Abstract
The mallard Anas platyrhynchos is the world’s most widespread and numerous dabbling duck. It is also farmed and released to the wild by the millions each year, but the effects of this on wild populations remain little studied. By using historical national ringing–recovery data from Sweden and Finland, we here address three predictions based on previous studies: (1) longevity is higher in wild than in hand-reared mallards, (2) wild mallards migrate longer than hand-reared, and (3) migration distance in wild ducks surviving long enough to start fall migration has decreased over the last 50 years. Indeed, wild mallards lived longer than hand-reared (19 versus 9 months in Swedish birds and 13 versus 4 months in Finnish birds). Compared to wild mallards, a smaller proportion of hand-reared birds survived long enough to have the chance to enter the wild breeding population; less than 25 % of the Swedish birds and less than 10 % of the Finnish birds lived a year or longer. Wild birds migrated farther than hand-reared (mean distance in Swedish birds, 676 versus 523 km; in Finnish birds, 1,213 versus 157 km), a pattern caused by both shorter life span and lower migration speed in hand-reared birds. Mean migration distance in wild Swedish mallards was 787 km in 1947–1972 but 591 km in 1977–1993. This difference was not statistically significant, though, possibly due to the limited sample size and lack of data from the last two decades. In general, our study provides a conservative test of the predictions addressed, calling for more research about the consequences of restocking duck populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mallard is the most widespread duck in the world, with an estimated global population of more than 18 million breeding birds (del Hoyo et al. 1992; Delany and Scott 2006). It is a model species in ecology, genetics, and epidemiology, as well as a flagship species in wetland conservation and management. Related to the latter, it is also one of the world’s most important game species, with annual harvests estimated at 4.5 million each in Europe and North America (Hirschfeld and Heyd 2005; Raftovich et al. 2011).
Like other economically important species, the mallard has been subject to large management efforts; for example, wetland restoration and restocking of wild populations with hand-reared birds. Release of hand-reared mallards to augment wild stock for conservation and hunting has a long history (e.g., Lincoln 1934; Brakhage 1953; Batt and Nelson 1990 and references therein), and it has increased strongly in parts of Europe during the last 30 years (Champagnon et al. 2009; Guillemain et al. 2010). Dependable statistics are lacking for most countries, but recent estimates indicate that the number of mallards released through restocking programs annually is remarkable compared to the size of wild populations. In France, ca. 1,400,000 mallards are produced and released annually, compared to an estimated wild winter population of 270,000 (Mondain-Monval and Girard 2000). In Sweden, the annual number of released birds may be almost as large as the wild breeding population (Wiberg and Gunnarsson 2007; Söderquist, unpublished data) and in Denmark the annually released stock (ca. 400,000 in 2007; cf. Noer et al. 2008) far outnumbers the breeding population, which amounts to some 20,000 pairs (Heldbjerg and Eskildsen 2010). Taken as a whole, mallard release programs may constitute one of the largest population manipulations of any migratory vertebrate.
Introduction and establishment of alien species is a major concern for biodiversity and basic ecosystem processes (Chapin et al. 2000; Lockwood et al. 2007). Ecological and genetic effects of restocking of native species are, however, much less studied (e.g., Laikre et al. 2006). This is a cause for serious concern, as restocking of native species nevertheless often involves “alien” non-native, genotypes. Having knowledge about and actively choosing certain provenances has long been commonplace in forestry and fisheries, but only more recently so in conservation restocking programs, and still to a very limited extent in game management. Here, too, the mallard may serve as an ideal model species; eggs, ducklings as well as adults have been subject to extensive—and probably increasing—international trade for decades, for example within the European Union. As a case in point, Swedish game managers have long used mallard eggs, ducklings, and adults imported from Denmark, which in turn imports large quantities from abroad (e.g., France). This trade creates a directed flux of captive mallard stock towards the northeast within the flyway.
For natural reasons, game management and applied research have long been interested in survival and return rates of hand-reared released mallards, during as well as after the hunting season (Brakhage 1953; Fog 1964, 1965; Batt and Nelson 1990; Yerkes and Bluhm 1998). However, this is far more than a game management issue. Depending on how many of the released birds that survive hunting and the following winter, an unknown fraction will get the opportunity to merge with the wild breeding population. In a North European perspective, this would result in introgression by genotypes from the breeding stock in countries in the southwest. The mallard has traditionally been regarded as a fairly panmictic species, but in reality its spatial genetic structure has only recently begun to be understood (Zeddeman et al. 2009; Kraus et al. 2011). There are undisputed regional adaptations, though; for example, different subpopulations within the western European flyways (sensu Scott and Rose 1996) have different migratory habits. Mallards breeding in the southern part of these flyways are nonmigratory, while most Swedish and Finnish are strongly migratory (e.g., Fransson and Pettersson 2001; Gunnarsson et al. 2012). Consequently, introgression by alien nonmigratory genotypes may, in the long term, reduce the migratory habits of wild recipient populations in northeastern Europe. In addition, hand-reared birds may carry other “burdens of captivity” manifested in behavior, physiology, disease, and morphology. This may make recipient populations less well adapted to natural conditions (Champagnon et al. 2010, 2011; Guillemain et al. 2010), which is a concern for conservationists, hunters, and epidemiologists alike.
To understand population trajectories and the magnitude of introgression, it is essential to present regional information about survival of hand-reared mallards in the wild, and how these rates compare to those of wild conspecifics (cf. Yerkes and Bluhm 1998). It is also important to find out if migratory habits differ between the two categories. Obviously, this is a huge undertaking involving many countries and one that has just started (Champagnon et al. 2009). The mallard’s genetic landscape may be changing quickly and it is essential to mine historical data sources for patterns, for baseline comparisons and to confirm which hypotheses are most relevant to address on a wider scale. A first attempt to compare survival and migratory habits between hand-reared and wild migratory mallards was made in the Swedish bird ringing atlas (Fransson and Pettersson 2001), but the patterns and interpretations presented in it are equivocal. For example, its “wild mallard” category included many individuals ringed in city parks, i.e., largely sedentary and fed birds. For increased generality and precision, we use recent and historical data from Sweden and Finland and apply consistent filtering criteria not only to standardize age and time of ringing but also to obtain a more clear-cut wild sample with which the hand-reared can be contrasted. Based on previous studies, we test three directional predictions: (1) longevity is higher in wild than in hand-reared mallards, (2) wild mallards migrate longer than hand-reared, and (3) migration distance in wild ducks surviving long enough to start fall migration has decreased the last 50 years.
Material and methods
Sources and selection of data
We used nationwide data on mallards ringed in Sweden (years 1919–2004) and Finland (years 1913–2006) obtained from The Bird Ringing Centre at The Swedish Museum of Natural History (Stockholm, Sweden), and The Finnish Ringing Centre at the Natural History Museum (Helsinki, Finland), respectively. The Swedish dataset comprised 4,864 mallards recovered dead, of which 509 were hand-reared and 4,355 were wild-caught birds. The Finnish dataset comprised 1,903 birds recovered dead, of which 426 were hand-reared and 1,477 were wild-caught. These datasets were subsequently filtered in several steps in order to obtain comparable groups of records that differed only in origin (wild versus hand-reared).
To ensure that birds in the “wild” mallard dataset were characterized by natural behavior and to reduce the probability of including individuals affected by handling, farming, winter feeding, and other anthropogenic activity, we deleted all records of individuals that: (1) were kept in captivity after marking and/or released more than 10 km from the ringing site, (2) were in poor condition when ringed, (3) had been used in experiments, (4) were influenced by other factors that may have biased encounter probabilities (Speek et al. 2001), (5) had been ringed in city parks in Malmö, Sweden (55° 34′–55° 37′ N, 12°58′–13°02′ E), Gothenburg, Sweden (57° 41′–57° 42′ N, 11° 55′–11° 57′ E), Turku, Finland (60° 26′ N, 22° 15′ E), and Helsinki, Finland (60° 11′ N, 24° 53′/24° 59′ E). Next filtration step was demographic; since all hand-reared birds are in their first calendar year when ringed, we only used data from wild birds marked in their first calendar year. After this step, 427 hand-reared Swedish, 1,146 wild Swedish, 227 hand-reared Finnish, and 264 wild Finnish mallards remained.
Another concern relevant for both groups is the actual ringing month. In the Finnish dataset, the distribution of month of ringing is rather similar for wild and hand-reared birds. However, the Swedish dataset is biased, with all hand-reared birds ringed in June–August and most wild ringed in fall, peaking in November. As survival rate is much higher in mallards ringed as juveniles than in those ringed as pulli (Gunnarsson et al. 2008), we subsequently used only data from birds ringed in June–August classified as “age group 3” according to the European Union for Bird Ringing manual (Speek et al. 2001). In other words, by excluding all pulli and unfledged birds, only full-grown bird hatched in the same breeding season were used.
Longevity
In this paper, we define ‘longevity’ as the time from ringing until recovery. To analyze this, we restricted the dataset further by only using data from birds found dead at least 20 days after ringing date and were recovered freshly dead (Speek et al. 2001). Sample sizes after this filtering step: Swedish hand-reared (n = 362), Swedish wild (n = 226), Finnish hand-reared (n = 217), and Finnish wild mallards (n = 161).
Migration distance
To analyze migratory habits, we added one filtering step to those described under “Sources and selection of data” section, namely that only birds recovered during their first winter (November–January) were considered. This let us explore differences in migratory habits between otherwise comparable groups of ducks of similar age to see how their movements differed during their first winter “migration”, here defined as the distance from ringing to recovery site. A first analysis concerned migration distance and all sampling years (Swedish hand-reared (n = 138), Swedish wild (n = 55), Finnish hand-reared (n = 17), and Finnish wild mallards (n = 44)). In a second analysis, we used recoveries from wild ducks only to see if migration distance in this category has changed over time by contrasting data from the period 1947–1972 with data from 1977 to 1993 (Swedish mallards, n = 25 in both groups; Finnish sample, too small to analyze).
Statistics
All analyses were run in SPSS 17, and all probabilities are two-tailed. Parametric tests (independent t test) were used unless assumptions of normality and equal variances could not be met, in which case nonparametric tests were used (Mann–Whitney U test).
Results
Longevity
Wild as well as hand-reared ducks showed great variation in longevity. Most of either category was recovered during the first fall and winter, while 2–9 % lived to be 4 years or older (Fig. 1). The time lapsed from ringing until recovery was significantly shorter in hand-reared birds than in wild, in Sweden (hand-reared, mean = 258 days (SE = 19); wild, mean = 575 days (SE = 47); P < 0.001; Mann–Whitney U = 27,949, df = 586) as well as in Finland (hand-reared, mean = 129 days (SE = 12); wild, mean = 388 days (SE = 44); P < 0.001, Mann–Whitney U = 11,707, df = 376). Seventy-seven percent of the Swedish and 90 % of Finnish hand-reared mallards were recovered as dead within 1 year. Corresponding values for wild birds in the present sample are 45 % for Swedish and 66 % for Finnish mallards.
Migration distance
As predicted, wild Swedish birds were recovered farther from the ringing site than were hand-reared (wild, mean = 676 km (SE = 58); hand-reared, mean = 523 km (SE = 36), P = 0.026; independent t test, t = −2.251; df = 191; Fig. 2). The smaller Finnish sample also showed a difference in distance to recovery site (wild, mean = 1,213 km (SE = 81); hand-reared, mean = 157 km (SE = 110), P < 0.001; independent t test, t = −7.131; df = 59; Fig. 3). Note, though, that individual variation was substantial in both groups; from sedentary birds to those migrating at least 1,700 km. Four of the six Swedish birds that had migrated the farthest were in fact hand-reared (Fig. 2). Also, mean migration distance per day (i.e., reflecting speed of migration) differed between groups in both countries (Sweden: wild, mean = 4.8 km day−1 (SE = 0.38, n = 55); hand-reared, mean = 3.5 km day−1 (SE = 0.25; n = 138), P < 0.01; independent t test, t = −2.713; df = 191; Finland: wild, mean = 8.5 km day−1 (SE = 0.59, n = 44); hand-reared, mean = 0.8 km day−1 (SE = 0.59, n = 17), P < 0.001; independent t test, t = −7.59, df = 59).
Frequencies of distance from ringing site (in June–August) to recovery site (November–January the same season) in Finnish mallards (hand-reared (n = 17) and wild (n = 44)). The gap between 100 and 800 km recovery distance is because of the open water in the Baltic Sea, which Finnish mallards have to cross when they migrate southwest in fall
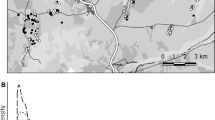
Recovery latitude did not differ significantly between the two study periods (P = 0.584; independent t test, t = 0.551; df = 48; inset in Fig. 4). However, birds in the early study period were ringed significantly farther north than those ringed in later years (P = 0.015, Mann–Whitney U = 194, df = 48; inset in Fig. 4). This might imply that present-day mallards migrate a shorter distance. Indeed, mean distance between ringing and recovery sites was 787 km (range, 4–1,372 km; SE = 75) in the early period 1947–1972, whereas it was 591 km (range, 6–1,479 km; SE = 96) in the late period 1977–1993 (Fig. 4). However, this difference was not statistically significant (P = 0.114; independent t test, t = 1.611, df = 48).
Ringing (circles) and recovery (squares) sites for wild mallards ringed in the time periods 1947–1972 (n = 25, red) and 1977–1993 (n = 25, blue). Inset map shows mean positions for ringing and recovery sites for the two periods. See “Material and methods” section for filtering criteria
Discussion
Longevity
In the present study, hand-reared Swedish and Finnish recovered birds showed 55 and 67 % reduction in mean total longevity (i.e., time until recovery), respectively, compared to wild. In a study carried out in the midcontinental North American mallard population, Brakhage (1953) also reported a dramatic reduction in survival (70 % of wild birds was recovered within 12 months, compared to 90 % of released hand-reared). The present study thus supports the prediction that wild mallards live longer than hand-reared, corroborating the pattern evident for mallard in earlier studies in Canada, Denmark, and the USA, (Brakhage 1953; Fog 1964; Lee and Kruse 1973; Soutiere 1989; Dunn et al. 1995). A similar pattern of reduced longevity has been documented in other species in which release of farmed stock is used to supplement wild-living populations (e.g., gray partridge Perdix perdix (Buner and Schaub 2008) and ring-necked pheasant Phasianus colchicus (Hill and Robertson 1986, 1987; Brittas et al. 1992)).
However, we here refrain from comparing mean longevity of hand-reared mallards among studies and countries. The main reasons for this are the large variation at what age ducklings are released, the extent to which ducklings are fed, and differences in subsequent hunting pressure (cf. Brakhage 1953; Lee and Kruse 1973; Soutiere 1989; Dunn et al. 1995). In this context, it is important to note that data presented in the present and many other studies do not concern true longevity, since these are based only on birds ringed and later recovered, and hence do not include those that were never recovered. Our estimates of longevity of recovered mallards should therefore not be compared with survival estimates generated by, e.g., traditional survival analyses with a capture–recapture approach according to Brownie et al. (1985). For example, ducks that die from natural causes rather than hunting are certainly underrepresented in the approach using recovery data only.
Inferring processes from these patterns is fraught with difficulty, but recent studies offer some insights. For example, Champagnon et al. (2011) demonstrated that farmed birds carry “a burden of captivity” in the sense that: (a) their bills are less efficient for sieving (extracting food from the water); (b) they have a smaller gizzard, at least for some time after release; and (c) they have a tendency to rely more on anthropogenic food sources. Further, despite similar time budgets and a gradual gizzard proliferation, released farmed mallards never caught up with the body condition of wild birds (Champagnon et al. 2011). In sum, this French study shows that farmed mallards had reduced physiological fitness, and that they had higher mortality than wild birds during fall and winter, even when not exposed to hunting. Although it needs further testing, differences in, e.g., bill morphology and foraging behavior may hypothetically also be important for the ultimate fate of ducks exposed to hunting, which was the case for the ducks in our study. Farmed ducks less adapted to utilizing natural food may have a preference to forage on hunting grounds subjected to food supplementation, which may result in higher hunting mortality as compared to wild conspecifics. There are no reasons to think that Fennoscandian-farmed birds should differ a lot from French, as they are largely farmed in a similar way. However, Swedish-farmed mallards are released at a younger and more vulnerable age than French, possibly leading to an even greater difference in early survival between farmed and wild birds. In this context, it should be noted that antipredator behaviors in ducklings and juveniles, wild as well as farmed, remain poorly studied. This is true with respect to natural predators as well as hunting. In other words, a physiological–behavioral interaction cannot be ruled out to explain the reduced longevity in hand-reared released mallards.
Regardless of process, the present study supports the conclusion in Champagnon et al. (2011) that only a small proportion (maximum 10–23 % in our study, <10 % in the French) of hand-reared released ducks survive long enough to get the chance to enter the breeding population. Arbitrarily reducing this proportion to compensate for unknown natural mortality, a 5 % survival of released birds would translate into an annual addition of 5,000–20,000 birds at minimum in Sweden as a crude estimate. This, in turn, would constitute some 1–5 % of the national breeding stock, but in reality a much higher fraction in the geographically limited areas to which releases are concentrated. Accordingly, in countries such as Denmark and France, released survivors may constitute a sizeable proportion of the wild breeding population in subsequent years. However, little is still known about how surviving released birds fare in the process of sexual selection and breeding success, so the effects on mean fitness of the wild population remain to be elucidated (Cheng et al. 1979).
Differences in ringing date may potentially introduce a bias on longevity if wild birds were ringed earlier than farmed. However, the pattern in median ringing date in our sample turns out to be the opposite, i.e., farmed mallards were ringed a bit earlier than wild ones, both in Sweden (July 11 versus August 1) and in Finland (July 12 versus July 25). This means that despite the fact that farmed mallards are protected from hunting mortality for a longer time than wild ones, until the opening of the hunting season in August, they have lower longevity. This strengthens our interpretation that hand-reared birds in general have lower longevity, and moreover makes the difference found by us a conservative estimate.
Migration distance
There was a striking difference in distance between ringing and recovery sites in wild and hand-reared birds, respectively, especially in the Finnish dataset. Wild mallards in the northern parts of Sweden and Finland migrate farther than their southern conspecifics (cf. Fransson and Pettersson 2001 p. 99). The present study thus corroborates previous research, as well as our second prediction by demonstrating that hand-reared mallards migrate shorter than wild (but see Brakhage 1953).
Distance to recovery site is not only a function of migratory strategy, but also one of survival. If hand-reared birds are in worse body condition because of inferior physiology and foraging behavior, or more likely to get shot or depredated because of less developed anti-predator behavior, they are not likely to get as far as wild birds, even if they do indeed have the same migratory strategy (cf. Välikangas 1933). As a case in point, four of the farthest-migrating individuals in our dataset were indeed hand-reared birds (Fig. 2). This highlights the necessity to separate the effects of migration strategy and survival when explaining spatial changes in recovery pattern, an insight rarely expressed in the ornithological literature to date. Interestingly, in the present study wild mallards showed a higher migratory speed than hand-reared in both national datasets. We thus tentatively conclude that the shorter migration distance observed in hand-reared mallards in Sweden and Finland is an effect of a combination of shorter life and slower migratory speed, the relative contributions of which need further study.
The third prediction was not supported by data, but we argue that this result needs to be interpreted with caution. Although we could not demonstrate a statistically significant reduction in migration distance over time, there are good reasons to re-address the issue in the near future. One is that our present analysis was based on a small sample (increasing the risk for a type II statistical error; cf. patterns in Fig. 4), and another is that our test was conservative in the sense that the “late” period does not contain data from the last 20 years, a period of dramatically increased releases of hand-reared mallards. Unfortunately, the difference in ringing latitude between periods complicates the interpretation, especially because it has been demonstrated previously that Swedish mallards generally migrate farther with increased natal latitude (Fransson and Pettersson 2001). This is to say that a more southerly sample of ringed birds can be expected to migrate shorter for natural reasons. A third reason to soon re-address migration distance in wild Swedish mallards in general is provided by Gunnarsson et al. (2012), who found a reduction over time in a geographically more limited sample. Although we can, as of yet, not conclude that introgression of hand-reared birds has led to reduced migration distance in the wild mallard stock, the topic is worth closer attention also because the processes behind a putative pattern of shorter winter migration are elusive. Two and mutually non-exclusive hypotheses need to be addressed in this context; (a) introgression of farmed-released birds with no or reduced migratory habits and (b) climate change, or more specifically shorter and milder winters at medium to high latitudes leading to delayed and/or shorter migration (cf. Korner-Nievergelt et al. 2010; Sauter et al. 2010; Lehikoinen and Jaatinen 2011; Gunnarsson et al. 2012).
Conclusion
We corroborate previous studies showing that hand-reared birds have shorter lives and migrate shorter than wild. At the same time, we caution that teasing apart the roles of within-season survival and migratory strategy for observed changes in recovery patterns requires further study. This is true not only for the mallard, but for many species in which there is growing concern about climate change. We do not find conclusive evidence for a long-term temporal trend of shorter fall–winter migration in wild mallards, but argue that this interpretation may be equivocal. Based on the fact that farming-release of mallards has become commonplace in Europe as late as during the last decades, all testing of patterns of migration distance to date, including the present, tend to be conservative. Surviving hand-reared birds may affect the wild stock also in other ways, not studied here. For example, they may carry “a burden of captivity” through the increased isolation of breeding stock from wild mallards, which is mainly due to epidemiological concerns in the wake of outbreaks of highly pathogenic avian influenza. Studies of several species demonstrate that breeding stock not in contact with wild conspecifics may develop physiological and behavioral maladaptations already after a few generations (cf. Ebinger and Lohmer 1984). In the case of the mallard, hand-reared released birds surviving hunting and winter weather might thus cause introgression, possibly leading to increased body weight, maladapted bill, underdeveloped gizzard, and possibly behavioral inferiorities with respect to foraging and predator avoidance in the wild population (Champagnon et al. 2010, 2011; Guillemain et al. 2010; Gunnarsson et al. 2011).
References
Batt BDJ, Nelson JW (1990) The role of hand-reared mallards in breeding waterfowl conservation. Trans of the N Am Wildl Nat Res Conf 55:558–568
Brakhage GK (1953) Migration and mortality of ducks hand-reared and wild-trapped at Delta, Manitoba. J Wildl Manage 17(4):465–477
Brittas R, Marcström V, Kenward RE, Karlbom M (1992) Survival and breeding success of reared and wild ring-necked pheasants in Sweden. J Wildl Manage 56(2):368–376
Brownie C, Anderson DR, Burnham KP, Robson DS (1985) Statistical inference from band recovery data—a handbook, 2nd edn. U.S. Fish and Wildlife Service, Resource Publication 156, Washington
Buner F, Schaub M (2008) How do different releasing techniques affect the survival of reintroduced grey partridges Perdix perdix? Wildl Biol 14(1):26–35
Champagnon J, Guillemain M, Gauthier-Clerc M, Lebreton J-D, Elmberg J (2009) Consequences of massive bird releases for hunting purposes: Mallard Anas platyrhynchos in the Camargue, Southern France. Wildfowl 2:184–191
Champagnon J, Guillemain M, Elmberg J, Folkesson K, Gauthier-Clerc M (2010) Changes in Mallard Anas platyrhynchos bill morphology after 30 years of supplemental stocking. Bird Study 57(3):1–8
Champagnon J, Guillemain M, Elmberg J, Massez G, Cavallo F, Gauthier-Clerc M (2011) Low survival after release into the wild: assessing the burden of captivity on Mallard physiology and behaviour. Eur J Wildl Res 58(1):255–267
Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Cheng KM, Shoffner RN, Phillips RE, Lee FB (1979) Mate preference in wild and domesticated (game-farm) mallards: II. Pairing success. Anim Behav 2:417–425
del Hoyo J, Elliot A, Sargatal J (1992) Handbook of the birds of the world, vol. 1. Lynx Edicions, Barcelona
Delany S, Scott DA (2006) Waterbird population estimates, 4th edn. Wetlands, Wageningen
Dunn JP, Diefenbach DR, Hartman FE (1995) Survival and recovery distribution of wild and captive-reared mallards in Pennsylvania. Norteast Wildl 52:8
Ebinger P, Lohmer R (1984) Comparative quantitative investigations on brains of rock doves, domestic and urban pigeons (Columba-l-livia). Z Zool Syst Evolutionsforschung 22(2):136–145
Fog J (1964) Dispersal and survival of released mallards (Anas platyrhynchos L.). Dan Rev Game Biol 4(3):1–57
Fog J (1965) The mallards from the estate of Kongsdal. Dan Rev Game Biol 4(3):61–94
Fransson T, Pettersson J (2001) Svensk ringmärkningsatlas [Swedish Bird Ringing Atlas], vol 1. Ljungföretagen Tryckeri AB. (In Swedish, English summary). Örebro
Guillemain M, Elmberg J, Gauthier-Clerc M, Massez G, Hearn R, Champagnon J, Simon G (2010) Wintering French mallard and teal are heavier and in better body condition than 30 years ago: effects of a changing environment? Ambio 39(2):170–180
Gunnarsson G, Elmberg J, Dessborn L, Jonzen N, Pöysä H, Valkama J (2008) Survival estimates, mortality patterns, and population growth of Fennoscandian mallards Anas platyrhynchos. Ann Zool Fenn 45(6):483–495
Gunnarsson G, Elmberg J, Waldenström J (2011) Trends in body mass of ducks over time: the hypotheses in Guillemain et. al. revisited. Ambio 40:338–340
Gunnarsson G, Waldenström J, Fransson T (2012) Direct and indirect effects of winter harshness on the survival of Mallard Anas platyrhynchos in Northwest Europe. Ibis 154:307–317
Heldbjerg H, Eskildsen A (2010) Overvågning af de almindelige fuglearter i Danmark 1975–2009. [Monitoring population changes of common birds in Denmark 1975–2009]. Årsrapport for Punkttællingsprojektet. Dansk Ornitologisk Forening. 56pp (in Danish, English summary)
Hill DA, Robertson P (1986) Hand reared pheasants: how do they compare with wild birds? Game Conser Ann Rep 17:76–84
Hill DA, Robertson P (1987) Hand reared pheasants: how they affect productivity. Game Conser Ann Rep 18:65–69
Hirschfeld A, Heyd A (2005) Mortality of migratory birds caused by hunting in Europe: bag statistics and proposals for the conservation of birds and animal welfare. Ber Vogelschutz 42:47–74
Korner-Nievergelt F, Sauter A, Atkinson PW, Guélat J, Kania W, Kéry M, Köppen U, Robinson RA, Schaub M, Thorup K, van der Jeug H, van Noordwijk AJ (2010) Improving the analysis of movement data from marked individuals through explicit estimation of observer heterogeneity. J Avian Biol 41:8–17
Kraus RHS, Zeddeman A, van Hooft P, Sartakov D, Soloview SA, Ydenberg RC, Prins HHT (2011) Evolution and connectivity in the world-wide migration system of the mallard: interferences from mitochondrial DNA. BMC Genet 12 (99)
Laikre L, Palmé A, Josefsson M, Utter F, Ryman N (2006) Release of alien populations in Sweden. Ambio 35(5):255–261
Lee FB, Kruse AD (1973) High survival and homing rate of hand-reared wild-strain mallards. J Wildl Manage 37(2):154–159
Lehikoinen A, Jaatinen K (2011) Delayed autumn migration in northern European waterfowl. J Ornithol 152:563–570
Lincoln FC (1934) Restocking of marshes with hand-reared mallards not proved practicable. Yearb Agric pp. 310–313
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion ecology. Blackwell, Malden
Mondain-Monval J-Y, Girard O (2000) Le canard colvert, la sarcelle dhiver et autres canards de surface. Faune Sauvage 251:124–139
Noer H, Søndergaard M, Jørgensen TB (2008) Udsætning af gråænder i Danmark og påvirkning af søers fosforindhold. Danmarks Miljøundersøgelser, Aarhus Universitet-Faglig rapport fra DMU nr. 687, 44pp (in Danish)
Raftovich RV, Wilkins KA, Williams SS, Spriggs HL, Richkus KD (2011) Migratory bird hunting activity and harvest during the 2009 and 2010 hunting seasons. U.S. Fish and Wildlife Service, Laurel, p 68
Sauter A, Korner-Nievergelt F, Jenni L (2010) Evidence of climate change effects on within-winter movements of European Mallards Anas platyrhynchos. Ibis 152(3):600–609
Scott DA, Rose P (1996) Atlas of anatidae populations in Africa and Western Eurasia. Wetland International Publication No.41, Wageningen
Soutiere EC (1989) Survival rates of hand-reared mallards released on 2 private farms. J Wildl Manag 53(1):114–118
Speek G, Clark JA, Rohde Z, Wassenaar RD, Van Noordwijk AJ (2001) The EURING exchange-code 2000. The European Union for Bird Ringing, Heteren
Välikangas I (1933) Finnische Zugvögel aus englischen Vogeleiern. Vogelzug 4:159–166
Wiberg S, Gunnarsson S (2007) Inventering av hållning och uppfödning av viltfågel i Sverige [A survey of game bird rearing in Sweden]. Sveriges Lantbruksuniversitet. Institutionen för husdjurens miljö och hälsa. Avdelningen för husdjurshygien. Rapport 17. [Swedish University of Agricultural Sciences. Department of Animal Environment and Health. Section of Animal Hygiene. Report 17], 66pp (in Swedish, English summary), Skara
Yerkes T, Bluhm C (1998) Return rates and reproductive output of captive-reared female mallards. J Wildl Manag 62(1):192–198
Zeddeman A, Van Hooft P, Prins HHT, Kraus RHS (2009) Mallard (Anas platyrhynchos) gene pool connectivity between Greenland, eastern Canada, Great Britain and the Netherlands. In: Jensen LM, Rasch M (eds) Nuuk ecological research operations, 2nd annual report, 2008. National Environmental Research Institute, Aarhus University, Denmark
Acknowledgments
We gratefully thank Henrik Svensson for help with the maps. This study was supported by grant V-205-09 from the Swedish Environmental Protection Agency. The Finnish Ringing Centre at the Natural History Museum (Helsinki, Finland) and The Bird Ringing Centre at The Swedish Museum of Natural History (Stockholm, Sweden) are gratefully acknowledged for access to data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Söderquist, P., Gunnarsson, G. & Elmberg, J. Longevity and migration distance differ between wild and hand-reared mallards Anas platyrhynchos in Northern Europe. Eur J Wildl Res 59, 159–166 (2013). https://doi.org/10.1007/s10344-012-0660-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-012-0660-0