Abstract
Faecal sexual steroids have been used in field studies evaluating the relationships between gender and the multiple factors influencing endocrine status of individuals. The determination of faecal steroids has been also proposed as an alternative, non-invasive sexing method when other methods were deemed impractical or risky for the health of birds. In this study, we quantified sexual steroid hormones in faeces of the great bustard (Otis tarda), a large and sexually dimorphic polyginic bird species that it is threatened and subjected to intense wildlife management. We evaluated differences between captivity and wild conditions, flocks and sexes, and used faecal steroids to develop sex determination procedures. We found similar steroid levels in captive and wild bustards, no differences between unisexual wild flocks and clear between-sexes differences in testosterone but not estradiol. Faecal steroids accurately discriminated gender in both captive and wild known-sex great bustards. Total testosterone concentration was always higher than estradiol concentration in faecal samples from males, but estradiol was not always higher than testosterone in females. Faecal steroids failed to reveal the presence of young males in female flocks during winter, despite faecal testosterone levels increased with age in a small sample of captive males. Our results show that faecal steroid measurement for both sexing and characterizing the endocrine status of great bustards is feasible, and therefore it should be valuable in wildlife management, especially in combination with additional information obtained from faeces as diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual steroid hormones (androgens, oestrogens and progestogens) are produced by both sexes in quantities different enough to modulate sexual dimorphism in ontogeny, behaviour and reproductive function, thus contributing to the evolution of species diversity in sex differences (Adkins-Regan 2007). Differences in sex hormone levels between males and females have largely contributed to our understanding of the physiology, sexual selection and mating systems in birds (Beletsky et al. 1995; Dawson 2008; Wingfield et al. 2000, 2008), also allowing gender attribution to individuals by determining steroid concentrations in blood, egg yolks and faecal samples (Petrie et al. 2001; Goymann 2005; Sasvári et al. 2008).
Identifying the sex of individuals is crucial for understanding the structure and dynamics of wildlife populations, which is of paramount importance to improve management actions in conservation programs of endangered species. The determination of faecal steroids may be useful in field studies evaluating the relationships between gender and the multiple factors influencing endocrine status of individuals, including age, development, social status, behaviour, stage in the breeding cycle, stress conditions, body condition etc. (Bishop and Hall 1991; Nelson 2005; Norris 2007; Schwarzenberger 2007). In fact, non-invasive monitoring of steroid hormone metabolites in bird droppings has recently become an increasingly popular technique revealing integrative and representative estimates of sexual and stress hormone concentrations without the undesirable effects caused by capture and handling (Palme 2005; Wasser and Hunt 2005; Schwarzenberger 2007; Herring and Gawlik 2009; Sheriff et al. 2011). Faecal steroids have been proposed as an alternative sexing method when other methods are impractical or risky for the health of birds (Tell and Lasley 1991; Swengel 1996; Goymann 2005). This method has the advantage over others that droppings are easily obtained, without interfering with the behaviour of individuals, and sampling does not require special or expensive materials. Despite other potential applications of faecal sexing in population studies (e.g. sex-ratio determination) when other sampling methods are logistically unaffordable or involve endangered species with legal capture restrictions, this sexing method has rarely been used in field studies of wild birds.
Faecal steroids should presumably differ between sexes in sexually size-dimorphic species with marked life-history differences between sexes (Wingfield 1994; Beletsky et al. 1995; Wingfield et al. 2000; Hirschenhauser et al. 2003). However, their use in sex determination of wild birds must be previously confirmed for each study species given potential species-specific differences in steroid metabolism and excretion (Goymann 2005; Schwarzenberger 2007). Thus, in this study we evaluated the adequacy of faecal steroids in sex determination procedures using the great bustard (Otis tarda) as a sexually size-dimorphic model species. The great bustard is a large bird and one of the most sexually dimorphic bird species: adult males weigh 10–15 kg and females 4–5 kg (Alonso et al. 2009). Males and females live in separate flocks throughout the year and meet only for mating, which allows the identification of flock gender and the collection of droppings from flocking individuals of known sex when the flock is observed and short time is elapsed until the sampling of faeces. These features and the availability of some captive individuals of known sex and age allowed us to test whether sexing procedures derived from faecal steroids may be applied in field studies without precise information on flock composition.
Previous studies have measured faecal steroids of adult male great bustards in captivity to explore relationships between seasonal steroid levels and sexual display activity (Biczo and Péczely 2007). Here, we assessed whether faecal steroids may provide important information about sex and endocrine flock differences during winter in a wild population. In addition, as a consequence of extreme sexual dimorphism and other marked life-history differences between sexes, the influence of age on faecal steroids should also differ between sexes due to potential differences in the age of sexual maturity and first breeding, as well as due to other physiological and behavioural differences between sexes (Ball and Ketterson 2008; Dawson 2008; Young et al. 2009). In great bustards, males’ first reproduction attempts take place at an age of 4 years or later and in females at the age of 2 years (Morales et al. 2002; Alonso et al. 2010). Before reaching breeding capability, juvenile females remain in female flocks after maternal independence (offspring are reared exclusively by females), while young males may remain in female flocks during their first and occasionally the second year of age and leave them afterwards to join male flocks (Martín et al. 2007). Given the prolonged maternal dependence or association with females of young males and their delayed first breeding, we assessed whether faecal steroids can identify young males in female flocks. Finally, we assessed whether faecal steroids varies with age in a small sample of captive bustards of known age.
Material and methods
Study area
We conducted research on agricultural fields near Madrid City (Spain). Fresh droppings (digested and undigested faecal material plus urine and urates, faeces hereafter) were collected from wild great bustards in Madrid (central Spain, hereafter Madrid). In the study area, great bustards lived in agro-steppe farmlands mainly cultivated (>80 %) with cereal (wheat and barley). Sheep grazed in stubbles and fallows. There were few fields with legumes, olives, sunflowers and grape vines, which complemented this farming system. A thorough description of the study area is available in previous studies (Lane et al. 1999, 2001).
Studied species and sample collection
The great bustard is a globally threatened species, considered in many areas of its distribution range as “vulnerable”. About 60–70 % of the world population occurs in Spain (ca. 30,000–35,000 individuals), where it is also vulnerable (Alonso and Palacín 2010). Hunting, agriculture intensification and habitat fragmentation have played a decisive role in the decline of great bustards in Spain. Although hunting was outlawed in 1980, agriculture intensification and habitat fragmentation are pervasive threats to great bustard conservation (Alonso and Palacín 2009).
The great bustard is a polyginic species, with males concentrating at traditional arenas (leks) where they fight to establish a hierarchical rank, perform elaborate sexual exhibitions directed toward females and copulate in an exploded lek mating system. Male flocks disperse in late March to start sexual exhibitions and are visited by females that inspect them and select one to mate with (Alonso et al. 2010). Besides agriculture intensification and habitat fragmentation, the population recovery and distribution in Spain are also limited by factors such as their sexually biased dispersal, conspecific attraction and sex-biased mortality (Martín et al. 2008; Martín et al. 2007). Therefore, sex ratio and other sex-related population traits are of particular importance when assessing the conservation status of this species (Alonso and Palacín 2009).
In February 2012, coinciding with the end of the winter period and the onset of the pre-mating period, two flocks of males (30 and 22 individuals) and three flocks of females (58, 27 and 41 individuals) were located and tracked from afternoon to sunset to situate their roosting locations. At the following sunrise (air temperature, 8 °C), fresh faeces were collected at these sites (male flocks, n = 31 and 27 faeces; female flocks, n = 26, 28 and 25 faeces, respectively), stored in plastic bags, transported to the laboratory in a chilled container, and frozen on the day of collection at −20 °C until analysed. Faeces collected at sunrise in the roosting sites correspond to the first defecation of individuals after the night resting period. Fresh faeces of this species at roosting sites are large (>6 cm3) and cannot be confused with faeces of other species. These faeces from flocks of known sex were used to evaluate whether faecal steroids can be used to noninvasively and accurately identify gender in this species.
Faeces of 13 captive great bustards were kindly provided by the Wildlife Recovery Center “Los Hornos” which belongs to the Environmental Department of Extremadura Regional Government (Junta de Extremadura, Spain). Captive great bustards were kept in large aviaries with natural weather conditions. They fed on natural fresh vegetation that sprouts in the enclosures. Faeces were sampled in March-May 2012, stored frozen and analysed for faecal steroids in order to develop sexing criteria derived from faeces of known sex, and to assess potential age differences in faecal steroids. Sampled individuals included 6 males (age 3.3 ± 1.9 years, range 1–5 years) and 7 females (age 2.7 ± 1.2 years, range 1–4 years).
Faecal steroid hormone extraction procedure
Extraction of steroid hormones from faecal frozen samples was performed by using previous established methods for bird species and steroid hormones (Kelemen et al. 2003; Wasser et al. 1994; Biczo and Péczely 2007). Frozen faecal samples were dried and pulverized, and then 0.1 g powered faeces were homogenized by adding 0.5 ml of distilled water and 100 μl of 10 % Sodium Dodecyl Sulphate (SDS) in order to emulgenate lipid content of faeces. Samples were then extracted for 30 min with 2.5 ml of diethyl-ether three times. Extracts were suspended in 1ml EIA buffer. The efficiency of extraction of each steroid hormone from faecal samples was tested by the addition of radiolabeled hormones (3H-testosterone and 3H-estradiol; 4,000–8,000 dpm, ICN, CA, USA) to a parallel set of faecal samples prior to extraction.
Faecal steroid hormone enzymeimmunoassays (EIAs)
Steroid hormones were analysed from faecal extracts by Enzymeimmunoassays developed in the Endocrine Laboratory of Animal Physiology Department UCM, Madrid, Spain, and validated for the species, fecal extracts and particular hormone. Polyclonal antibodies were raised in rabbits against testosterone-6CMO:BSA (C9003), and 6-keto-17beta-oestradiol-6CMO:BSA (C9506). All antibodies were then purified and characterized for cross-reactivity against related steroid hormones. Hormone conjugates: testosterone-3HS and estradiol-3HS were labelled by horseradish peroxidase (Sigma, MO, USA). All steroids were obtained from Steraloids Inc. (Wilton, NH, USA).
Enzyme immunoassays were performed following the same assay protocol: 96-well flat-bottomed polystyrene microtiter plates (Immulon 1B, Dynex, CA, USA) were coated with 100 μl/well of each purified antibody solution (1:4,000 in coating buffer: sodium carbonate, 50 mM, pH 9.6) except for the first well which acted as plate/assay blank, and incubated overnight at 4 °C. Afterwards, non-bound antibodies were removed from the wells by washing plates five times with wash solution (NaCl, 150 M/l, Tween 20, 0.5 ml−1), inverted and dried.
Standards were solubilized in ethanol, evaporating the solvent and solubilizing them in assay buffer (sodium phosphate, 100 mM, pH 7.0, with sodium chloride, 8.7 g l−1; BSA, 1g l−1). Standard curve covered a range between 0 to 1 ng/well, and was constructed by using 10 standard solutions, 0.0, 0.1, 0.5, 1, 5, 10, 50, 100, 500, and 1,000 pg/well. The wells of the first and last rows were called B0 (maximum binding of enzyme conjugate to the antibody), and 100 μl of conjugate dilutions (1:40,000 in assay buffer) were added to the wells. Standards and faecal extracts were analysed in duplicates. For standard curve each standard was diluted in 150 μl of conjugates, mixed and 50 μl were pipetted into the wells from the 2 and 6 rows. For faecal samples: 50 μl for testosterone or 100 μl for estradiol of each extract were mixed with 250 μl of diluted conjugates, and 60 μl were pipetted into the wells of 7 to 11 rows. Then volume of the wells was completed until 100 μl. Plates were covered and incubated for 2 hours at room temperature. Bound/free separation was achieved emptying plates by inversion and washing them five times with wash solution. To evaluate the amount of labelled hormone bound to the antibody, 100 μl of substrate solution (3,3′,5,5′-tetramethylbenzidine dihydrochloride, pH 5.0, Neogen, KY, USA) and hydrogen peroxide were added to all wells and incubated for 15 min at room temperature, this reaction was stopped by the addition of 100 μl of 10 % sulphuric acid. Absorbance was read at 450–600 nm in an automatic microplate reader.
Hormone concentrations were calculated by means of software developed for these techniques. Standard-dose response curve was constructed by plotting the binding percentage (B/Bo × 100) against hormone standard concentrations added. Faecal steroid hormone concentrations are expressed as ng/g dry faecal matter.
Validation of the EIA technique was performed according to the methods described by Munro and Lasley (1988). Cross-reactivities of polyclonal testosterone antibodies were: testosterone 100 %, 5-alpha-dyhidrotestosterone 20 %, 4-androstenediol 11.5 %, 5-beta-dihydrotestosterone 5 %, androstenediol 3.5 %, androstenelone 3.2 %, 5-alpha-androstan-3-alpha, 17-beta diol 1 % and <1 % with cortisol, progesterone and estradiol. Cross-reactivities of polyclonal 17 beta estradiol antibodies were: 17 beta-estradiol 100 %, keto-estradiol 6-CMO 100 %, 16 keto-estradiol 16.70 %, 6 keto-estradiol 20.00 %, estriol 8.73 %, estradiol 3-benzoate 3.28 %, estrone 0.50 %, equilin 0.3 % and <1 % with equilenin, progesterone and testosterone. Assay sensitivities were the following: testosterone 4.9 pg/well and 17 beta estradiol 2.9 pg/well. Intra- and inter-assay coefficients of variation were calculated by assaying a pooled faecal sample 10 replicates in the same assay and the 10 replicates of the same sample in ten consecutive assays: testosterone 5.5 % and 8.9 %, and 17 beta estradiol 7.4 % and 9.9 %, respectively.
Parallelism was performed by comparing serial dilutions of pooled faecal samples and the standard curve demonstrating that binding inhibition curves of serially diluted pools of samples were parallel to the standard curve in a range of 1:1 to 1:50-fold dilutions. While not a final proof of authenticity, parallelism is routinely calculated to show the specificity of the assay. Linearity was demonstrated by performing serial dilutions of a pool of faecal samples (1:1 to 1:32-fold), and comparing them to the percentage of binding inhibition to antibody in the assay (% B/B0) being linear in the range of 1:1 to 1:16-fold dilutions.
Data analysis
Differences between sexes in the concentration of faecal steroids were evaluated according to the effect size theorem (Cohen 1988), which allows the assessment of the magnitude of differences (Cohen’s d and their 95 % confidence intervals). Unlike significance tests based on sample size-dependant P values, effect size provides an estimation of the magnitude of biologically relevant differences independently of sample size (Nakagawa and Cuthill 2007). To determine what magnitude of effect size constitutes a biologically relevant difference, we assessed whether lower and upper 95 % confidence intervals overlapped with the zero Cohen’s d reference value indicating no difference between groups. This approach incorporates the information on the precision of the estimate provided by confidence intervals (Nakagawa and Cuthill 2007) to the conventional benchmarks considering the rule of thumb that effect sizes <0.2 (i.e., <14.7 % of non-overlap between two data sets), 0.5 (33.0 % of non-overlap) and >0.8 (>47.4 % of non-overlap) are small, medium and large, respectively (Cohen 1988). Differences between steroid levels in faeces collected in different flocks were assessed by considering partial Eta squared (η 2 p ) as a measure of effect size in ANOVA (Cohen 1988).
We used discriminant function analysis (DFA) to determine the sex of faeces based on concentration of faecal steroids. We applied a leaving-one-out resampling method (jack-knife) to test the performance of the discriminant functions for faeces from captive and wild individuals. We transformed (log10) faecal steroids concentrations prior to calculate DFA and verified that frequency distributions did not differ from the normal distribution (Shapiro–Wilk W test; log10 testosterone, W = 0.99, P = 0.204; log10 estradiol, W = 0.99, P = 0.183).
Results
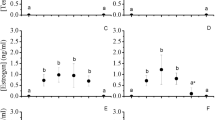
Concentrations of testosterone and estradiol were similar between wild flocks of the same sex (males: comparison of two flocks, testosterone: Cohen’s d = 0.07, 95 % CI = −0.45, 0.59, estradiol: Cohen’s d = 0.17, 95 % CI = −0.35, 0.68; females, comparison of three flocks ANOVA, testosterone: F 2,76 = 1.00, η 2 p = 0.04, R 2 = 0.02, estradiol: F 2,76 = 2.00, η 2 p = 0.06, R 2 = 0.03). Therefore, we pooled data from flocks of each sex in subsequent analyses. Concentrations of testosterone and estradiol in faeces were different enough between sexes, especially in wild great bustards (testosterone in wild males, 21.8 ± 10.3 ng/g; testosterone in wild females, 11.3 ± 5.8 ng/g, Cohen’s d = 1.31, 95 % CI = 0.93, 1.68; estradiol in wild males, 8.4 ± 3.6 ng/g, estradiol in wild females, 13.1 ± 9.2 ng/g, Cohen’s d = −0.64, 95 % CI = −0.98, –0.28). The differences between sexes were also relevant for testosterone but not for estradiol in faeces from captive bustards (i.e., faeces from males and females did not differ greatly in estradiol), as indicated the effect sizes and their 95 % CI of these comparisons (testosterone in captive males, 19.0 ± 9.4 ng/g, testosterone in captive females, 9.9 ± 3.1 ng/g, Cohen’s d = 1.35, 95 % CI = 0.06, 2.45; estradiol in captive males, 7.5 ± 3.5 ng/g, estradiol in captive females, 10.0 ± 4.4 ng/g, Cohen’s d = −0.63, 95 % CI = −1.69, 0.53). The differences between sexes in both sexual steroids were translated into large effect sizes for the ratio of testosterone to estradiol in wild great bustards (males, 2.9 ± 1.5 ng/g, females, 1.0 ± 0.3 ng/g, Cohen’s d = 1.84, 95 % CI = 1.42, 2.23) and captive great bustards (males, 2.7 ± 1.1 ng/g, females, 1.1 ± 0.4 ng/g, Cohen’s d = 2.14, 95 % CI = 0.66, 3.33). Concentrations of testosterone, estradiol and their ratio were similar between captive and wild bustards faeces in each sex (i.e., no Cohen’s d was significantly different to zero). A straightforward scatterplot of testosterone and estradiol suggested that it was feasible to discriminate the sex of the faeces in captive and wild great bustards (Fig. 1). Discriminant function analysis (DFA) distinguished the sex of 92.0 % of faeces (n = 137) from wild great bustards with a major proportion of correct sex assignation for females (Table 1). Although sample size was scarcer in captive than in wild great bustards, DFA calculated quite similar equations with both samples (Table 1).
All faeces from captive males and wild male flocks showed testosterone levels higher than estradiol levels (Fig. 1), while most faeces from captive females (71.4 %, n = 7) and about the half of faeces from wild females (49.4 %, n = 79) showed testosterone levels higher than estradiol levels (Fig. 2). These faeces showed similar estradiol levels (Cohen’s d = −0.40, 95 % CI = −0.80, 0.02) but clearly lower testosterone levels (Cohen’s d = 1.03, 95 % CI = 0.59, 1.45) than faeces collected from wild males (Fig. 1b).
Testosterone increased with age in males (Spearman correlation coefficient r s = 0.93, P = 0.008, n = 6) and decreased in females (r s = −0.84, P = 0.017, n = 7). No clear association of estradiol and age was apparent in males (r s = 0.49, P = 0.32, n = 6) or females (r s = 0.06, P = 0.91, n = 7). The ratio of testosterone to estradiol was not correlated with age of captive birds neither in males nor in females.
Discussion
Faecal steroids accurately discriminated individuals’ gender from faeces of both captive and wild great bustards of known sex. We suggest that this sexing method may be widely applied in field studies with monomorphic species as a suitable alternative to other sexing methods requiring capture and handling. Typical cases are demographic studies where sex composition is required and sexes are difficult or impossible to distinguish in the field. This sexing method is based on the rule that total testosterone concentration should be higher than estradiol concentration in faecal samples from individual males, and the opposite in females, although obviously variations of these patterns should depend on age, breeding status, stage in the reproductive cycle or other factors influencing steroid level variability among individuals (Sturkie 1990; Norris 2007). In fact, we found that while testosterone levels were always higher than estradiol levels in both captive and wild males, a proportion of faeces from captive and wild females showed also higher levels of testosterone than estradiol, although these faeces showed lower levels of testosterone than those from male faeces. These results suggest that a few young males should have been present in flocks of females (Alonso et al. 2009) and consequently, the testosterone to estradiol ratio could be somewhat inflated in female flocks. This is further supported by the apparent increase of testosterone with age in a small sample of captive male bustards of known age.
Determination of age-classes associated with reproductive and developmental status is other potential application of faecal steroids, especially when individuals of different ages or reproductive status are not distinguishable in the field. Testosterone but not estradiol contents in faeces of a small sample of captive great bustards of known age differed with age, especially between juveniles and older individuals, likely as a consequence of different gonadal development and gonadotropin releasing hormone levels between sexually mature and immature individuals (Sharp and Ciccone 2005; Ball and Ketterson 2008; Dawson 2008; Blas et al. 2010). This finding opens the possibility of ageing wild great bustards by determining faecal steroids, especially regarding the ageing of juveniles (i.e., immature individuals in their first year) vs. older individuals. Current guidelines to assign age to mating males discriminate four main age classes based on plumage features, while female ages are extremely difficult to identify even for expert observers (Alonso et al. 2006, and references therein). Data on faecal steroids from faeces collected in the wild did not show any apparent grouping steroids to discriminate faeces of juvenile, immature and older individuals. This does not invalidate likely differences in steroid levels between ages, but confirms our current inability to age great bustards in the wild based upon faecal steroids, at least during winter when steroid levels may remain at basal levels. To evaluate potential relationships between faecal steroids and age, faecal steroids of additional captive individuals or wild individuals of known age should be measured.
Faecal steroid contents may also be a useful tool in breeding behavioural studies. Mating behaviour of great bustards markedly differs between sexes: males conduct elaborate displays through which they express their status and condition to other males and females of the lek, while females temporarily visit leks to select a mate (Alonso et al. 2010). This sexual activity shows marked seasonality over a relatively short time period (Alonso et al. 2010), which has been related with gonadal activity determining the levels of faecal steroids in captivity conditions (Biczo and Peczely 2007). We found that differences between sexes in faecal steroids concentrations were especially relevant for testosterone but not for estradiol in both captive and wild bustards. This suggests a major role for testosterone in the activation of sexual activity in both sexes, and a minor role of estradiol in males just prior to the breeding season (see also Biczo and Peczely 2007 for similar results in captive males). A note of caution should be added, because testosterone in the faeces may also originate from 4-androstenedione (in females) as inter-conversions of metabolites may take place (e.g. Moehle et al. 2002). Further research is needed to quantify the effect of inter-conversion of metabolites in sex typing of faecal sexual steroids in birds.
No biologically relevant differences in faecal steroids concentrations were found between flocks, both of males and females, sampled at the same time period in a relatively small geographic area. This suggests the lack of a clear potential between-flocks segregation by endocrine status or other individual features reflecting it, such as age, social status, stage in the breeding cycle, stress conditions, body condition, etc. (Bishop and Hall 1991; Nelson 2005; Norris 2007; Schwarzenberger 2007). Differences between faecal steroids between winter in the wild (this study) and pre-mating and mating season in captivity (Biczo and Peczely 2007) suggest that faecal steroids may also provide important information about the stage of the breeding cycle of great bustards in the wild, which should be assessed in future studies. Potential differences in testosterone and estradiol levels could be applied as a non-invasive technique to study the level and timing of sexual activity among individual non-marked bustards using different display territories within the exploded lek (Alonso et al. 2010), or between different leks or geographically distant breeding areas. Eventually, it could also be used as a correlate of the sexual state of marked males through the breeding season, and compared with parameters such as display activity or mating success (Alonso et al. 2010).
The use of faeces as a source of suitable genetic material has been previously attempted with success in great bustards (Idaghdour et al. 2003) and other bird species (Segelbacher and Steinbrück 2001). The main application of sexing by faecal steroids, as an alternative to molecular faecal sexing, arises when the physiological measurement of these hormones are specifically required for the research objectives or when steroids are related to additional information obtained from faeces. Gender may be an important, but often neglected source of variation among individuals in a wide array of physiological variables analysed in faeces of wild birds. For instance, information on great bustard diet has been previously obtained from faeces of individuals of unknown sex (Lane et al. 1999; Rocha et al. 2005), despite the fact that gender may be an important source of variation in diet studies (Ruckstuhl and Neuhaus 2005).
In conclusion, an essential step in the field research of great bustards is to determine if non-invasive methods of sexing are feasible. The measurement of faecal steroids offer valuable insight about gender, and potentially about age, reproductive status and stage in the reproductive season of great bustards and likely in other birds, and thus may constitute a valuable tool in to avian ecology, evolution and conservation studies. Faecal steroid measurements for both sexing and characterizing the endocrine status of individuals may be especially helpful in combination with additional information obtained from faeces in a wide array of avian studies, thus contributing to the integration of this multi-level knowledge in fruitful areas of future research.
References
Adkins-Regan E (2007) Hormones and the development of sex differences in behaviour. J Ornith 148(Suppl 1):S17–S26
Alonso JC, Palacín C (2009) Avutarda Otis tarda. In: Salvador A, Bautista LM. (eds.) Enciclopedia virtual de los vertebrados españoles. Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org. Accessed 15 June 2012. [In Spanish.]
Alonso JC, Palacín C (2010) The world status and population trends of the Great Bustard: 2010 update. Chinese Birds 1:141–147
Alonso JC, Magaña M, Martín CA, Palacín C, Alonso JA (2006) Field determination of age in male great bustards (Otis tarda) in spring. Eur J Wildl Res 52:43–47
Alonso JC, Magaña M, Alonso JA, Palacín C, Martín CA (2009) The most extreme sexual size dimorphism among birds: allometry, selection, and early juvenile development in the great bustard (Otis tarda). Auk 126:657–665
Alonso JC, Magaña M, Palacín C, Martín CA (2010) Correlates of male mating success in great bustard leks: the effects of age, weight and display effort. Behav Ecol Sociobiol 64:1589–1600
Ball GF, Ketterson ED (2008) Sex differences in the response to environmental cues regulating seasonal reproduction in birds. Phil Trans R Soc B 363:231–246
Beletsky LD, Gori DF, Freeman S, Wingfield JC (1995) Testosterone and polyginy in birds. Curr Ornithol 12:1–41
Biczo A, Péczely P (2007) Display activity and seasonality of faecal sexual steroids in male great bustard (Otis tarda L.). Acta Biol Hung 58:21–33
Bishop CM, Hall MR (1991) Non-invasive monitoring of avian reproduction by simplified faecal steroid analysis. J Zool 224:649–668
Blas J, López L, Tanferna A, Sergio F, Hiraldo F (2010) Reproductive endocrinology of wild, long-lived raptors. Gen Comp Endocr 168:22–28
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum, Hillsdale, New Jersey
Dawson A (2008) Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Phil Trans R Soc B 363:1621–1633
Goymann W (2005) Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and influences of diet on hormone metabolite levels. Ann N Y Acad Sci 1046:35–53
Herring G, Gawlik DE (2009) Stability of avian fecal corticosterone metabolite levels in frozen avian feces. J Wildl Manage 73:1010–1013
Hirschenhauser K, Winkler H, Oliveira RF (2003) Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Hormones Behav 43:508–519
Idaghdour Y, Broderick D, Korrida A (2003) Feces as a source of DNA for molecular studies in a threatened population of great bustards. Conserv Genetics 4:789–792
Kelemen K, Péczely P, Szöke ZS, Ladjánszky V (2003) A comparative methodical study of the faecal steroid analysis on birds: looking for a valid method of testosterone determination. Acta Biol Hung 54:285–298
Lane SJ, Alonso JC, Alonso JA, Naveso MA (1999) Seasonal changes in diet and diet selection of great bustards (Otis tarda) in north-west Spain. J Zool 247:201–214
Lane SJ, Alonso JC, Martín CA (2001) Habitat preferences of great bustard Otis tarda flocks in the arable steppes of central Spain: are potentially suitable areas unoccupied? J Appl Ecol 38:193–203
Martín CA, Alonso JC, Alonso JA, Palacín C, Magaña M, Martín B (2007) Sex-biased juvenile survival in a bird with extreme size dimorphism, the great bustard Otis tarda. J Avian Biol 38:335–346
Martín CA, Alonso JC, Alonso J, Palacín C, Magaña M, Martín B (2008) Natal dispersal in great bustards: the effect of sex, local population size and spatial isolation. J Anim Ecol 77:326–334
Möhle U, Heistermann M, Palme R, Hodges JK (2002) Characterization of urinary and fecal metabolites of testosterone and their measurement for assessing gonadal endocrine function in male nonhuman primates. Gen Comp Endocrinol 129:135–145
Morales MB, Alonso JC, Alonso JA (2002) Annual productivity and individual female reproductive success in a great bustard Otis tarda population. Ibis 144:293–300
Munro CJ, Lasley BL (1988) Non-radiometric methods for immunoassay of steroid hormones. Prog Clin Biol Res 285:289–329
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–601
Nelson RJ (2005) An introduction to behavioral endocrinology. Sinauer, Sunderland, MA
Norris DO (2007) Vertebrate endocrinology, 4th edn. Elsevier Academic, London
Palme R (2005) Measuring fecal steroids: guidelines for practical application. Ann N Y Acad Sci 1046:75–80
Petrie M, Schwabl H, Brande-Lavridsen N, Burke T (2001) Sex differences in avian yolk hormone levels. Nature 412:498
Rocha P, Marques AT, Moreira F (2005) Seasonal variation in Great Bustard Otis tarda diet in south Portugal with a focus on the animal component. Ardeola 52:371–376
Ruckstuhl KE, Neuhaus P (2005) Sexual segregation in vertebrates: ecology of the two sexes, 1st edn. Cambridge University, Cambridge
Sasvári L, Péczely P, Hegyi Z (2008) Parental testosterone and estradiol concentrations in the early nestling period correlate with the age-dependent breeding performance in Tawny Owls Strix aluco. Orn Fenn 85:46–54
Schwarzenberger F (2007) The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int Zoo Yearbook 41:52–74
Segelbacher G, Steinbrück G (2001) Birds feces for sex identification and microsatellites analysis. Vogelwarte 41:139–142
Sharp PJ, Ciccone N (2005) The gonadotrophin releasing hormone neurone: Key to avian reproductive function. In: Dawson A, Sharp PJ (eds) Functional avian endocrinology. Narosa Publishing House PVT LTD. Daryaganj, India, pp 59–72
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–887
Sturkie PD (1990) Avian physiology, 3rd edn. Springerverlag New York Inc, New York, USA
Swengel SR (1996) Special techniques, C: Sex determination. In: Ellis DH, Gee GF, Mirande CM (eds) Cranes: their biology, husbandry, and conservation. National Biological Service/International Crane Foundation, USA, pp 223–231
Tell LA, Lasley BL (1991) An automated assay for fecal estrogen conjugates in the determination of sex in avian species. Zoo Biol 10:361–367
Wasser SK, Hunt KE (2005) Noninvasive measures of reproductive function and disturbance in the barred owl, great horned owl, and northern spotted owl. Ann N Y Acad Sci 1046:1–29
Wasser SK, Monfort SL, Southers J, Wildt DE (1994) Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) feces. J Reprod Fert 101:213–220
Wingfield JC (1994) Hormone-behavior interactions and mating systems in male and female birds. In: Short RV, Balaban E (eds) The differences between the sexes. Cambridge University, Cambridge, pp 303–330
Wingfield JC, Jacobs JD, Tramontin AD, Perfito N, Meddle S, Maney DL, Soma K (2000) Toward an ecological basis of hormone-behavior interactions in reproduction of birds. In: Wallen K, Schneider JE (eds) Reproduction in context: social and environmental influences on reproduction. MIT, Cambridge, pp 85–128
Wingfield JC, Williams TD, Visser ME (2008) Introduction. Integration of ecology and endocrinology in avian reproduction: a new synthesis. Phil Trans R Soc B 363:1581–1588
Young GR, Dawson A, Newton I, Walker L (2009) The timing of gonadal development and moult in three raptors with different breeding seasons: effects of gender, age and body condition. Ibis 151:654–666
Acknowledgments
We thank J. Caldera and C. Giner-Abati (Centro de Recuperación Los Hornos, Junta de Extremadura) for providing faeces of captive bustards. The Dirección General de Investigación of Spain provided funding through research projects CGL2009-12753-C02-01 and CGL2008-02567.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Bautista, L.M., Silván, G., Cáceres, S. et al. Faecal sexual steroids in sex typing and endocrine status of great bustards. Eur J Wildl Res 59, 815–822 (2013). https://doi.org/10.1007/s10344-013-0735-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-013-0735-6