Abstract
The American mink (Neovison vison) is responsible for the widespread decline of its prey species in the regions where it is an invasive species. The current expansion of the mink in the Iberian Peninsula has aroused concern among conservationists about its negative impact on the rich native fauna. However, evidence for this is still scarce, although there are several studies establishing a direct causal relationship between declining native species and the presence of the American mink. Thus, it is important to further investigate the responses of native species to the American mink in several habitats and locations to enhance our knowledge about the patterns of the effect of the mink in Spain, as well as to inform conservation actions. A field study of the impact of the American mink on a mountainous vertebrate community in central Spain is presented. We studied six species: two fish, one amphibian, one bird, and two mammals. The general results showed a species-specific sensitivity to mink presence, with the Mediterranean water shrew (Neomys anomalus) and the southern water vole (Arvicola sapidus) being the most affected because their ranges were significantly decreased after the introduction of the mink. Regarding the other species, neither their abundance nor range was apparently affected by the American mink. The predatory behavior of the mink and interactions with other carnivores could account for these results. These data aid in shedding light about the current impact of the mink on invaded areas of the Iberian Peninsula and highlight the variability of its effects, as well as the urgent need to establish a general program of control of the mink to avoid negative effects upon native prey communities. Furthermore, given the different responses of native species, we propose that measures to protect native species should be based on species-specific goals and attributes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The American mink (Neovison vison) is one of the most widespread invasive mammals, particularly in Europe (Dunstone 1993; Macdonald and Harrington 2003; Bonesi and Palazón 2007). It has been frequently claimed to be a critical threat to endemic and autochthonous vertebrates (Macdonald and Harrington 2003; Bonesi and Palazón 2007; Genovesi et al. 2012), both through consumptive and nonconsumptive effects (Lima 2002; Sih et al. 2010). Evidence from a number of studies on the impact of mink on invaded communities shows a quite different response of each species to its presence (Craik 1997; Ferreras and Macdonald 1999; Macdonald and Strachan 1999; Sidorovich and Macdonald 2001; Nordström et al. 2003; Ahola et al. 2006; Peris et al. 2009; Fey et al. 2010; Melero et al. 2012; Zuberogoitia et al. 2013). For example, some species were highly affected by the presence of the mink, while others were not, leading to a debate about the cause of such species-specific responses (Cox and Lima 2006; Fey et al. 2010; Sih et al. 2010).
The impact of American mink in central Spain has been the subject of various reports, sometimes published in the media, giving rise to social alarm, as in the UK (Dunstone 1993). A typical example comes from the relationship between fishermen, environmental managers, and the mink. The first group believes that the mink is the main cause of the decline in some recreational freshwater fish, for instance, the brown trout (Salmo trutta) or the rainbow trout (Oncorhynchus mykiss), leading to demands for control measures.
There is also general concern among scientific and environmental managers about the negative pressure of the mink on a variety of species in Spain (Delibes et al. 2004; Palomo et al. 2007; Melero et al. 2012; Zuberogoitia et al. 2013). A recent work by Melero et al. (2012) indicated that the American mink was impacting on native communities in Mediterranean Spain, with species-specific effects being rather variable. It is, therefore, crucial to achieve a robust empirical test of the mink problem in other areas of Spain to gain further and complementary insights into the problems caused by the mink on other species and habitats in Spain (Pullin et al. 2004; Sutherland et al. 2004).
The current policy of managing American mink in Spain consists usually of trapping campaigns that lack any spatial or temporal continuity (except in the areas inhabited by European mink and in Catalonia; Melero et al. 2010; Zuberogoitia et al. 2010) without a well-defined management objective. Management strategies involving mink are expected to improve under better scientific knowledge (Pullin et al. 2004).
Therefore, the aim of this work was to contribute to the knowledge of the short-term effects of the colonization and establishment of the American mink on the susceptible autochthonous fauna of a Mediterranean mountain assemblage.
Material and methods
Study area
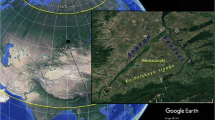
The study was carried out in 2006 and 2010 in the Sierra de Francia, a mountain range in the province of Salamanca, central Spain (geographical coordinates of the central study area are −6°9′59.20″ N 40°29′56.00″ W; Fig. 1). The area is in the western range of the Sistema Central, with a medium to low altitude (maximum, about 1,735 m; mean, 1,200 m), in a zone of transition between Mediterranean and Atlantic climates. Winters are cold (mean temperature in December, 6 °C) and rainy (mean rainfall in December, 160 mm), while summers are hot (23 °C in August) and dry (15 mm in August; Hijmans et al. 2005), resulting in Atlantic oak forests in the northern slopes (mainly composed by the oak Quercus pyrenaica) and Mediterranean-like forests in the southern ones (dominated by Quercus ilex and a dense layer of scrubs like Arbutus unedo). Above 1,300 m, there are typical high mountain vegetation, scrubland of Cytisus oromediterraneus, and some pasture lands dominated by Nardus stricta.
Broad surfaces of the mountains were transformed to allow wood exploitation, mainly monospecific pine plantations (widely Pinus nigra). All the river and stream sections sampled during the fieldwork shared common vegetation and ecological dynamics: narrow (>4 m), steep stretches of fast-flowing mountainous rivers scarcely or not altered at all by human disturbance (i.e., almost pristine rivers with scarce exceptions). The bed and the banks are mainly composed of boulders and rocks, with sedimentation being rare, although there are well-developed riparian forests at the banks (composed principally of black alders, Alnus glutinosa, and brooms, Cytisus spp.).
Study design
It is not ethically acceptable to introduce mink deliberately to study their impact. Hence, we planned a field study with similar characteristics and scope of a researcher-manipulated experiment (Krebs 1999) to gain similar insights into the dynamics of the system under scrutiny. In our case, mink began to colonize the study area in 2007 and we had good information about the composition of the native vertebrate community in 2006 obtained by our own fieldwork. We designed a before–after control–impact (BACI)-based study to test the effects of the mink on this community (Smith et al. 1993; McDonald et al. 2000).
The study areas were a 32-km-long stretch of the rivers Francia and Alagón successfully colonized by the mink (impact sites) and a control area including several rivers and streams where the mink was not already present and which was relatively isolated from the rivers Francia and Alagón by the Sierra de Francia mountain range (Fig. 1).
Surveys were performed over 400-m-length sections in these rivers and streams, separated from each other by at least 500 m to ensure independence. The distance covered in each section (400 m) was chosen as a good distance to adequately survey all the species researched. Twenty-six transects were made in the control area and 27 transects in the impact (mink presence) area (Fig. 1). Altogether, we sampled 53 sections (21.2 km). The fieldwork was undertaken in July–August 2006 and repeated in July–August 2010 (3 years after the establishment of the mink). The methods employed were the same in 2006 and in 2010. As previously stated, control and impact sections shared similar ecological attributes, and therefore, there were no apparent differences between impact and control sites. Environmental conditions did not change between 2006 and 2010.
Hair traps baited with fresh chicken wings were used to accurately estimate the distribution of the American mink (González-Esteban et al. 2006; Pauli et al. 2008; García and Mateos 2009; Mullins et al. 2010) and thus ensure that mink were present only at the impact sites and absent from control ones. During the two study periods (2006 and 2010), we deployed two hair traps per sampling section, which remained active for collecting hairs over six consecutive nights to ensure mink detection (Lynch et al. 2006; García and Mateos 2009; Mullins et al. 2010). After that, hair traps were collected and the hairs removed and identified using light microscopy (Teerink 1991; Toth 2002; González-Esteban et al. 2006). If there are mink hairs, we concluded that the species was present, and if not, that the species was absent. Further details about the hair trap design and procedures can be found elsewhere (González-Esteban et al. 2006; García and Mateos 2009).
We initially aimed to test changes in the whole native vertebrate community; however, we focused on six species at the end (two fish, one amphibian, one bird, and two small mammals) for which we had enough data (i.e., sufficient number of captures to confidently estimate abundance) to check the effects of the mink. For instance, we know the occurrence of the Iberian desman (Galemys pyrenaicus) in the impact area but not in the control area (García-Díaz 2012), so it would be impossible to study the impact of the mink. However, in the case of fish, the two species studied were the only ones present in up to 95 % of both the control and impact sites. All the species studied have been found in the scats of American mink collected in the area (own data).
The two fish were the brown trout (S. trutta) and the chub (Squalius spp.; in the study area, there were two species of chub Squalius carolitertii and Squalius alburnoides, but these were very difficult to distinguish in hand). Fish were surveyed using commercial nonbaited wire mesh net traps (from Trap Man UK; http://www.trapman.co.uk) placed in narrow locations of the rivers and streams, so the fish present in the sections were required to enter the traps when moving along the bed. Traps were deployed at regular intervals of 80 m, covering the total 400-m distance of the sampling sections (there were five traps per section). The traps were active for 24 consecutive hours and were checked every 3 h. Fish abundance was measured as the number of fish captured per unit effort (no. of fish/no. of traps/hours of effort).
The only amphibian included in the study was the Iberian brown frog (Rana iberica), whose population was estimated by counting the number of individuals found along the 400-m surveying sections. The researchers walked slowly along the sections (~200 m/h), recording all the individuals detected. Frog counts were always carried out at midday (1100–1300 hours GMT).
One bird (the dipper, Cinclus cinclus) and two mammalian species (the Mediterranean water shrew, Neomys anomalus, and the southern water vole, Arvicola sapidus) were also studied, taking data only on the occurrence (i.e., the proportion of sites occupied over all the sections sampled; Mackenzie et al. 2006) of the three species along each sampling section.
Small mammalian species were detected by intensive searching for scats (Queiroz et al. 1998; Fedriani et al. 2002). Scat sampling consists of looking for species’ scats carefully, searching it in the bed and the banks of the sampling sections. Scats can also be found in holes or among rocks, so the researchers carried a portable torch to examine these sites. The total length of the sampling sections (400 m) was usually surveyed within 2 h. All the scats found were collected and identified in the laboratory to the species level by dissecting them under a binocular microscope and identifying the hairs found with a light microscope (Teerink 1991; De Marinis and Agnelli 1993). Scat surveys were repeated at least three times per study period (2006 and 2010) to avoid false absences (Mackenzie et al. 2006; García-Díaz 2012).
The time between the arrival of the mink and the fieldwork to determine the status of its influence on native species was only 3 years (2007 to 2010). Three years exceed or equal the mean life expectancy of the potentially affected species studied, so we were confident that the effects of the mink were tested on more than one generation of their prey (Churchfield 1990; Esteban and Sanchiz 2000; Belica 2007; Román 2007). Indeed, Ahola et al. (2006) investigated the trends of amphibian populations after 3 to 4 years of mink removal. Moreover, in our study, the two small mammalian species did not undergo cyclical population changes (Churchfield 1990; Román 2007).
Therefore, our data are not flawed because these life history traits and seems to adequately reflect the short-term changes in the populations due to the presence of the mink. However, delayed effects of factors affecting population dynamics of the species under study, such as weather, parasites, food limitation, and even predation by mink, can emerge after long time spans (Rockwood 2006). Hence, our work only reflects the short-term impact of the American mink.
Statistical analysis
Our data came from different sources and sampling designs and did not meet the assumptions of classical ANOVA-based BACI designs (Smith et al. 1993). Thus, we used two different statistical generalized linear models (GLM). In all the statistical models, we tested the before–after and control–impact effects, as well as its potential interaction (BA × CI).
In the case of birds and mammals, we only had data about occurrence. Given the type of data (occurrence in a BACI framework), we used a log-linear analysis of 2 × 2 contingency tables. It should be emphasized that, in this kind of analysis, a nonsignificant value indicates good performance of the model in replicating the 2 × 2 table. This means that if, for example, the occurrence of the water shrew depends on the before–after factor, then the log-linear significance should be p > 0.05 rather than p < 0.05. The statistical test used in the log-linear analysis was the maximum likelihood χ 2, which also evaluates the adjustment of the model.
For the other species, the abundance data were also skewed, with many zeros. Due to this, we employed a hurdle model (Potts and Elith 2006; Zeileis et al. 2008), which consists of merging two GLM, a Poisson log analysis for nonzero values and a logistic regression for modeling zeros. Thus, it was possible to control through the same statistical approach the origin of variations in abundance and the source of variability in zero values (Potts and Elith 2006; Zeileis et al. 2008
All the analyses were performed using the R statistical software (R Development Core Team 2010), particularly with the pscl package (http://cran.r-project.org/web/packages/pscl/index.html; Zeileis et al. 2008).
Results
The results of the analysis for the two species of fish and frog are summarized in Table 1. The fitted hurdle models showed statistically significant effects of the BA × CI interaction on the presence/absence and abundance of both chub and brown trout in the study area (p < 0.05 in the logit and Poisson models for these species; Fig. 2) but not when considering BA factor alone. In the other studied species, the Iberian brown frog, there were no statistically significant differences between control and impact areas or between before and after time (Table 1; Fig. 2), which indicates that there were no detectable impacts of the mink. Nonetheless, the depiction of abundance estimates for frogs in Fig. 2 suggests that frogs may have declined in the impact areas but not in the control ones.
Regarding the effects on the occurrence of birds and mammals, the best fitted log-linear models (those with higher p values; Table 2) indicated a reduction in the range occupied by the two small mammals (the water vole and the water shrew; Table 2) but not the dipper (Table 2). The water shrew did not show any trend in control areas (occupancy before, 65.4 %; occupancy after, 61.5 %) but disappeared from 33.4 % of the sections after the arrival of the mink in the impact area (occupancy before, 66.7 %; occupancy after, 33.3 %). Meanwhile, the trend in the range of the water vole was even more acute: no trends in control sites (occupancy before, 42.3 %; occupancy after, 42.2 %) and a strong population crash of up to 48.2 % in impact sections (occupancy before, 59.3 %; occupancy after, 11.1 %).
Discussion
Our study adds to previous works on the effects of the invasion of the American mink on vertebrate communities in Spain (Melero et al. 2012; Zuberogoitia et al. 2013). Indeed, it is unusual in explicitly testing the effects of alien mink on fish (but see also Melero et al. 2012). Generally, our results support the idea of a differential impact of the mink on native species (Nordström et al. 2003; Ahola et al. 2006; Peris et al. 2009; Genovesi et al. 2012; Melero et al. 2012). This is critical in disentangling the ecological processes affected by the establishment of the mink (Cox and Lima 2006; Fey et al. 2010; Sih et al. 2010). Nonetheless, it should be recalled that our conclusions only apply to the short-term trends of the impact of the mink.
The abundance of the fish and amphibian species studied did not show a common response to the invasion. Effects on the brown trout and chubs displayed a statistically significant BA × CI interaction. However, a careful inspection of the statistics (Table 1) indicated that the effects of the interaction were lower than those of the control–impact factor alone and that there were no before–after significant results (the effect size was just 0.00 in the Poisson log model for the brown trout). This means that there seems to be no impacts of American mink on the species. The significant interaction term is likely to be due to differences in the abundance among the control and impact sites.
Our results regarding fish species contrast with those of Melero et al. (2012) who found that some chub species declined due to the American mink, although other fish species did not. This difference could be attributed to different dynamics of the study areas; interspecific interactions among fish species and human disturbance are cornerstones in shaping the structure of fish assemblages (Moyle and Cech 2000; Olden and Poff 2004). The fish community in the study of Melero et al. (2012) was composed of up to 24 species inhabiting an area with high human disturbance, whereas in our study, there were only 2 fish species (in the vast majority of the sampling sections) and the rivers and streams are almost pristine. Furthermore, their research encompassed 10 years, while ours was only 3 years.
Fish endemic to the Iberian Peninsula are particularly threatened and declining due to a number of factors (Doadrio 2001; Smith and Darwall 2006). The disparity of the results published to date implies the necessity of more research about this particular aspect to clarify the role of the mink in decreasing fish populations. Therefore, caution should be taken when assigning a negative effect of mink on a particular fish species until support is available
The abundance of Iberian brown frogs did not differ between control and impact areas or before and after the arrival of the American mink (Table 1). The case of the Iberian brown frog, endemic to the Iberian Peninsula, is of particular interest (Pleguezuelos et al. 2002). Our results did not show negative trends related to the mink, something that concerns amphibian conservationists in Spain (Pleguezuelos et al. 2002). However, it should be emphasized that there was some decline of frogs (Fig. 2) that was associated with the American mink (there were low frog numbers in the impact sites after the arrival of the mink), but this could also be due to chance variations. Thus, there remains some concern about the impact of mink on native frogs, perhaps sufficient to attempt to protect them from the invader following the precautionary principle in conservation biology (Primack 2002).
In their research in outer Finnish islands, Ahola et al. (2006) indicated huge reductions in amphibian populations due to mink pressure during a similar temporal period (3 to 4 years after the removal of mink). Such differences with respect to our results are difficult to explain, but perhaps depend on the species being monitored and on the behavioral ecology of the mink (Lima 2002), particularly in reference to their foraging ecology (Stephens et al. 2008). Thus, the availability of potential alternative preys is likely to play an important role (Oliver et al. 2009).
Mammals and birds appear to be the most sensitive groups to the mink, both in our study and in others across the world (Dunstone 1993; Craik 1997; Macdonald and Strachan 1999; Ferreras and Macdonald 1999; Macdonald and Harrington 2003; Nordström et al. 2003; Peris et al. 2009; Schüttler et al. 2009; Genovesi et al. 2012; Melero et al. 2012; Zuberogoitia et al. 2013). Range contractions of small mammals were evident in our analyses (Table 2), for both the water vole and the water shrew, following the expansion of mink. Schüttler et al. (2009) evinced the high vulnerability of solitary ground-nesting birds that rear their chick in concealed nests. European dippers tightly met these criteria (i.e., Smiddy et al. 1995) but showed no definite tendency in its range in the impact sections. Again, this confirms the fact that extrapolations of the known negative effects of mink to predict impact on newly invaded communities are not particularly suitable.
The common water vole (Arvicola terrestris) has been widely found to be a victim of the expansion of the mink (Macdonald and Strachan 1999; Macdonald et al. 2002; see a short review in Macdonald and Harrington 2003), and these results have been used to explain recent declines in Spanish populations of the southern water vole (Palomo et al. 2007). However, no strong evidence was provided until our study. The impact of the mink on water shrews was never assessed, even though the Eurasian water shrew (Neomys fodiens) is widely present in the UK, where the American mink is common (Dunstone 1993; Bonesi and Palazón 2007), and much research has been devoted to the impact of mink on riparian communities. This impact should now be considered as very negative.
Negative effects of the mink on any vertebrate species could be due to high predation pressure (Macdonald and Harrington 2003), which involves devastating consequences only for current mink prey or nonlethal effects (i.e., reduced movement rates to avoid predation could result in lower rates of foraging gain that eventually affects survival of the individuals; review of this foraging–risk trade-off in Stephens et al. 2008). Other species could stay as a “virtual refugee” (Berryman and Hawkins 2006) and show no response to mink colonization, at least at the first stages of the invasion.
This background helps in understanding this invasive ecological process and in postulating potential negative effects on newly invaded communities, constructing predictive vulnerability profiles (Schüttler et al. 2009). In fact, this hypothesis fitted our results. The study area is inhabited by a large population of Eurasian otters (Lutra lutra; García et al. 2009; García-Díaz et al. 2011) and European polecats (Mustela putorius; own data), whose presence could lead the American mink to capture a larger quantity of bank-living small mammals to avoid resource competition (Bueno 1996; Bonesi et al. 2004; Harrington et al. 2009; Melero et al. 2012), in our case, the water shrew and the water vole, with fish and amphibians being scarcely targeted by the mink. Scats of both otters (n = 75) and polecats (n = 18) were collected during the study, and we only found remains of one water vole in one scat of an otter, matching the previous hypothesis. This is the well-known mesopredator release effect (Polis et al. 1989; Sergio et al. 2008), where a top predator prevents the mesopredator from sharing some resources with them (Polis et al. 1989; Sergio et al. 2008), thereby protecting these resources. Nonetheless, although otters could be indirectly protecting some of their prey from the mink, this also means they are sentencing other potential prey inhabiting the areas to death (as shown in our study; see Oliver et al. 2009).
Our results have important conservation and management implications. Fishermen or administration managers usually cite the impact of mink on a variety of species (for example, the brown trout stocks or amphibians) to explain their decline, usually without lacking any rigorous support. Such biased conclusions could result in inappropriate decision-making in conservation.
We used an empirical field design to show that the magnitude of the impact was species-specific, and therefore, management strategies need to be carefully designed to mitigate the invasion of an area by the American mink. In effect, a recently approved legislation about invasive species (Spanish List and Catalog of Invasive Alien Species, Royal Decree 1628/2011) compels environmental administrations to carry out actions to reduce the impact of feral species.
A good conservation strategy should have a general technique to control the invasive mink (i.e., trapping; Zuberogoitia et al. 2010), as well as a species-specific and site-specific intervention schedule for species likely to be affected. (Different strategies are needed to preserve frogs compared to those required for water voles for example.) A working example of this might be the conservation of frogs. New ponds can be constructed to keep mink out of there (i.e., through fencing or intensive trapping of arriving mink; Balharry 1998; Jay et al. 2008). It should be noted that, without controlling mink numbers, any other complementary actions are doomed to failure.
The current policy in Spain, based on legislation, requires evidence to implement programs against feral species included in the official catalog of invaders. Hence, the empirical demonstration of negative effects should be sufficient to trigger a generalized strategy dealing with this problem in Spain. However, we are facing the effects of economic crisis and it seems difficult that sufficient resources will be achieved to develop species-specific and site-specific management actions (as proposed here based on our results). In this scenario, the best way to protect affected species is to begin a control campaign of mink encompassing all the invaded places (Macdonald and Harrington 2003; Zuberogoitia et al. 2010; Bryce et al. 2010; Genovesi et al. 2012). Population control has been demonstrated as efficient in preserving some endemic vertebrates from the pressure of the mink (Bonesi et al. 2007) and in reducing mink density (Melero et al. 2010).
Finally, we would like to call for more investigations into the impact of the mink in Spain, which must be done as soon as possible.
References
Ahola M, Nordström M, Banks PB, Laanetu N, Korpimäki E (2006) Alien mink predation induces prolonged declines in archipelago amphibians. Proc R Soc B 273:1261–1265
Belica L (2007) Brown trout (Salmo trutta): a technical conservation assessment. USDA Forest Service, Rocky Mountain Region
Balharry E (1998) How to exclude pine martens from game and poultry pens and an introduction to the species in Scotland. The Vincent Wildlife Trust, Herefordshire
Berryman AA, Hawkins BA (2006) The refuge as an integrating concept in ecology and evolution. Oikos 115:192–196
Bonesi L, Palazón S (2007) The American mink in Europe: status, impacts and control. Biol Conserv 134:470–483
Bonesi L, Chanin P, Macdonald DW (2004) Competition between Eurasian otter Lutra lutra and American mink Mustela vison probed by niche shift. Oikos 106:19–26
Bonesi L, Rushton SP, Macdonald DW (2007) Trapping for mink control and water vole survival: identifying key criteria using a spatially explicit individual based model. Biol Conserv 136:636–650
Bryce R, Oliver MK, Davies L, Gray H, Urquhart J, Lambin X (2010) Turning back the tide of American mink invasion at an unprecedented scale through community participation and adaptive management. Biol Conserv 144:575–583
Bueno F (1996) Competition between American mink Mustela vison and otter Lutra lutra during winter. Acta Theriol 41:149–154
Churchfield S (1990) The natural history of shrews. Comstock Publication Associates, Ithaca
Cox JG, Lima SL (2006) Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
Craik C (1997) Long-term effects of North American mink Mustela vison on seabirds in western Scotland. Bird Study 44:303–309
Delibes M, Clavero M, Prenda J, Blázquez MC, Ferreras P (2004) Potential impact of an exotic mammal on rocky intertidal communities of northwestern Spain. Biol Invasions 6:213–219
De Marinis AM, Agnelli P (1993) Guide to the microscope analysis of Italian mammal hairs: Insectivora, Rodentia and Lagomorpha. Boll Zool 60:225–232
Doadrio I (ed) (2001) Atlas y libro rojo de los peces continentales de España. DGCN-MNCN, Madrid
Dunstone N (1993) The mink. T and AD Poyser, London
Esteban M, Sanchiz B (2000) Differential growth and longevity in low and high altitude Rana iberica (Anura, Ranidae). Herpetol J 10:19–26
Fedriani JM, Delibes M, Ferreras P, Román J (2002) Local and landscape habitat determinants of water vole distribution in a patchy Mediterranean environment. Ecoscience 9:12–19
Ferreras P, Macdonald DW (1999) The impact of American mink Mustela vison on water birds in the upper Thames. J Appl Ecol 36:701–708
Fey K, Banks PB, Korpimäki E (2010) Alien mink predation and colonisation processes of rodent prey on small islands of the Baltic Sea: does prey naïveté matter? Int J Ecol. doi:10.1155/2010/984396
García-Díaz P (2012) Situación actual del desmán ibérico, Galemys pyrenaicus (E. Geoffroy Saint Hilaire, 1811), en la provincia de Salamanca. M.Sc. thesis, University of Salamanca, Salamanca
García P, Mateos I (2009) Evaluation of three indirect methods for surveying the distribution of the Least Weasel Mustela nivalis in a Mediterranean area. Small Carniv Conserv 40:22–26
García P, Lizana M, Morales J, Gutiérrez J, Acera F, Báez R, García-González A, Pérez-Alonso JC, Prieto O, Díez-Frontón D (2009) Nuevos datos sobre la distribución y dieta de la nutria paleártica (Lutra lutra) en la provincia de Salamanca. Ecología 22:117–125
García-Díaz P, Arévalo V, Lizana M (2011) Comparison of track and direct observation estimations for assessing abundance of the Eurasian otter (Lutra lutra). Folia Zool 60:36–41
Genovesi P, Carnevalli L, Alonzi A, Scalera R (2012) Alien mammals in Europe: updated numbers and trends, and assessment of the effects on biodiversity. Integr Zool 7:247–253
González-Esteban J, Villate I, Irizar I (2006) Differentiating hair samples of the European mink (Mustela lutreola), the American mink (Mustela vison) and the European polecat (Mustela putorius) using light microscopy. J Zool 270:458–461
Harrington LA, Harrington AL, Yamaguchi N, Thom MD, Ferreras P, Windham TR, Macdonald DW (2009) The impact of native competitors on an alien invasive: temporal niche shifts to avoid interspecific aggression? Ecology 90:1207–1216
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Jay S, Lane M-R, O’Hara K, Precey P, Hamblin M (2008) Otters and stillwater fisheries. The Wildlife Trusts, London
Krebs CJ (1999) Ecological methodology, 2nd edn. Addison-Wesley Educational Publishers Inc., Menlo Park
Lima SL (2002) Putting predators back into behavioral predator–prey interactions. Trends Ecol Evol 117:70–75
Lynch AB, Brown MJ, Rochford JM (2006) Fur snagging as a method of evaluating the presence and abundance of a small carnivore, the pine marten (Martes martes). J Zool 270:330–339
Macdonald DW, Strachan R (1999) The mink and the water vole: analyses for conservation. WildCru, London
Macdonald DW, Harrington LA (2003) The American mink: the triumph and tragedy of adaptation out of context. New Zeal J Zool 30:421–441
Macdonald DW, Sidorovich VE, Anisomova EI, Sidorovich NV, Johnson PJ (2002) The impact of American mink Mustela vison and European mink Mustela lutreola on water voles Arvicola terrestris in Belarus. Ecography 25:295–302
Mackenzie DL, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modelling. Inferring patterns and dynamics of species occurrence. Academic, Burlington
McDonald TL, Erickson WP, Mcdonald LL (2000) Analysis of count data from before–after control–impact studies. J Agric Biol Environ Stat 5:262–279
Melero Y, Palazón S, Bonesi L, Gosàlbez J (2010) Relative abundance of culled and not culled American mink populations in Northeast Spain and their potential distribution: are culling campaigns effective? Biol Invasions 12:3877–3885
Melero Y, Plaza M, Santulli G, Saavedra D, Ruiz-Olmo J, Gosàlbez J, Palazón S (2012) Evaluating the effect of American mink, an alien invasive species, on the abundance of a native community: is coexistence possible? Biodivers Conserv 21:1975–1809
Moyle PB, Cech JJ (2000) Fishes: an introduction to ichthyology, 4th edn. Prentice Hall, Upper Saddle River
Mullins J, Statham MJ, Roche T, Turner PD, O’Reilly C (2010) Remotely plucked hair genotyping: a reliable and non-invasive method for censusing pine marten (Martes martes, L. 1758) populations. Eur J Wildl Res 56:443–453
Nordström M, Högmander J, Laine J, Nummelin J, Laanetu N, Korpimäki E (2003) Effects of feral mink removal on seabirds, waders and passerines on small islands of the Baltic Sea. Biol Conserv 109:359–368
Olden JD, Poff NL (2004) Ecological processes driving biotic homogenization: testing a mechanistic model using fish faunas. Ecology 85:1867–1875
Oliver M, Luque-Larena JJ, Lambin X (2009) Do rabbits eat voles? Apparent competition, habitat heterogeneity and large-scale coexistence under mink predation. Ecol Lett 12:1201–1209
Palomo LJ, Gisbert J, Blanco JC (eds) (2007) Atlas y libro rojo de los mamíferos terrestres de España. DGB-SECEM-SECEMU, Madrid
Pauli JN, Hamilton MB, Crain EB, Buskirk SW (2008) A single-sampling hair trap for mesocarnivores. J Wildl Manag 72:1650–1652
Peris SJ, Sanguinetti J, Pescador M (2009) Have Patagonian waterfowl been affected by the introduction of the American mink Mustela vison? Oryx 43:648–654
Pleguezuelos JM, Márquez R, Lizana M (eds) (2002) Atlas y libro rojo de los anfibios y reptiles de España. DGCN-AHE, Madrid
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation—potential competitors that eat each other. Ann Rev Ecol Evol Syst 20:297–300
Potts JM, Elith J (2006) Comparing species abundance models. Ecol Model 199:153–163
Primack RB (2002) Essentials of conservation biology, 3rd edn. Sinauer Associates, Sunderland
Pullin AS, Knight TM, Stone DA, Charman K (2004) Do conservation managers use scientific evidence to support their decision-making? Biol Conserv 119:245–252
Queiroz AI, Quaresma CM, Santos CP, Barbosa AJ, Carvalho HM (1998) Bases para a conservação da Toupeira-de-água (Galemys pyrenaicus). Estudos de Biologia e Conservação da Natureza 27:1–118
Rockwood LL (2006) Introduction to population ecology. Blackwell, Maiden
Román J (2007) Historia natural de la rata de agua (Arvicola sapidus) en Doñana. Ph.D. thesis, Autonomous University of Madrid, Madrid
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org/
Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F (2008) Top predators as conservation tools: ecological rationale, assumptions and efficacy. Ann Rev Ecol Evol Syst 39:1–19
Schüttler E, Klenke R, McGehee S, Rozzi R, Jax K (2009) Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol Conserv 142:1450–1460
Sidorovich V, Macdonald DW (2001) Density dynamics and changes in habitat use by the European mink and other native Mustelids in connection with the American mink expansion in Belarus. Neth J Zool 51:107–126
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Smiddy P, O’Halloran J, O’Mahony B, Taylor AJ (1995) The breeding biology of the Dipper Cinclus cinclus in south-west Ireland. Bird Study 42:76–81
Smith EP, Orvos DR, Cairns J Jr (1993) Impact assessment using the Before-After-Control-Impact (BACI) model: concerns and comments. Can J Fish Aquat Sci 50:627–637
Smith KG, Darwall WRT (eds) (2006) The status and distribution of freshwater fish endemic to the Mediterranean basin. Mediterranean regional assessment no. 1. IUCN, Gland
Stephens DW, Brown JS, Ydenberg RC (eds) (2008) Foraging. Behavior and ecology. University of Chicago Press, Chicago
Sutherland WJ, Pullin AS, Dolman PM, Knight TM (2004) The need for evidence-based conservation. Trends Ecol Evol 19:305–308
Teerink BJ (1991) Hair of west European mammals. Atlas and identification key. Cambridge University Press, Cambridge
Toth M (2002) Identification of Hungarian Mustelidae and other small carnivores using guard hair analysis. Acta Zool Acad Sci Hung 48:237–250
Zeileis A, Kleiber C, Jackman S (2008) Regression models for count data in R. J Stat Soft 27:1–25
Zuberogoitia I, González-Oreja JA, Zabala J, Rodríguez-Refojos C (2010) Assessing the control/eradication of an invasive species, the American mink, based on field data; how much would it cost? Biodivers Conserv 19:1455–1469
Zuberogoitia I, Zalewska H, Zabala J, Zalewski A (2013) The impact of river fragmentation on the population persistence of native and alien mink: an ecological trap for the endangered European mink. Biodivers Conserv 22:169–186
Acknowledgments
We wish to thank Paul Chanin and Johnny Birks for their respective critical reviews of our drafts, Christian Gortázar and two anonymous reviewers, whose comments highly improved our work, G. Hernández, and I. Mateos for their help and support during the fieldwork. This work was partially supported by the projects “Distribución y estado de conservación de los vertebrados bioindicadores y amenazados de los medios acuáticos de las sierras de la provincia de Salamanca (Key: 18.JCY4 463A.C.03.Orden EDU/940/2009)” of the Government of the Junta de Castilla y León, Spain and “Estudio de la distribución y estado de conservación del desmán ibérico (Galemys pyrenaicus) en la provincia de Salamanca” Asociación Galemia–Universidad de Salamanca. PG-D is funded by an IPRS/APA scholarship by the Commonwealth Government of Australia (Department of Education, Employment and Workplace Relations) and an Invasive Animals Cooperative Research Centre scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
García-Díaz, P., Arévalo, V., Vicente, R. et al. The impact of the American mink (Neovison vison) on native vertebrates in mountainous streams in Central Spain. Eur J Wildl Res 59, 823–831 (2013). https://doi.org/10.1007/s10344-013-0736-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-013-0736-5