Abstract
Changes in agricultural management have been identified as the most probable cause for the decline of Skylark (Alauda arvensis) populations in Europe. However, parasitic infections have not been considered as a possible factor influencing this process. Four hundred and thirty-four Skylarks from the Southern Italy and the Netherlands were screened for haemosporidian parasites (Haemosporida) using the microscopy and polymerase chain reaction (PCR)-based methods. The overall prevalence of infection was 19.5%; it was 41.8% in Italian birds and 8.3% in Dutch birds. The prevalence of Plasmodium spp. was 34.1% and 6.5% in Skylarks from Italy and Netherlands, respectively. Approximately 15% of all recorded haemosporidian infections were simultaneous infections both in Italian and Dutch populations. Six different mitochondrial cytochrome b (cyt b) lineages of Plasmodium spp. and three lineages of Haemoproteus tartakovskyi were found. The lineage SGS1 of Plasmodium relictum was the most prevalent at both study sites; it was recorded in 24.7% of birds in Italy and 5.5% in the Netherlands. The lineages SYAT05 of Plasmodium vaughani and GRW11 of P. relictum were also identified with a prevalence of <2% at both study sites. Two Plasmodium spp. lineages (SW2 and DELURB4) and three H. tartakovskyi lineages have been found only in Skylarks from Italy. Mitochondrial cyt b lineages SYAT05 are suggested for molecular identification of P. vaughani, a cosmopolitan malaria parasite of birds. This study reports the greatest overall prevalence of malaria infection in Skylarks during the last 100 years and shows that both Plasmodium and Haemoproteus spp. haemosporidian infections are expanding in Skylarks so it might contribute to a decrease of these bird populations in Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigment-forming haemosporidians (Haemosporida) are a clearly phylogenetically defined group of obligate heterogeneous parasites, which inhabit in birds all over the world except the Antarctic (Greiner et al. 1975; McClure et al. 1978; Atkinson and van Riper 1991; Bishop and Bennett 1992). Over 45% of bird species of the world fauna have been currently investigated with respect to infection with haemosporidians (Valkiūnas 2005). Haemoproteus spp. was recorded in approximately 50% and Plasmodium and Leucocytozoon spp. in approximately 30% of the investigated bird species (Valkiūnas 2005). Although these parasites are widespread geographically, their prevalence varies markedly between different regions, host species and populations (Greiner et al. 1975; Valkiūnas 2005).
The Skylark (Alauda arvensis) is largely distributed in the temperate zone of Europe, Asia and Northern Africa, with 13 subspecies described (Cramp 1988; Donald 2004). In Western Europe, the Skylark is undergoing a rapid population decline in recent years, as are many other farmland birds (BirdLife International 2004; Donald et al. 2006). The decline of farmland birds is frequently associated with changed conditions during the breeding season and deterioration of the breeding habitat (Donald 2004; Newton 2004). Although the impact of parasites on bird populations is often overlooked in wildlife ornithology, parasitism certainly is an important factor in conservation biology and should therefore be considered in biodiversity preservation studies (Valkiūnas 2005; Parker et al. 2006). The establishment of parasites in new hosts and geographic areas is often associated with changes in virulence and might lead to devastating outbreaks among resident bird populations, which is particularly well documented in relatively simple island ecosystems in Hawaii islands (Atkinson and van Riper 1991) and recently recorded in Galápagos (Levin et al. 2009). A possible role of blood parasites as a factor influencing the decline of Skylark populations has not been considered because of limited knowledge about haemosporidian infections in this bird species (Bennett et al. 1982; Valkiūnas 2005). The aim of this study was to describe distribution, diversity, prevalence and intensity of haemosporidian parasites in Skylarks from two sites in Europe.
Materials and methods
Study sites and collection of blood samples
One hundred and forty-six Skylarks were caught in the Volturno Plain (41°02′ N, 13°55′ E) located 40 km north of Naples, Italy between 1 and 30 of October in 2006 and 2007. Two hundred and eighty-eight Skylarks were sampled in the northern part of the Netherlands (52°55 N, 006°18 E) between May 2006 and December 2007: 12 of them were caught during the period of establishment of breeding territory, 145 adult birds—during the breeding season, 9—during moult, 45—during autumn migration and 2—during winter. Additionally, 71 nestlings have been sampled in nests; they were 5–7 days old. Skylarks were caught using mist nets or traps. The birds were banded to avoid resampling. Blood samples were taken by puncturing the brachial vein. Blood films were air-dried, fixed in absolute methanol in the field and stained with Giemsa solution in the laboratory as described by Valkiūnas et al. (2008b).
A complementary blood sample (20–50 μl) was collected using heparinized microcapillaries and stored in non-lysis SET buffer (Waldenström et al. 2004) or in 96% ethanol (only Dutch samples). In the field, the samples were stored at ambient temperature (Italy) or on ice (the Netherlands) and later stored at −20°C in the laboratory. The blood samples were analysed by molecular methods between 1 and 24 months after their collection. In total, 434 samples were collected at both study sites (Table 1).
Examination of blood films and parasite morphology
An Olympus BX51 light microscope equipped with an Olympus DP12 digital camera and imaging software DP-SOFT was used to examine blood slides, prepare illustrations and to take measurements. Blood films were examined for 10–15 min at low magnification (×400), and then at least 100 fields were studied at high magnification (×1,000), as described by Valkiūnas et al. (2008b). We used the morphometric features (Table 2) and identified parasites according to Valkiūnas (2005). The intensity of infection was estimated as a percentage by actual counting of the number of parasites per 1,000 red blood cells or per 10,000 red blood cells if infections were light (i.e. <0.1%), as recommended by Godfrey et al. (1987).
The morphology of gametocytes of Haemoproteus tartakovskyi from Skylarks was compared with the type and voucher specimens of H. tartakovskyi from its type host the Common crossbill (Loxia curvirostra) (accession no. 413.91) at the Collection of the Institute of Ecology, Nature Research Centre, Vilnius. The morphology of Plasmodium (Novyella) vaughani from the Skylark was compared with the type and voucher material of the same parasite from its type vertebrate host the American robin (Turdus migratorius) and additional vertebrate host the Blackbird (Turdus merula) (accession nos. 635, 639, 654 and 655) in the Garnham Collection at the Natural History Museum, London.
Extraction of DNA, PCR, sequencing and analysis of molecular data
The DNA was extracted using a standard ammonium acetate method (Sambrook et al. 2002). Diluted total DNA was used as the template in PCR assays for detection of the parasites, using primers and temperature profiles as in Hellgren et al. (2004). The method consists of a nested PCR assay that amplifies a part of the parasites mitochondrial cytochrome b (cyt b) gene in two steps, first an initial PCR (primers HaemNFI/HaemNR3; 570 bp excluding primers) that amplifies parasites from all of the three genera followed by a second step that separates Leucocytozoon spp. (primer HaemFL/HaemR2L; 478 bp excluding primers) from parasites of the genera Plasmodium and Haemoproteus spp. (primers HaemF/HaemR2; 480 bp excluding primers). By amplifying the parasite DNA in two PCRs, the sensitivity of the screening is increased (Waldenström et al. 2004; Hellgren et al. 2004). Positive or negative infections were seen as presence or absence of bands on a 2% agarose gel using 1.5 μl of the final PCR product. Samples which showed positive amplification were sequenced using the procedures described by Bensch et al. (2000). Amplified fragments were sequenced from the 5′ end with the primer HaemF. We used dye terminator cycling sequencing (big dye) kit and the samples were loaded on an ABI PRISM ™ 3100 sequencing robot (Applied Biosystems, Florida, USA).
The obtained sequences were edited and aligned using the BioEdit programme (Hall 1999). The appearance of double peaks in the sequence was considered as mix infection. All unique lineages, i.e. sequences differing by one or more nucleotide base pair, were sequenced in the reversed direction with the complement primer HaemR2. The 38 taxa (total 474 nucleotides) in the final alignment were used for Bayesian analysis. Bayesian phylogeny was constructed using mrBayes version 3.1.2 (Ronquist and Huelsenbeck 2003). We used the General Time Reversible model including invariable sites and variation among sites (GTR+I+G) as suggested by the software mrModeltest 2.2 (Nylander 2004, software available from <http://www.ebc.uu.se/systzoo/staff/nylander.html>). Two simultaneous runs were conducted with a sample frequency of every 100th generation over three million generations. Convergence in phylogeny estimation for each analysis was assessed using the programme Tracer (Rambaut, A. & Drummond, A., available at http://evolve.zoo.ox.ac.uk) and used to indicate the appropriate “burn-in” period. The 25% of the trees were discarded as burn-in period. The remaining trees were used to construct a majority rule consensus tree. The phylogenies were visualised using Tree View 1.6.6. (software available from <http://evolution.genetics.washington.edu/phylip/software.html>). We used sequences of haemosporidian parasites, which species were positively identified (for linkage of parasite lineages with their morphospecies, see Križanauskienė et al. 2006; Palinauskas et al. 2007; Krone et al. 2008; Martinsen et al. 2008; Valkiūnas et al. 2008a, b; Zehtindjiev et al. 2008; Bensch et al. 2009; Križanauskienė et al. 2010). GenBank accession numbers and MalAvi reference names (see Bensch et al. 2009) of all lineages mentioned in this article are given in Fig. 1
Bayesian phylogeny of cytochrome b gene lineages of positively identified species of avian pigment-forming haemosporidian parasites. Lineages recorded in the Skylark A. arvensis are given in bold. Names of the lineages (when available) are given before the species names of parasites; GenBank accession numbers of the lineages are provided after the parasite species names. Nodal support values (circles—80–100%; triangles—60–70%) indicate posterior clade probabilities
The sequence divergence between the different lineages was calculated with the use of a Jukes–Cantor model of substitution, with all substitution weighted equally, implemented in the programme MEGA version 4 (Tamura et al. 2007) where the pairwise deletion was selected.
Student’s t test for independent samples was used to determine statistical significance between mean linear parameters. Prevalences were compared by Yates corrected Chi-square test. A P value of 0.05 or less was considered significant.
The representative blood slides were deposited in the Nature Research Centre, Vilnius, Lithuania (accession nos. 47733–47736 NS). Sequences of new parasite lineages were deposited in GenBank (nos. GU289671, GU289672 and GU289673).
Results
Molecular analysis of blood samples
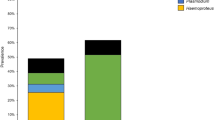
Only pigment-forming haemosporidian parasites of the genera Haemoproteus and Plasmodium were found using molecular techniques; that is in accord to microscopy data (see below) (Table 1). The overall prevalence of haemosporidian infection was 19.5%; it was 41.8% in Italian and 8.3% in Dutch Skylarks (P < 0.001). We detected three lineages of Haemoproteus spp. and six lineages of Plasmodium spp. in 85 infected Skylarks (Fig. 1). Plasmodium parasites were found in 34.1% of Italian and 6.5% of Dutch Skylarks (P < 0.001). Haemoproteids were present in 2% of the Italian birds and absent from the Dutch Skylarks. In Dutch Skylarks, the prevalence of Plasmodium spp. was 4.6% for adults and 9.9% in nestlings (P < 0.05, not significant), indicating the active malaria transmission at the study site. According to the PCR diagnostics, 5.5% and 1.7% simultaneous infections have been detected in Italian and Dutch populations, respectively. All recorded malarial infections were simultaneous in nestlings.
At both study sites, the lineage SGS1 of Plasmodium relictum was most prevalent; it was recorded approximately in 24.7% of birds in Italy and 5.5% in the Netherlands (Table 1). This lineage together with the lineages pGRW11, pMOALB1, pDELURB4 and pGRW4 form well-supported clade of P. relictum morphospecies (Fig. 1). Lineages pGRW11, pDELURB4 and pSW2 has been recorded only in Italian birds and lineage pMOALB1 was recorded only in Dutch Skylarks; these lineages were rare (prevalence < 5%). Plasmodium lineage pSYAT05 was found in both studied populations (Table 1).
Three Haemoproteus lineages (hALARV1, hALARV2 and hALARV3) were found only in Skylarks from Italy. These lineages cluster together with lineage hSISKIN1 of H. tartakovskyi (the p distances between these lineages ranged from 0.2% to 2.3% with a total mean distance of 1.25%) and form well-supported clade with the latter parasite (Fig. 1).
Microscopic investigation
All samples were examined microscopically, and the PCR-based diagnostics (both positive and negative results) was confirmed by microscopic observations. Microscopic examination revealed undetected by PCR simultaneous haemosporidian infections, which were present approximately in 15% of infected birds in both Italian and Dutch populations. Over 60% of all recorded infections were light (<0.001%), so it could be regarded as chronic. For some of the detected cyt b lineages (pSW2, pMOTALB1 and pDELURB4), we were unable to identify species due to low intensity of parasitemia and absence of all necessary blood stages on the slides.
H. tartakovskyi (Figs. 1 and 2e–h) (lineages hALARV1, hALARV2 and hALARV3), P. (Novyella) vaughani (Figs. 1 and 2i–l) (lineage pSYAT05) and P. relictum (lineage pSGS1) were identified using morphological features of blood stages of the parasites. Lineages pMOALB1 and pDELURB4 of Plasmodium spp. are closely related to the lineages pSGS1, pGRW11 and pGRW4 of P. (Haemamoeba) relictum (Palinauskas et al. 2007; Knowles et al. 2010) with genetic difference among them between 0.2% and 2.3% (Fig. 1); these lineages probably belong to this morphospecies. However, our material is incomplete for these parasites’ unequivocal identification using morphological characters because the recorded infections were too light. The unidentified Plasmodium lineage (pSW2) is genetically distant from other Plasmodium spp. lineages (from 6.6% to 12.3%); based on available morphological features, it belongs to subgenus Novyella. Additional material is needed for identification of this parasite species.
H. tartakovskyi from the blood of its type vertebrate host, the crossbill L. curvirostra (a–d, lineage hSISKIN1) and the Skylark A. arvensis (e–h, lineage hALARV03), and P. (Novyella) vaughani (i–l, lineage pSYAT05) from the Skylark: a–b, i, e young gametocytes; c, g, j—macrogametocytes; d, h, k—microgametocytes; l—erythrocytic meront. Arrow—a refractive colourless globule. Giemsa-stained thin blood films. Bar = 10 μm
The parasites of the lineages hALARV1, hALARV2 and hALARV3 (Figs. 1 and 2e–h) are indistinguishable morphologically among each other and from H. tartakovskyi; they are also genetically similar to the lineage pSISKIN1 of H. tartakovskyi (Figs. 1 and 2a–d) from its type host, the Common crossbill (L. curvirostra) and additional host the Siskin (Spinus spinus). Genetic divergence between these lineages varies between 0.2% and 2.1%. We consider all these lineages as intraspecies genetic variation of the same morphospecies, i. e. H. tartakovskyi.
The intensity of parasitemia was light in all infected birds; it was <0.05% in the great majority of our samples. The highest intensity of malaria infection (0.77%) was recorded in one bird infected with the P. relictum lineage SGS1 in Italy on 27 October 2006. All Haemoproteus infections were of low intensity (between 0.015% and 0.018%). Because (1) H. tartakovskyi has been recorded in Skylarks for the first time and (2) lineages for molecular identification of P. vaughani, a widespread agent of avian malaria have not been determined, we describe recorded in this bird parasites briefly and link these morphospecies with their cyt b lineages.
Description of parasites
P. (Novyella) vaughani Novy and MacNeal, 1904 (Fig. 2i–l, Table 2)
Avian host: Skylark (A. arvensis) (Passeriformes, Alaudidae)
DNA sequences: Mitochondrial cyt b gene lineage pSYAT05 (479 bp), GenBank accession no. DQ847271
Prevalence: five of 434 (1.2%) (Table 1)
Additional hosts: The lineage pSYAT05 of P. vaughani has been recorded in seven bird species: American robin (T. migratorius), Blackbird (T. merula), Blackcap (Sylvia atricapilla), Sardinian warbler (Sylvia melanocephala) and Tomtit (Petroica macrocephala) (Bensch et al. 2009; Bentz et al. 2007; Hellgren et al. 2007; Martinsen et al. 2008).
Geographical distribution: the lineage pSYAT05 was recorded in the USA, Europe and New Zealand (Bensch et al. 2009; Bentz et al. 2007; Hellgren et al. 2007; Martinsen et al. 2008), so it seems to be cosmopolitan in distribution.
Representative blood films: Voucher specimen (accession number 47736 NS A. arvensis, 4 October 2007, collected by P. Zehtindjiev) is deposited in the Nature Research Centre, Vilnius, Lithuania. Simultaneous infection of P. relictum is present in the voucher slide 47736 NS.
Erythrocytic meronts (Fig. 2l): Develop in mature erythrocytes; they were seen anywhere in the host cells. Fully grown meronts are variable in form, most frequently are roundish, oval or irregular; mature meronts contain between four and eight merozoites (Fig. 2l, Table 2); one clearly defined round refractive colourless globule is present in each meront (Fig. 2l, Table 2); it is frequently located close to a clump of pigment granules. The influence of meronts on infected erythrocytes is not pronounced.
Macrogametocytes (Fig. 2j) develop in mature erythrocytes and are elongated in form. The cytoplasm is homogeneous in appearance, sometimes contains large vacuoles. Parasite nucleus is prominent (Table 2), variable in shape and usually median in position (Fig. 2j). Pigment granules are few (Table 2), elongated or sometimes roundish, of medium size (0.5–1.0 μm), randomly scattered throughout the cytoplasm or clumped in small groups. The influence of gametocytes on infected erythrocytes is not pronounced.
Microgametocytes (Fig. 2i, k): The general configuration is as for macrogametocytes with the usual haemosporidian sexual dimorphic characters. The parasite nucleus is diffuse, and its size is markedly variable in different gametocytes (Fig. 2i, k).
H. tartakovskyi Valkiūnas 1986 (Fig. 2e–h, Table 2)
Avian host: Skylark (A. arvensis) (Passeriformes, Alaudidae)
DNA sequences: Mitochondrial cyt b gene lineages hALARV1 (479 bp, GenBank accession no. GU289671), hALARV2 (479 bp, GenBank accession no. GU289672) and hALARV3 (479 bp, GenBank accession no. GU289673).
Prevalence: four of 434 (1.0%) (Table 1)
Additional hosts: The lineages hALARV1, hALARV2 and hALARV3 have been recorded only in Skylarks so far. Closely related lineage hSISKIN1 is prevalent in crossbills and siskins in Europe (Fig. 1).
Geographical distribution: The lineages hALARV1, hALARV2 and hALARV3 have been recorded only in Italy.
Representative blood films: Voucher specimens (accession numbers 47733 NS, 47734NS and 47735 NS, A. arvensis, 23–26 October 2006, collected by P. Zehtindjiev) are deposited in the Institute of Ecology, Nature Research Centre, Vilnius, Lithuania.
Young gametocytes (Fig. 2e, f): The earliest forms (Fig. 2e) can be seen anywhere in the infected erythrocytes; they are roundish or oval, each possesses a large nucleus and prominent cytoplasm. As parasite develops, gametocytes adhere to the erythrocyte nuclei and extend longitudinally along the nuclei markedly displacing them laterally (Fig. 2f).
Macrogametocytes (Fig. 2g): The cytoplasm is homogeneous in appearance, sometimes contains small vacuoles. Gametocytes markedly displace the nucleus of erythrocytes laterally, frequently to the envelope of the host cells (Fig. 2c, g). Parasite nucleus is oval or roundish usually more or less median in position (Fig. 2g); pigment granules are numerous (Table 2), oval and roundish, of medium size (0.5–1.0 μm), usually randomly scattered throughout the cytoplasm.
Microgametocytes (Fig. 2h): The general configuration is as for macrogametocytes with the usual haemosporidian sexual dimorphic characters. The parasite nucleus is diffuse, and its size is variable in different gametocytes (Fig. 2h).
Discussion
Molecular and microscopy approaches were combined in investigations of haemosporidian parasites of Skylarks for the first time during this study. The PCR-based methods were less sensitive in determining simultaneous infections than microscopic examination of blood films. That should be taken in consideration in field studies of blood parasites using general primers, as previously discussed by Valkiūnas et al. (2006) and Martínez et al. (2009).
P. relictum (lineages pSGS1) was the most prevalent haemosporidian parasite in Skylarks. That was expected because this lineage is widespread and actively transmitted in the Old World (Palinauskas et al. 2007; Bensch et al. 2009). Unidentified to species level, Plasmodium lineages pDELURB4 and pMOALB1 are closely related to pSGS1 and pGRW11 (Fig. 1), so probably belong to the same morphospecies P. relictum, but further morphological studies are needed to prove that.
Linkage between DNA sequences and identifications based on traditional morphological species can provide important information about life history strategies for parasitologists and evolutionary biologists studying phylogenetic relationships of these organisms; it can also be used for molecular identification of parasites (Križanauskienė et al. 2010). Unfortunately, the number of incorrectly identified species is increasing in GenBank (Valkiūnas et al. 2008a). To ensure parasites’ species identification, we used museum type specimens in our identifications and also provided brief description of reported parasites in this study.
P. vaughani is second only to P. relictum in frequency of occurrence in birds. According to the previous studies, this avian malaria parasite has been reported in numerous bird species belonging to many host families and even orders, but is particularly common in passerines (Garnham 1966; Valkiūnas 2005), so the record of P. vaughani in Skylarks was not unexpected. In spite of worldwide distribution, molecular identification of P. vaughani has not been developed yet. That is important to do because the majority of natural malarial infections are light, so frequently are difficult to identify to species level in single blood films. We suggest using the lineage pSYAT05 for molecular identification of P. vaughani. Morphological features and measurements of blood stages of parasites of the lineage pSYAT05 are indistinguishable from P. vaughani from its type vertebrate hosts, the American robin. Gametocytes (Fig. 2i–k) and erythrocytic meronts (Fig. 2l), which are typical for P. vaughani, predominate among parasites of the pSYAT05 lineage in our material. Importantly, the same lineage was found in the USA in the American robin, the type host of P. vaughani (Martinsen et al. 2008); it also present in the black bird, which is a common host of this parasite in Europe (Valkiūnas 2005; Hellgren et al. 2007). These data are in accord with former microscopic investigations, which showed cosmopolitan distribution and broad avian host range of P. vaughani (Garnham 1966; Corradetti and Scanga 1973; Bennett et al. 1982; Valkiūnas 2005). Formerly, P. vaughani was found in Skylarks only in Kazakhstan (prevalence is 3%, see Yakunin and Zhazyltaev 1977). This parasite has been reported in European Skylarks for the first time during this study.
We found the lineage SW2 of Plasmodium (Novyella) sp. only in Italian birds. This lineage is common in sedge warblers (Acrocephalus shoenobaenus) in Africa (Waldenström et al. 2002); it was found in Skylarks for the first time during this study. We were unable to identify this parasite to species level because intensity of infection was light.
The gametocytes of the lineages hALARV1, hALARV2 and hALARV3 are indistinguishable from each other in all their main qualitative and morphometric parameters. Comparison of blood stages of these Skylark parasites with the type specimens of H. tartakovskyi (lineage hSISKIN1, Fig. 2a–d) from its type vertebrate host, the Common crossbill (Valkiūnas 1986) showed that all these haemoproteids are indistinguishable. We consider all these lineages as intraspecies variation of H. tartakovskyi and attribute them this species. That is in accord to our phylogenetic analysis and is similar to the level of intaspecies variation reported, for instance in Haemoproteus balmorali (Fig. 1). Lineages of H. tartakovskyi are prevalent in Common crossbills, Hawfinches and Siskins in Europe (Bensch et al. 2009). This haemoproteid has been reported in Skylarks for the first time during this study. Because H. tartakovskyi normally is prevalent in fringillid birds (Valkiūnas 1986), it might be that our report of this parasite is a case of new emerging haemosporidian infection in European Skylarks. H. tartakovskyi is transmitted by biting midge Culicoides impunctatus (Valkiūnas and Liutkevičius 2002); its development in avian host and virulence remains unknown.
Plasmodium (Novyella) sp. (lineage pSW2) infection has been recorded in African migrating Sage Warbler (Acrocephalus schoenobaenus) (Waldenström et al. 2002). According to our study, this malaria parasite has been reported for the first time in Skylarks, so it should be considered as a possible new pathogen in this bird species, so it might contribute to decrease of Skylarks’ population in Europe. It is interesting that the lineage pSW2 have been reported in non-migrant Tawny owl (Strix aluco) in Germany (Krone et al. 2008), so transmission certainly takes place in Europe.
Only two haemosporidian parasite species have been identified in Skylarks in Western Europe in the twentieth century: Haemoproteus alaudae and Plasmodium (Haemamoeba) supraecox (Bennett et al. 1982; Valkiūnas 2005). Malaria parasites have been recorded incidentally in this bird, and the overall prevalence of haemoproteids was reported to be <10% before massive decline of Skylarks populations in Western Europe (Peirce 1981). Interestingly, these two haemosporidian parasites were not recorded during this study. However, we found at least four additional species (three Plasmodium and one Haemoproteus) and nine different mitochondrial DNA lineages of haemosporidians, which have not been reported in Skylarks in Western Europe before. It is worth noting that P. relictum has been reported in one of five examined Skylarks in Georgia (Burtikashvili 1978) and P. vaughani was seen in two of 65 examined Skylarks in Kazakhstan (Yakunin and Zhazyltaev 1977), so these parasites have broad range of distribution in Skylarks. Recently, Plasmodium spp. were found in 20% of Skylarks in France (Chavatte et al. 2009). However, during this study, we report highest prevalences and genetic diversity of malaria parasites and haemoproteids than ever been reported in this bird before. Additionally, H. tartakovskyi seems to be an emerging parasite in Italian Skylarks because it has been reported only in birds belonging to the Fringillidae so far; it is prevalent in Siskins, Common crossbills and Hawfinch in Europe (Valkiūnas et al. 2003; Valkiūnas 2005). These data indicate possible ongoing colonization of Skylarks by blood parasites in changing environment conditions. That warrants further investigation, particularly due to recent reports on mortality of birds caused by emerging Haemoproteus spp. infections in Europe (Olias et al. 2011). It worth noting that transmission of the same lineages of haemoproteids between passerines of different families certainly occurs in Europe (Križanauskienė et al. 2006, 2010), but epidemiological significance of this phenomenon remains insufficiently understood.
It is important to note that significantly greater prevalence of pigment-forming haemosporidian infections was recorded in Skylarks sampled in Italy than in the Netherlands (Table 1). The birds sampled in Italy were caught during their seasonal migration; they probably came from Northeastern Europe to the study site and were on their way to wintering quarters in the Mediterranean.
Importantly, malarial parasites were prevalent in 5–7 days old nestlings during this study. Furthermore, all recorded Plasmodium spp. infections were simultaneous in nestlings; such infections have been reported to be particularly virulent in birds (Zehtindjiev et al. 2008; Palinauskas et al. 2009). Because Plasmodium spp. are particularly virulent and even lethal in juveniles (Garnham 1966; Valkiūnas 2005), it seems probable that malaria might be a factor contributing to mortality in European Skylarks due to direct or indirect (via predators) elimination of nestlings and/or fledglings.
The Skylarks are “species of Conservation Concern in Europe”, owing to the populations’ decline of over 50%, particularly in West Europe since 1960s and mainly since 1980s (BirdLife International 2004). Although there are examples of dramatic impact of malaria parasites and other haemosporidians on wildlife, particularly in ecosystems where malaria has been emerging (Garnham 1966; Atkinson et al. 2001; Valkiūnas 2005; Levin et al. 2009), blood parasites have not yet been considered in understanding the decline of Skylark populations. This study does not provide final answer if blood parasites contribute to the decline of Skylarks populations; additional field studies comparing distribution and virulence of certain species of haemosporidians between areas where Skylarks are decreasing and where populations are stable are needed to answer this questions satisfactorily. However, our data indicate that haemosporidian parasites are likely emerging in Skylarks, so should be considered in conservation programmes. That is particularly true due to recent reports about mortality in birds caused by emerging haemosporidian infections in Europe (Olias et al. 2011; Valkiūnas 2011). Thus, the recent decline of Skylark populations in Western Europe is accompanied by increased prevalence and diversity of haemosporidian parasites, which should be considered as possible factors contributing to the population decline process and need further research.
References
Atkinson CT, van Riper IIIC (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) ird-parasite interactions: Ecology, evolution, and behaviour. Oxford University Press, Oxford, U.K., pp 19–48
Atkinson CT, Lease JK, Drake BM, Shema NP (2001) Pathogenicity, serological responses, and diagnosis of experimental and natural malarial infections in native Hawaiian thrushes. Condor 103:209–218
Bennett GF, Whiteway M, Woodworth-Lynas C (1982) A host-parasite catalogue of the avian haematozoa. Occas Pap Biol, Memorial University of Newfoundland 5:1–243
Bensch S, Stjenman M, Hasselquist D, Östman Ö, Hansson B, Westerdahl H, Torres-Pinheiro R (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. P Roy Soc Lond B Bio 276:1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related pigment-forming haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
BirdLife International (2004) Birds in Europe: population estimates, trends and conservation status. Cambridge, UK: BirdLife International. (BirdLife conservation series No. 12)
Bishop MA, Bennett GF (1992) Host-parasite catalogue of the avian haematozoa: supplement 1, and bibliography of the avian blood-inhabiting haematozoa: supplement 2. Occas Pap Biol, Memorial University of Newfoundland 15:1–244
Burtikashvili LP (1978) Blood parasites of wild birds in Georgia. Tbilisi, Metsniereba (in Russian)
Chavatte JM, Gres V, Snounou G, Chabaud A, Landau I (2009) Plasmodium (Apicomplexa) of the skylark (Alauda arvensis). Zoosystema 31:369–383
Corradetti A, Scanga M (1973) The Plasmodium vaughani—complex. Exp Parasitol 34:344–349
Cramp S (1988) The birds of the Western Palearctic, vol 5. Oxford University Press, Oxford
Donald PF (2004) The skylark. T&AD Poyser, London
Donald PF, Sanderson FJ, Burfield IJ, van Bommel FPJ (2006) Further evidence of continent-wide impacts of agriculture intensification on European farmland birds, 1990–2000. Agr Ecosyst Environ 116:189–196. doi:10.1016/j.agee.2006.02.007
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell, Oxford
Godfrey RD, Fedynich AM, Pence DB (1987) Quantification of hematozoa in blood smears. J Wildlife Dis 23:558–565
Greiner EC, Bennett GF, White EM, Coombs RF (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor v. 5.0.9. Nucl Acid S 41:95–98
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon spp., Plasmodium spp. and Haemoproteus spp. from avian blood. J Parasitol 90:797–802
Hellgren O, Waldenström J, Pérez-Tris J, Szöll ÖE, Hasselquist D, Križanauskienė A, Ottosson U, Bensch S (2007) Detecting shifts of transmission area in avian blood parasites—a phylogenetic approach. Mol Ecol 16:1281–1290. doi:10.1111/j.1365-294X.2007.03227.x
Knowles SCL, Palinauskas V, Sheldon BC (2010) Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evol Biol 23:557–569
Križanauskienė A, Hellgren O, Kosarev V, Sokolov LV, Bensch S, Valkiūnas G (2006) Variation in host specificity between species of avian haemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. J Parasitol 92:1319–1324
Križanauskienė A, Pérez-Tris J, Palinauskas V, Hellgren O, Bensch S, Valkiūnas G (2010) Molecular phylogenetic and morphological analysis of haemosporidian parasites (Haemosporida) in a naturally infected European songbird, the blackcap Sylvia atricapilla, with description of Haemoproteus pallidulus sp. nov. Parasitology 137:217–227
Krone O, Waldenström J, Valkiūnas G, Lessow O, Muller K, Iezhova TA, Fickel J, Bensch S (2008) Haemosporidian blood parasites in European birds of prey and owls. J Parasitol 94:709–715. doi:10.1645/GE-1357.1
Levin II, Outlaw DC, Vargas FH, Parker PG (2009) Plasmodium blood parasite found in endangered galapagos penguins (Spheniscus mendiculus). Biol Conserv 142:3191–3195. doi:10.1016/j.biocon.2009.06.017
Martínez J, Martínez-De La Puente J, Herrero J, Del Cerro S, Lobato E, Rivero-De Aguilar J, Vásquez RA, Merino S (2009) A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitol 136:713–722. doi:10.1017/S0031182009006118
Martinsen ES, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273. doi:10.1016/j.ympev.2007.11.012
McClure HE, Poonswad P, Greiner EC, Laird M (1978) Haematozoa in the birds of Eastern and Southern Asia. Memorial University of Newfoundland, St. John’s, p 296
Newton I (2004) The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. IBIS 146:579–600. doi:10.1111/j.1474-919X.2004.00375.x
Nylander JAA (2004) MrModeltest v2. Evolutionary Biology Centre, Uppsala, Program distributed by the author
Olias P, Wegelin M, Freter S, Gruber AD, Klopfleischer R (2011) Avian malaria deaths in parrots, Europe. Emerg Infect Dis 17:950–952
Palinauskas V, Kosarev V, Shapoval A, Bensch S, Valkiūnas G (2007) Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida: Plasmodiidae). Zootaxa 1626:39–50
Palinauskas V, Valkiūnas G, Križanauskienė A, Bensch S, Bolshakov CV (2009) Plasmodium relictum (lineage P-SGS1): further observation of effects on experimentally infected passeriform birds, with remarks on the treatment with MalaroneTM. Exp Parasitol 123:134–139
Parker PG, Whiteman NK, Miller E (2006) Conservation medicine on Galápagos Islands: partnership among behavioral, population, and veterinary scientists. Auk 123:625–638
Peirce MA (1981) Distribution and host-parasite check-list of the haematozoa of birds in Western Europe. J Nat Hist 15:419–458
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Sambrook J, Fritch FJ, Maniatis T (2002) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NewYork
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
Valkiūnas G (1986) Haemoproteus tartakovskyi sp. n. (Haemosporidia, Haemoproteidae) from crossbill. Parasitologia 20:307–310, In Russian
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton, 934 p
Valkiūnas G (2011) Haemosporidian vector research: marriage of molecular and microscopical approaches is essential. Mol Ecol 20:3084–3086
Valkiūnas G, Liutkevičius G (2002) Complete development of three species of Haemoproteus (Haemosporida, Haemoproteidae) in the biting midge Culicoides impunctatus (Diptera, Ceratopogonidae). J Parasitol 88:864–868. doi:10.1645/0022-3395(2002)088[0864:CDOTSO]2.0.CO;2
Valkiūnas G, Iezhova TA, Shapoval AP (2003) High prevalence of blood parasites in hawfinch Coccothraustes coccothraustes. J Nat Hist 37:2647–2652
Valkiūnas G, Bensch S, Iezhova TA, Križanauskienė A, Hellgren O, Bolshakov CV (2006) Nested cytochrome b PCR diagnostics underestimate mixed infections of avian blood hemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422. doi:10.1645/GE-3547RN.1
Valkiūnas G, Atkinson CT, Bensch S, Sehgal RNM, Ricklefs RE (2008a) Parasite misidentifications in GenBank: how to minimize their number? Tends Parasitol 24:247–248
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Bensch S (2008b) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401
Waldenström J, Bensch S, Kiboi S, Hasselquist D, Ottosson U (2002) Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol Ecol 11:1545–1554. doi:10.1046/j.1365-294X.2002.01523.x
Waldenström J, Hasselquist D, Bensch S, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting Haemoproteus and Plasmodium infections from avian blood. J Parasitol 90:191–194. doi:10.1645/GE-3221RN
Yakunin MP, Zhazyltaev TA (1977) The blood parasite fauna of wild and domestic birds from Kazakhstan Trudi Instituta Zoooogii. Akademii Nauk Kazakskoi SSR 37:124–148 (in Russian)
Zehtindjiev P, Ilieva M, Westerdahl H, Hansson B, Valkiūnas G, Bensch S (2008) Dynamics of parasitemia of malaria parasites in a naturally and experimentally infected migratory songbird, the great reed warbler Acrocephalus arundinaceus. Exp Parasitol 119:99–110. doi:10.1016/j.exppara.2007.12.018
Acknowledgements
We thank A. Warren, the Natural History Museum, London, U.K. for providing the type and voucher material of P. vaughani. The authors are grateful to Vaidas Palinauskas for the help with images of parasites. Tatjana A. Iezhova is gratefully acknowledged for assistance during identification of parasites. Thanks are due to Najden Chukerov for participation during field studies. This study was partly funded by FP7 Capacities project WETLANET (PZ). The field work was supported by a grant from the Associazione dei Migratoristi Italiani per la Conservazione dell’Ambiente Naturale (ANUU). Laboratory work was supported by the Swedish Research Council (SB). The investigations described herein comply with the current laws of Italy and Netherlands.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Zehtindjiev, P., Križanauskienė, A., Scebba, S. et al. Haemosporidian infections in skylarks (Alauda arvensis): a comparative PCR-based and microscopy study on the parasite diversity and prevalence in southern Italy and the Netherlands. Eur J Wildl Res 58, 335–344 (2012). https://doi.org/10.1007/s10344-011-0586-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-011-0586-y