What were the most important prey groups in 1921–1928? (3) Did the occurrence of the main prey groups changed systematically along the last decade? (4) Was the occurrence of garbage in the diet different among years and colonies?
Material and methods
Study area
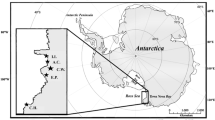
The archipelago of the Azores is located in the subtropical northeast Atlantic (36–39° N, 25–31° W) situated ca. 1,400 km west from the Portuguese mainland, and represents an ornithological transition between the tropical and temperate areas (Monteiro et al. 1996b). It comprises nine volcanic islands, with over 26 small islets (0.1–10 ha) and adjacent stacks (<1 km), forming three groups (western, central and eastern) along a tectonic zone running about 600 km WNW–ESE (Monteiro et al. 1996b; Fig. 1). The climate is subtropical and oceanic (Monteiro et al. 1996a) and the sea waters are of low productivity. However, the ocean circulation in the North Atlantic generates a north–south productivity gradient and the conjunction of equatorial and tropical waters transported by the Gulf Stream with colder northern waters creates frontal zones (Gould 1985; Pingree et al. 1999) where marine productivity is higher (Santos et al. 1995).
Agriculture and fishing are the main activities practised in the archipelago, having therefore an important socio-economic impact. The fleet is mainly artisanal, with 85 % of the fleet composed of small open or close-deck boats under 12 m long (INE 2007). These activities should influence the Yellow-legged Gulls diet due to their feeding plasticity and opportunistic behaviour. The access of gulls to urban waste deposited in open-air refuse dumps is other important aspect to have in consideration, since these places provide vast amounts of supplementary food throughout the year and relatively easy to obtain by gulls (Blockpoel and Spaans 1991; Bosh et al. 1994). In the central Islands group of Azores, there are several dump sites relatively close to our study colonies (Neves et al. 2006).
The present study was conducted on four of the main colonies of the Yellow-legged Gull in the Azores, located on the central island group of the archipelago (Fig. 1): (1) Baixo islet (off Graciosa Island), (2) Topo Islet (off São Jorge Island), (3) Mistério da Prainha (Pico Island) and (4) Capelinhos (Faial Island).
Sample collection
Between late April and early of May 2009 and 2010, a sample of six to ten feathers was collected from each of the 17 incubating Yellow-legged Gulls captured with nest traps on Mistério da Prainha. Sampling was performed conforming to current guidelines for use of wild birds in research (Lewis et al. 1988). We also used samples of three to four feathers from museum specimens collected at Capelinhos colony during the winter and the breeding seasons of 1921–1928 and held in the American Museum of Natural History. Such feathers were sampled from 17 specimens by Monteiro (1996).
Each tissue has a different turnover rate and therefore can reflect diets integrated over different timescales (Dalerum and Angerbjorn 2005; Bond and Jones 2009). As the Yellow-legged Gulls perform a partial pre-breeding moult (body feathers and some coverts) and a complete post-breeding moult (Arcos et al. 2002), the isotopic values from the feathers we used will reflect an annual average diet of adult gulls. Pico and Faial Islands are only 6 km apart, which is well within the range of movements by resident Yellow-legged Gull (authors, unpublished data) and Audouin’s gull (Larus audouinii; Christel et al. 2012). Moreover, we frequently observed Yellow-legged Gulls from Mistério da Prainha flying towards Faial Island. Therefore, as breast feathers represent a year round isotopic signature, it is appropriate to compare historical feathers from Capelinhos with contemporaneous feathers from Mistério da Prainha. Isotopic signatures were also obtained from samples of the main prey group of Yellow-legged Gulls in the Azores: fish (muscle of Capros aper and Trachurus picturatus), mammals (muscle from Rattus rattus), terrestrial invertebrates (three to six individual arthropods from Diplopoda and Isoptera) and refuse (a mixture of chicken, beef and pork meat), which were collected in the Azores archipelago. In addition, we used the isotopic signatures of soft tissue from goose barnacles Lepas anatifera collected in the Azores (Pajuelo et al. 2010), and from the first primary feathers of three seabird species, the Cory’s Shearwater Calonectris diomedea borealis, the Madeiran Strom-petrel Oceanodroma castro and the Macaronesian Shearwater Puffinus baroli baroli, also from the Azores (Roscales et al. 2011)
Decadal variation in the diet of adult gulls was studied using regurgitated pellets collected in three of the main breeding colonies: Topo Islet, Baixo islet and Mistério da Prainha. Data obtained previously by Hamer et al. (1994) in 1989, by Ramos et al. (1998) in 1995–1996 and by Neves et al. (2006) in 2004 were assembled with our data obtained for 2009–2010. Samples were thus collected in different years among the three colonies, with the exception of 2004 when all the colonies were sampled. All data were representative of the diet of breeding adults because: (a) Hamer et al. (1994) collected pellets from roosting areas used by adults birds, (b) Ramos et al. (1998) and Neves et al. (2006) collected pellets during the incubation and early hatching stages, and from rocky roosting areas used only by adults, and (c) in 2009–2010, pellets were randomly collected around the nests during the incubation and early hatching periods (April–May). Pellets were stored in individual plastic bags, labeled with the date of collection and colony name and frozen for later examination in the laboratory.
The prey items found were identified to the lowest possible taxonomic level and classified according to the following categories defined in the previous studies: fish, goose barnacles, squids, unidentified Crustacea, gastropod molluscs, birds, mammals, terrestrial invertebrates, vegetable matter, refuse and unidentified. Sagittae otoliths were identified using the reference collections of the Department of Oceanography and Fisheries (University of the Azores) and reference books (Nolf 1985; Cohen et al. 1990; Smale et al. 1995; Queró et al. 2003; Veen and Hoedmakers 2005). Data from all the studies comprised a total of 2,240 pellets (679 pellets from Topo Islet colony, 470 from Baixo islet colony and 1,091 from Mistério da Prainha colony). Vegetable matter was excluded from the analysis of diet variation because vegetables could be ingested accidentally when gulls feed on earthworms and other invertebrates (Noordhuis and Spaans 1992; Neves et al. 2006). Inorganic refuse material (e.g. plastic, paper) is also ingested unintentionally by gulls; however, it was not excluded from diet analysis because it is presented together with organic refuge material by previous studies in the Azores (Ramos et al. 1998, Neves et al. 2006). Diet composition is described by frequency of occurrence, calculated as the percentage of pellets containing a given prey type (e.g. fish, bird, etc.).
The main foraging habitats used were identified according to the frequencies of items and their presumed origin. Marine prey included fish, goose barnacles, squids, unidentified Crustacea and gastropods molluscs; terrestrial prey comprised birds, terrestrial invertebrates and mammals; refuse items corresponded to inorganic materials and to chicken and pork scraps, including eggshells. Despite refuse items may contain fish remains from human consumption (Duhem et al. 2003), in the Azores it is more likely that the fish come from the sea, thereby in our study all the fish remains found in the regurgitated pellets were considered marine prey.
Stable isotope analysis
Consumers are typically enriched in 15N relative to their diet, therefore 15δN values of consumers’ tissues can be used as reliable proxy of the trophic position (Post 2002; Caut et al. 2009). 13δC reflects the various dietary sources of the consumers’ tissue, being commonly used to discriminate among feeding habitats (e.g. marine versus terrestrial habitats) (Kelly 2000; Bearhop et al. 2003). As feather keratin is metabolically inert after synthesis, its isotopic composition reflects diet at the time feathers are grown (e.g. Mizutani et al. 1992). Therefore, isotopic analysis of historical feathers can be applied to study long-term variations in the isotopic signature of predators due to changes in the marine environment and thus their food sources (Newsone et al. 2007; Quillfeldt et al. 2010).
On the other hand, stable isotope analysis (SIA) of the main groups of prey will provide important information to calculate the relative contribution of each prey group to the diet of the adult gulls, during the 1921–1928 and 2009–2010 time periods, using isotopic mixing models (Schwarcz 1991). Such analysis will thus provide estimated proportions for small-sized and soft prey items, as well as for the larger and harder prey, which are usually underestimated and overestimated, respectively, by conventional diet analysis (Duffy and Jackson 1986; Hobson and Clark 1992).
At the laboratory, feathers were cleaned of surface contaminants and external lipid layer resulting from preening through vigorous washes with a sonicator (Sonimass S20) in triple baths of 0.25 M sodium hydroxide solution for 15 min each, alternated with triple baths of deionized water. Feathers were then cut into the smallest fragments possible, dried in an oven for 48 h at 50 °C to a constant mass and well mixed to homogenise the samples for isotopic analysis. Muscle samples were dried in an oven for two days, and then each sample was ground into a homogeneous powder and lipids were extracted using four repeated rinses (the first during 1 h and the others during 15 min) of 2:1 chloroform/methanol. Prey samples were dried in an oven for 48 h at 50 °C to remove residual solvent.
The procedures to perform SIA followed standard protocols reported in the literature (e.g. Bearhop et al. 2000; Valladares et al. 2010; Ceia et al. 2012). Briefly, stable carbon and nitrogen isotope assays were carried out on 0.40 ± 0.03 mg (range, 0.35–0.45 mg) sub-samples for gulls’ feathers and 0.34 ± 0.02 mg (range, 0.31–0.37) for prey groups loaded into tin cups. Relative abundance of stables isotopes of carbon (13C/12C) and nitrogen (15N/14N) were determined by continuous-flow isotope-ratio mass spectrometry (CF-IRMS). Analyses were conducted using a Euro EA 3024. Results are presented in the usual delta notation (δ) values in parts per thousand (in per mille) according to the following equation:
where X (in per mille) is 13C or 15N and R is the ratio of corresponding element (13C/12C or 15N/14N), in sample or standard. The standard values were Pee Dee Belemnite (PDB) for δ 13C, and atmospheric nitrogen (N2) for δ 15N.
Statistical analysis
Both δ13C and δ15N signatures were analysed in the framework of stable isotope mixing models. We adopted a Bayesian multisource stable isotope mixing model [Stable Isotope Analyses in R (SIAR); Parnell et al. 2010] using diverse function within the siar package from R 2.15 (R Development Core Team 2013). This allowed us to assess the gulls’ trophic position for 1921–1928 and 2009–2010 (using the isotopic signatures from historical and contemporaneous feathers) and to estimate the contribution (i.e. proportion) of each prey group to the diet of adult gulls (using the mean isotopic values and the respective standard deviation of each prey group source). Based on the literature, we applied mean enrichment factors of 3 and 1 ‰ to δ15N and δ13C values, respectively, between each group of prey and the breast feathers of adult gulls (Kelly 2000; Cherel et al. 2005a; Caut et al. 2009). A standard deviation of ±0.5 % was taken into account, considering potential differences in fractionation factors among species.
We used a multivariate ANOVA (MANOVA; Wilks’ lambda test) to evaluate the relation between the past and the present trophic ecology of gulls, using the overall carbon and nitrogen isotopic signatures for historical and contemporary feathers. A one-way ANOVA was used to compare both isotope signatures between historical and contemporary feathers.
Decadal variation in the adult gulls’ diet was analysed using chi-square tests, with Yates correction when df = 1. We applied the Bonferroni correction to these chi-square tests because multiple tests were performed with the same data.
Results
Temporal changes in carbon and nitrogen isotopic signatures
The stable isotope analysis showed that Yellow-legged Gulls during 1921–1928 and 2009–2010 were segregated by their overall isotopic signatures in feathers (MANOVA, Wilk’s lambda = 0.30, P < 0.001). Between 1921–1928 and 2009–2010, both δ13C (ANOVA, F 1,32 = 35.02, P < 0.001) and δ15N isotopic signatures (ANOVA, F 1,32 = 63.00, P < 0.001) decreased significantly (Fig. 2).The isotopic mixing model revealed that in the past (1921–1928) Yellow-legged Gulls apparently consumed a higher proportion of fish and seabirds, while at the present (2009–2010) they consumed a higher proportion of marine invertebrates, represented by goose barnacles, followed by seabirds, refuse and fish (Fig. 3).
Stable isotope plot of nitrogen–carbon showing the isotopic signatures of adult Yellow-legged Gull breast feathers for 1921–1928 (empty circles) and for 2009–2010 (empty triangles) and, of its main food prey (marine invertebrates represented by L. anatifera, mammals, seabirds, terrestrial invertebrates, refuse and fish; mean ± SD). Samples were collected on the Azores archipelago and the mean fractionation used for δ13C and δ15N were 1.0 and 3.0 ‰, respectively
Estimated contributions of each of the main prey groups (marine invertebrates, mammals, seabirds, terrestrial invertebrates, refuse and fish) to the diet of adult Yellow-legged Gulls resident on the Central Islands group of the Azores archipelago in 1921–1928 and in 2009–2010, calculated with SIAR (95, 75, and 50 % credibility intervals) using δ15N and δ13C values of adult growing feathers and of their main prey groups
Decadal variation in the diet of adult gulls
The present study assembles data from 2,240 pellets to evaluate decadal variation in the diet of adult Yellow-legged Gull in three of the main colonies of the Azores archipelago. The diet came from three different sources: the marine environment, the terrestrial environment and refuse dumps. The most frequent prey groups in the diet of gulls from the three colonies were fish, goose barnacles, birds, mammals and refuse tips (Table 1).
Fish was highly diverse with a total of 44 species identified from otoliths and vertebrae (see Appendix 1). Pellets from Mistério da Prainha in 2009 contained two fish species (Diaphus rafinesquei and Scomber japonicus) and one fish family (Argentinidae) that had not been found in the previous dietary studies of Yellow-legged Gulls in the Azores. Appendix 1 presents a list of all fish families and species found in the diet of this gull species (this study, Hamer et al. 1994, Ramos et al. 1998, Neves et al. 2006). The proportion of ingested fish decreased over a decade in the diet from all the study colonies, and this was significant for Topo Islet and for Mistério da Prainha (χ 2 = 400.50 and χ 2 = 108.30, respectively, df = 1, P < 0.001; Table 1). In contrast, goose barnacles increased significantly on the diet from these two colonies (Topo Islet, χ 2 = 60.79; Mistério da Prainha, χ 2 = 13.17, df = 1, P < 0.001). During 2004, goose barnacles were an important prey group on the Baixo islet and gastropod molluscs were important on the Topo Islet (Table 1). Moreover, in that year, birds were found for the first time in the diet of gulls from Topo Islet and Mistério da Prainha and were more frequent in the diet from Baixo islet during all the study years (Table 1). Birds increased significantly in the diet of Yellow-legged Gulls from the Baixo islet colony (χ 2 = 11.12, df = 1, P < 0.001). Similar to what happened with goose barnacles, mammals increased significantly in the diets from Topo islet and Mistério da Prainha colonies (χ 2 = 33.40 and χ 2 = 34.67, respectively df = 1, P < 0.001), representing the prey group with the higher increase in the diet from Mistério da Prainha (21.8 %). During the 2000s, terrestrial invertebrates were a very important prey to the gulls breeding on Topo and Baixo islets having a decadal increase of 31.4 and 19.4 %, respectively, which was significant for both colonies (χ 2 = 169.15 and χ 2 = 55.77, respectively, df = 1, P < 0.001). In addition, the analysis and quantification of garbage in the diet of gulls shows that: (1) it became part of the birds’ diet over a period of 15 years on Topo islet and over a period of 14 years on Mistério da Prainha (Table 1) and (2) on Baixo islet colony, despite its significant increase in the birds’ diet between 1995 and 2004 (χ 2 = 17.47, df = 1, P < 0.001), garbage declined significantly over the 15 years period (χ 2 = 5.59, df = 1, P = 0.0181).
In 2004, garbage ingestion accounted for 16.0 % on Topo islet and 24.0 % on Baixo islet (Table 1), which were not significantly different (χ 2 = 2.25, df = 1, P = 0.13) but were significantly lower than the 35.3 % on Mistério da Prainha for the same year (χ 2 = 21.52, df = 2, P < 0.001). During this year (2004), there were significant differences in the gulls’ diet among the three colonies: the proportion of fish ingested on Topo islet was lower when compared to that on Mistério da Prainha and Baixo islet (χ 2 = 52.68, df = 2, P < 0.001), the occurrence of goose barnacles was lower on the Mistério da Prainha (χ 2 = 43.68, df = 2, P < 0.001), while mammals occurred in a higher frequency on this last mentioned colony than on Topo islet (χ 2 = 11.21, df = 1, P < 0.001).
In terms of food sources, prey from both marine and terrestrial environments occurred in the diets of gulls from all the study colonies and in all years. The frequency of marine prey was higher during the 1990s or early 2000s for all colonies: at Topo islet in 1989 (97.3 %), Baixo islet in 2004 (82.3 %) and Mistério da Prainha in 1996 (94.8 %). The proportion of fish was the main contributor for the higher occurrence of marine food in the diets on Topo Islet and Mistério da Prainha; on the Baixo Islet the main contributors were the marine gastropods and, especially the goose barnacles (Table 1). Marine food declined significantly in the birds’ diet on Topo islet and Mistério da Prainha over the study time (χ 2 = 164.53 and χ 2 = 63.11, respectively, df = 1, P < 0.001). Terrestrial prey reached the higher proportion in the diets from Topo islet (47.4 %) and Mistério da Prainha (34.3 %) in 2004 and, from Baixo islet (71.0 %) in 2010. Such prey increased significantly in the diets of birds from the three colonies (Topo islet: χ 2 = 270.83; Baixo islet: χ 2 = 38.99; Mistério da Prainha: χ 2 = 55.15, for all df = 1 and P < 0.001; Table 1), essentially due to the rise in the consumption of terrestrial invertebrates on Topo islet, of terrestrial invertebrates, birds and mammals on Baixo islet, and of mammals on Mistério da Prainha (Table 1). All the significant differences found for prey items analysed remained significant after sequential Bonferroni correction.
Discussion
According to the stable isotope mixing model, in the past (1921–1928), the Yellow-legged Gull from the central Islands group of the Azores archipelago should have foraged more on seabirds and fish, and at the present (2009–2010), they should forage more on marine invertebrates (goose barnacles), refuse and also on seabirds and fish. Our study comprises a limitation due to the absence of past isotopic signatures of the main prey groups that were impossible to obtain. However, as the changes in the gulls’ isotopic signatures from 1921–1928 to 2009–2010 were very high, it is unlikely that those differences in prey isotopic signatures would change our results. Therefore, over the last 89 years, there was a significant depletion of nitrogen and carbon signatures indicating that gulls have been feeding on lower trophic level and on coastal/ terrestrial prey in recent years, respectively (see Fig. 2). Our mixing model suggests that seabirds should have been a very important component of the past diet of Yellow-legged Gull in the Azores, and presumably throughout the entire North Atlantic Ocean. During the winter, the sea conditions are often unfavorable for foraging gulls and our results suggest that seabirds are a good alternative prey to fish not only in the past but also in the present; two seabird species, Madeiran Storm petrel and Macaronesian Shearwater, are winter breeders in the Azores (Monteiro et al. 1996a). In a small colony of Yellow-legged Gulls (n = 12 breeding pairs) situated on the Selvagem Grande Island (North Atlantic) where fishery discards and refuse tips are unavailable, Yellow-legged Gulls feed mostly on seabirds, particularly on the endemic White-faced storm-petrel (Pelagodroma marina), which was present on 40.8 % of all pellets analysed by Matias and Catry (2010). In the Azores, historical population trends inferred from a comparison of sixteenth and seventeenth century chronicles with modern late twentieth century census indicate a drastic decline for most of the seabird species breeding regularly in the Azores (Monteiro et al. 1996a). Currently, these are species of European conservation concern and have an unfavorable conservation status (Tucker and Head 1994). On Baixo islet, birds were important in the diet of gulls, including small petrel species such as Madeiran Storm-petrel (Ramos et al. 1998), because this islet holds important colonies of small petrel species (Monteiro et al. 1996b).
The main pattern resulting from the stable isotope mixing model is in accordance with the results provided from the analysis of pellets, supporting the existence of a relatively recent and significant change in the diet of the Yellow-legged Gull in the Azores. In fact, the direct analysis of diet composition over a decade, from the 1990s to the 2000s showed that the proportion of ingested fish decreased significantly. In the Laurentian Great Lakes (Canada), the Herring Gull (Larus argentatus) presented also a significant decline in its trophic position over 25 years due to a temporal decrease in fish abundance (Hebert et al. 2008). The stable isotope mixing model suggests that seabirds and marine invertebrates (goose barnacles) should comprise a higher proportion of contemporaneous diet than that inferred from the pellet analysis. This difference should be attributed mainly to the fact that breast feathers reflect the diet throughout the year, while the pellets represent the diet over the time they were collected, i.e. the breeding season in this study. In addition, a higher ingestion of seabirds, particularly during winter, is a possible explanation for the difference between the two dietary methods in the proportion of birds consumed.
Fish and offal discarded by fishing vessels and the bait used in longline fisheries provide an important portion of food to the Yellow-legged Gull and to other seabird species (Cooper et al. 2003; González-Zevallos and Yorio 2006). The decline in the availability of seabirds (Monteiro et al. 1996a) and presumably of fish (at sea and/or discarded by fishing vessels) in the Azores could have induced a recent shift in the gulls’ diet towards alternative food sources from terrestrial habitats and refuse dumps. In relation to the fish prey, the landings of the total baitfish catch, which are the most likely to be taken by the Yellow-legged Gull, particularly Blue-jacked Mackerel (T. picturatus) have declined by about 30 % since the 1990s (Pham et al. 2013). Despite the fact that the total official catches of small pelagic and demersal fishes in the Azores did not show a marked decrease since 1950 (Pham et al. 2013), the strong decline in baitfish suggests that the total fish availability for gulls may have decreased. Further studies of the spatial and temporal variation in fish availability and feeding areas at a finer scale in relation to Yellow-legged Gull diet and breeding performance are necessary.
The population of Yellow-legged Gull increased 58.3 % on Mistério da Prainha, 41.7 % on Topo islet and 23.0 % on Baixo islet between 1984 and 2004 (Neves et al. 2006). These populations may still have increased gradually after 2004, contributing to increase the intra-specific competition for food at sea and, therefore, enlarging the number of gulls that use alternative food sources. According to our results, terrestrial prey occurred in larger percentages than garbage in the diet of gulls from these three colonies. These results are explained by the existence of plenty of terrestrial food in the Azores provided from agriculture habitats, where gulls can be observed feeding on rats, earthworms and insects (Ramos et al. 1998, pers. observations). In the Lagoon of Venice, Soldatini et al. (2005) also found a constant but not preponderant use of refuse tips as a food source by the Yellow-legged Gull. The accessibility of gulls to open-air refuse dumps is usually a determinant factor for the consumption of garbage (Duhem et al. 2003). In the Berlengas Island (western Portuguese coast), the foraging range of this bird species reached 100 km from its colony (Ceia, unpublished data), and our three study colonies have refuse dumps and/or landfills within this distance. Therefore, it is not surprising the presence of garbage and its decadal increase in the diet of Yellow-legged Gulls in the Azores.
Despite the human population resident in Graciosa, São Jorge and Pico Islands have decreased 15, 12 and 7 %, respectively, between 1991 and 2011 (SREA 2011), there was an increase in the amount of refuse produced by human in response to changes in consumption habits and to a massive increment of tourism in the archipelago (Neves et al. 2006), which should also explain such increase in refuse items (mainly chicken, beef and pork scraps, paper, plastic and glass) found in the pellets. Neves et al. (2006) showed that the percentage of pellets containing refuse was higher for gulls breeding closest to larger human populations. In some overpopulated colonies (e.g. western Mediterranean), refuse dumps are the main foraging habitat used by gulls, followed by terrestrial habitat, while marine habitat is the least frequent (Duhem et al. 2003). This suggests that even if not essential for population survival, food waste seems to be a useful supplements in the gulls’ diet and is likely to play a role in the growth of colonies. The drastic expansion of gull colonies may have a strong negative impact on sympatric seabird species, affecting survival, fecundity, foraging ecology and nesting habitat availability for many species such as terns Sterna spp. (Martínez-Abraín et al. 2003; Oro and Martínez 2007; Paracuellos and Nevado 2010) and also on vegetation structure (Ellis 2005). In the Azores, Neves et al. (2006) pointed out that Yellow-legged Gull at Topo and Baixo islets is probably limiting the distribution of terns because in 2004 these islets had none or only small numbers of breeding terns. Therefore, it is important to monitor the Azorean Yellow-legged Gull colonies, in order to detect demographic changes and control their population size.
Regular coverage of all the open-air refuse dumps in the Azores became mandatory since the early 2000s when the European Union legislation to urban waste management was applied, decreasing by this way the access of gulls to garbage. However, in some islands, the situation is still precarious, because there are some small refuse dumps that remain uncovered. The refuse dumps of the entire archipelago should be replaced by properly managed landfills. Culling breeding adults and destruction of clutches are two of the main control measures that have been implemented in several gull colonies (Harris and Wanless 1997; Martínez-Abraín et al. 2003; Oro and Martínez-Abraín 2007). On small gull colonies, some alternative solutions to prevent negative effects on other breeding seabird species such as terns have been tested, through the installation of exclosures and the removal of gull nests (Blockpoel et al. 1997). In conclusion, our study revealed the feeding plasticity and opportunistic foraging behavior of Yellow-legged Gull in the Azores, and that it is in accordance with patterns described for continental Europe.
References
Arcos JM, Oro D, Sol D (2001) Competition between the yellow-legged gull Larus cachinnans and Audouin’s gull Larus audouinii associated with commercial fishing vessels: the influence of season and fishing fleet. Mar Biol 139:807–816
Arcos JM, Ruiz X, Bearhop S, Furness RW (2002) Mercury levels in seabirds and their fish prey at the Ebro Delta (NW Mediterranean): the role of trawler discards as a source of contamination. Mar Ecol Prog Ser 232:281–290
Bearhop S, Phillips RA, Thompson DR, Waldron S, Furness RW (2000) Variability in mercury concentrations of Great Skuas Catharacta skua: the influence of colony diet and trophic status inferred from stable isotope signatures. Mar Ecol Prog Ser 195:261–268
Bearhop S, Furness RW, Hilton GM, Votier SC, Waldron S (2003) A forensic approach to understanding diet and habitat use from stable isotope analysis of (avian) claw material. Funct Ecol 17:270–275
BirdLife International (2013) Species factsheet: Larus michahellis. http://www.birdlife.org. Accessed 15 April 2013
Blokpoel H, Scharf WC (1991) The ring-billed gull in the great lakes of North America. In: Bell BP, Cossee RO, Flux JEC, Heather BD, Hitchmough RA, Robertson CJR, Williams MJ (eds) Acta 20 Congressus Internationalis Ornithologici, vol 4, New Zealand Ornithological Congress Trust Board. Wellington, New Zealand, pp 2372–2377
Blokpoel H, Spaans AL (1991) Introductory remarks: superabundance in gulls: causes, problems and solutions. In: Bell BP, Cossee RO, Flux JEC, Heather BD, Hitchmough RA, Robertson CJR, Williams MJ (eds) Acta 20 Congressus Internationalis Ornithologici, vol 4, New Zealand Ornithological Congress Trust Board. Wellington, New Zealand, pp 2359–2361
Blokpoel H, Tessier GD (1992) Control of ring-billed gulls and herring gulls nesting at urban and industrial sites in Ontario, 1987–1990. Proceedings Eastern Wildlife Damage Control Conference 5:51–57
Blokpoel H, Tessier GD, Andress RA (1997) Successfull restoration of the Ice Island common tern colony requires on-going control of ring-billed gulls. Colon Waterbirds 20:98–101
Bond A, Jones IL (2009) A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar Ornith 37:183–188
Bosch M, Oro D, Ruiz X (1994) Dependence of Yellow-legged Gull Larus cachinnans on food from human activity in two western Mediterranean colonies. Avocetta 18:135–139
Bosch M, Oro D, Cantos FJ, Zabala M (2000) Short-term effects of culling on the ecology and population dynamics of the yellow-legged gull. J Appl Ecol 37:369–385
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Ceia FR, Phillips RA, Ramos JA et al (2012) Short- and long-term consistency in the foraging niche of wandering albatrosses. Mar Biol 159:1581–1591. doi:10.1007/s00227-012-1946-1
Cherel Y, Hobson KA, Hassani S (2005) Isotopic discrimination between food and blood and feathers of captive penguins: Implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115
Christel I, Navarro J, del Castillo M, Cama A, Ferrer X (2012) Foraging movements of Audouin’s gull (Larus audouinii) in the Ebro Delta, NW Mediterranean: a preliminar satellite-tracking study. Estuar Coast Shelf S 96:257–261
Cohen DM, Inada T, Iwamoto T, Scialabba N (1990) Gadiform fishes of the world (order Gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date. FAO Fisheries Synopsis, no. 125, vol. 10. FAO, Rome, p 442
Cooper J, Baccetti N, Belda EJ et al (2003) Seabird mortality from longline fishing in the Mediterranean Sea and Macaronesian waters: a review and a way forward. Sci Mar 67(Supplement 2):57–64
Dalerum F, Angerbjorn A (2005) Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia 144:647–658. doi:10.1007/s00442-005-0118-0
Development Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Dolbeer RA (1990) Ornithology and integrated pest management: red-winged Blackbirds Agelaius phoeniceus and corn. Ibis 132:309–322
Dolbeer RA, Belant JL, Bernhardt GE (1997) Aerial photography techniques to estimate populations of Laughing Gull nests in Jamaica Bay, New York, 1992–1995. Col Wat 20:8–13
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colon Waterbirds 9:1–17
Duhem C, Vidal E, Legrand J, Tatoni T (2003) Opportunistic feeding responses of the Yellow-legged Gull Larus michahellis to accessibility of refuse dumps. Bird Study 50:61–67
Duhem C, Roche P, Vidal E, Tatoni T (2008) Effects of anthropogenic food resources on yellow-legged gull colony size on Mediterranean islands. Pop Ecol 50:91–100
Ellis JC (2005) Marine birds on land: a review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecol 181:227–241
Ferns PN, Mudge GP (2000) Abundance, diet and Salmonella contamination of gulls feeding at sewage outfalls. Water Res 34:2653–2660
Finney SK, Harris MP, Keller LF et al (2003) Reducing the density of breeding gulls influences the pattern of recruitment of immature Atlantic puffins Fratercula arctica to a breeding colony. Biol Conserv 40:545–552
Forero MG, Hobson KA, Bortolotti GR et al (2002) Food resource utilization by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299
France RL (1995) Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar Ecol Prog Series 124:307–312
Garthe S, Camphuysen CJ, Furness RW (1996) Amounts of discards by commercial fisheries and their significance as food for seabirds in the North Sea. Mar Ecol Prog Ser 136:1–11
González-Zevallos D, Yorio P (2006) Seabird use of discards and incidental captures at the Argentine hake trawl fishery in the Golfo San Jorge, Argentina. Mar Ecol Prog Ser 316:175–183
Gould WJ (1985) Physical Oceanography of the Azores Front. Progr Oceanogr 14:167–190
Hamer KC, Thompson DR, Rudle AJ, Lewis SA, Stewart FM (1994) Mesopelagic fish eaten by Yellow-legged Herring Gulls Larus argentatus atlantis in the Azores. Seabird 16:30–33
Harris MP, Wanless S (1997) The effect of removing large numbers of gulls Larus spp. on an island population of oystercatchers Haematopus ostralegus: implications for management. Biol Conserv 82:167–171
Hebert CE, Weseloh DVC, Idrissi A et al (2008) Restoring piscivorous fish populations in the Laurentian Great Lakes causes seabird dietary change. Ecology 89:891–897
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94:189–197
Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Mar Ecol Prog Ser 84:9–18
Hobson KA, Fisk A, Karnovsky N et al (2002) A stable isotope (δ13C, δ15N) model for the North Water food web: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep-Sea Res PT II 49:5131–5150
INE (2007) Estatísticas da Pesca 2006. Instituto Nacional de Estatística. Direcção-Geral das Pescas e Aquicultura, Portugal
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Kress S (1983) The use of decoys, sound recordings and gull control for re-establishing a tern colony in Maine. Colonial Waterbirds 6:185–196
Lewis WO, Able KP, Anderson DW et al (1988) Guidelines for the use of wild birds in research. Auk 105(Suppl):1–41
Martínez-Abraín A, Gonzalez-Solís J, Pedrocchi V et al. (2003) Predation, kleptoparasitism and disturbances of yellow-legged gull on Audouin’s gull in three western Mediterranean colonies. In: Mínguez E, Oro D, De Juana E, Martínez-Abraín A (eds) Mediterranean seabirds and their conservation. Sci Mar 67:89–94
Matias R, Catry P (2010) The diet of Atlantic Yellow-legged Gulls (Larus michahellis atlantis) at an oceanic seabird colony: estimating predatory impact upon breeding petrels. Eur J Wildl Res 56:861–869. doi:10.1007/s10344-010-0384-y
Mizutani H, Fukuda M, Kabaya Y (1992) δ13C and δ15N enrichment factors of feathers of adult birds. Ecology 73:1391–1395
Monaghan P, Shedden CB, Ensor K, Fricker CR, Girdwood RWA (1985) Salmonella carriage by herring gulls in the Clyde area of Scotland in relation to their feeding ecology. J Appl Ecol 22:669–680
Monteiro LR (1996) Seabirds as monitors of mercury contamination in the Portuguese Atlantic. PhD Thesis, University of Glasgow
Monteiro LR, Ramos JA, Furness RW (1996a) Past and present status and conservation of the seabirds breeding in the Azores Archipelago. Biol Conserv 78:319–328
Monteiro LR, Ramos JA, Furness RW, del Nevo AJ (1996b) Movements, morphology, breeding, molt, diet and feeding of seabirds in the Azores. Colon Waterbird 19:82–97
Morais L, Santos C, Vicente L (1998) Population increase of yellow-legged gulls Larus cachinnans breeding on Berlenga Island (Portugal), 1974–1994. Sula 12:27–37
Nelson JS (1984) Fishes of the world, 2nd edn. Wiley-Interscience, New York, p 523
Neves VC, Murdoch N, Furness RW (2006) Population status and diet of the Yellow-legged Gull in the Azores. Arquipélago. Life and Marine Sciences 23A:59–73
Newsome SD, Martínez del Rio C, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Evol 5:429–436
Nolf D (1985) Otolithi piscium. In: Schultz HP (ed) Handbook of Paleoichthyology, vol 10. Fisher, Stuttgart, p 145
Noordhuis R, Spaans AL (1992) Interspecific competition for food between Herring Gull Larus argentatus and Lesser Black-backed Gull L fuscus in the Dutch Wadden Sea area. Ardea 80:115–132
Oro D, Martínez-Abraín A (2007) Deconstructing myths on large gulls and their impact on threatened sympatric waterbirds. Anim Conserv 10:117–126
Oro D, Bosch M, Ruiz X (1995) Effects of a trawling moratorium on the breeding success of the yellow-legged gull Larus cachinnans. Ibis 137:547–549
Oro D, de León A, Mínguez E, Furness RW (2005) Estimating predation on breeding European storm-petrels by Yellow-legged Gulls. J Zool (Lond) 265:421–429
Pajuelo M, Bjorndal KA, Alfaro-Shigueto J et al (2010) Stable isotope variation in loggerhead turtles reveals Pacific–Atlantic oceanographic differences. Mar Ecol Prog Ser 417:277–285
Paracuellos M, Nevado JC (2010) Culling Yellow-legged Gulls Larus michahellis benefits Audouin’s Gulls Larus audouinii at a small and remote colony. Bird Study 57:26–30
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS One 5:e9672
Pérennou C, Sadoul N, Pineau O, Johnson A, Hafner H (1996) Gestion des sites de nidification des oiseaux d’eaux coloniaux. Tour du Valat, Arles
Pham CK, Canha A, Diogo H et al (2013) Total marine fishery catch for the Azores (1950–2010) ICES. J Mar Sci 70(3):564–577. doi:10.1093/icesjms/fst024
Pingree RD, Garcia-Soto C, Sinha B (1999) Position and structure of the subtropical/Azores Front region from combined Lagrangian and remote sensing (IR/altimeter/SeaWiFS) measurements. J Mar Biol Assoc UK 79:769–792
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Queró Jean-Claude, Porehe P, Vayne Jean-Jacques (2003) Guide des poissons de l’Atlantique européen. Delachaux et Niestlé S.A., pp 465
Quillfeldt P, Masello JF, McGill RAR, Adams M, Furness R (2010) Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front Zool 7:15
Ramos JA, Sola E, Porteiro FM, Monteiro LR (1998) Prey of Yellow-legged Gull, Roseate Tern and Common Tern in the Azores. Seabird 20:31–40
Ramos R, Ramírez F, Sanpera C, Jover L, Ruiz X (2009) Feeding ecology of yellow-legged gulls Larus michahellis in the western Mediterranean: a comparative assessment using conventional and isotopic methods. Mar Ecol Prog Ser 377:289–297
Ramos R, Ramírez F, Carrasco JL, Jover L (2011) Insights into the spatiotemporal component of feeding ecology: an isotopic approach for conservation management sciences. Divers Distrib 17:338–349
Richards SA, Wilson WG (2000) Adaptive feeding across environmental gradients and its effects on population dynamics. Theor Popul Biol 57:377–390
Roscales JL, Gómez-Diaz E, Neves V, González-Solíz J (2011) Trophic versus geographic structure in stable signatures of pelagic seabirds breeding in the northeast Atlantic. Mar Ecol Prog Ser 434:1–13. doi:10.3354/meps09211
Rutz C, Bijlsma RG (2006) Food-limitation in a generalist predator. P Roy Soc B-Biol Sci 273:2069–2073
Santos RS, Hawkins S, Monteiro LR, Alves M, Isidro EJ (1995) Marine research, resources and conservation in the Azores. Aquat Conserv 5:311–354
Schwarcz HP (1991) Some theoretical aspects of isotope paleodiet studies. J Archaeol Sci 18:261–275
Sinclair ARE, Krebs CJ (2003) Complex numerical responses to top-down and bottom-up processes in vertebrate populations. In: Sibly RM, Hone J, Clutton-Brock T (eds) Wildlife population growth rates. Cambridge University Press, Cambridge
Smale MJ, Watson G, Hecht T (1995) Otolith atlas of Southern African marine fishes. Ichthyological Monographs, vol 1. JLB Smith Institute of Ichthyology, Grahamstown
Smith GC, Carlile N (1993) Methods for population control within a silver gull colony. Wildl Res 20:219–226
Sol D, Arcos JM, Senar JC (1995) The influence of refuse tips on the winter distribution of Yellow-legged Gulls Larus cachinnans. Bird Study 42:216–221
Soldatini C, Riccato F, Torricelli P, Mainardi D (2005) Yellow legged gulls’ diet and foraging locations. XV Congresso della Società Italiana di. Ecologia, Torino
Spaans AL (1971) On the feeding ecology of the Herring Gull Larus argentatus Pont. in the northern part of the Netherlands. Ardea 59:73–188
Spaans AL, Coulson JC, Migot P et al (1991) The herring gull in north-west Europe. In: Bell BP, Cossee RO, Flux JEC, Heather BD, Hitchmough RA, Robertson CJR, Williams MJ (eds) Acta 20 Congressus Internationalis Ornithologici, vol 4, New Zealand Ornithological Congress Trust Board. Wellington, New Zealand, pp 2365–2371
SREA (2011) Estimativa da população residente em 1991 e 2011. http://estatistica.azores.gov.pt. Accessed 26 March 2013
Thibault J-C, Zotier R, Guyot I, Bretagnolle V (1996) Recent trends in breeding marine birds of the Mediteranean region with special reference to Corsica. Colon Waterbird 19:31–40
Tucker GM, Heath MF (1994) Birds in Europe: their conservation status. BirdLife International, Cambridge
Valladares S, Moreno R, Jover L, Sanpera C (2010) Evaluating cleansing effects on trace elements and stable isotope values in feathers of oiled birds. Ecotoxycology 19:223–227
Veen J, Hoedmakers K (2005). Synopsis iconographique des otoliths de quelques espèces de poisons des côtes ouest africaines. Wetlands International, pp 40
Vidal E, Médail F, Tatoni T (1998) Is the yellow-legged gull a superabundant bird species in the Mediterranean? Impact on fauna and flora, conservation measures and research priorities. Biodivers Conserv 7:1013–1026
Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (1984) Fishes of the North-eastern Atlantic and the Mediterranean (FNAM), vol 1. UNESCO, Paris, p 510
Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (1986) Fishes of the North-eastern Atlantic and the Mediterranean, vols 1 and 3. UNESCO, Paris, p 1473
Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (1989) Fishes of the North-eastern Atlantic and the Mediterranean, vol 2. UNESCO, Paris, p 1473
Witt H-H, Crespo J, de Juana E, Varela J (1981) Comparative feeding ecology of Audouin’s Gull Larus audouinii and the Herring gull L. argentatus in the Mediterranean. Ibis 123:519–526
Yésou P, Beaubrun PC (1995) Le goéland leucophée Larus cachinnans. In: Yeatman-Berthelot D, Jarry G (eds) Nouvel atlas des oiseaux nicheurs de France 1985–1989. S.O.F, Paris, pp 328–329
Acknowledgments
We acknowledge the support given by Fundação para a Ciência e Tecnologia, Portugal, to Patricia Pedro (SFRH/BD/40095/2007). We are grateful to the Department of Oceanography and Fisheries (DOP, Azores University), especially to Ricardo Serrão Santos, for the authorisation to use the feathers from museum specimens obtained by Luís Monteiro and for logistical support. We thank the Secretaria Regional do Ambiente e do Mar (SRAM) for issuing permits for fieldwork and sample collection. For assistance with sample collection we thank Justin Hart, Maria Magalhães, Hugo Diogo, Lídia Silva, Lune Poeki, Henri Pegeot, Paola Visicchio and Fausto André.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Pedro, P.I., Ramos, J.A., Neves, V.C. et al. Past and present trophic position and decadal changes in diet of Yellow-legged Gull in the Azores Archipelago, NE Atlantic. Eur J Wildl Res 59, 833–845 (2013). https://doi.org/10.1007/s10344-013-0737-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-013-0737-4