Abstract
Bovine tuberculosis (bTB), widely considered a disease of livestock, can also affect large game species despite their poorly understood role in the epidemiology of the endemic state of the disease. The Idanha-a-Nova County, situated in the eastern region of central Portugal, is one of the most important regions for large game hunting activity. In order to investigate the role of large game and the presence of bTB in Idanha-a-Nova, 132 wild boar (Sus scrofa) and 339 red deer (Cervus elaphus) were inspected for bTB-compatible lesions (bTBCL) during the 2008–2009 hunting season. Tissue samples with bTBCL were collected and processed for bacteriological confirmation and spoligotyping of Mycobacterium bovis isolates. In wild boar, bTB lesions were single, mostly calcified (21/29, 72.4%) and situated in the mandibular lymph nodes. Lesions were more severe and largely non-calcified (36/40, 90%) in red deer. Wild boar presented a consistently higher bTB infection rate than the red deer; however, the difference was only significant in the southern B area. This area was significantly affected by bTB for both animal species (up to 72.7% in wild boar and 34.8% in red deer) and requires the urgent implementation of intervention measures to contain the disease. Wild boar may assume an important role in the emergence of new disease foci in distant areas that infected red deer cannot reach. Spoligotyping of M. bovis isolates grouped the strains into three clusters, revealing patterns common to wild boar, red deer, and also to local cattle, indicating possible transmission among different animal species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycobacterium bovis causes bovine tuberculosis (bTB), an infectious and usually chronic disease of livestock that can also affect a wide range of wild animal species (Phillips et al. 2003). As a zoonotic disease, bTB poses a special risk for humans, specifically hunters, veterinarians, game, and game meat handlers as well as consumers.
Bovine tuberculosis has become a major worldwide disease affecting wildlife on every continent (Corner 2006). In Mediterranean Europe, it is an issue of increasing importance in wild ungulates, such as wild boar (Sus scrofa) and red deer (Cervus elaphus), especially in populations that suffer from increasing artificial management, such as fencing, feeding, and watering (Bollo et al. 2000; Vicente et al. 2006). The importance of the infection in wild animals relies mainly on their role in maintaining tuberculosis and acting as a reservoir of infection for livestock (Aranaz et al. 2004). Emergence of bTB in these wild species constitutes a severe barrier to its eradication in livestock (Gortázar et al. 2007). The morbidity and mortality that the infection can cause in protected and endangered species is also an aspect that deserves concern.
In Idanha-a-Nova County, a region in central-east Portugal, a remarkable increase, both in number and spatial distribution of wild ungulates sharing feed and water resources with livestock have been observed during the last few decades. This increase is associated with the development of an important commercial hunting industry. Wildlife management and spatial densities in Idanha-a-Nova have already raised concerns about their repercussion in the effectiveness of the bTB eradication plan in Portugal. In fact, bTB herd prevalence in the central region of Portugal, raised from 0.12% to 0.14% in the last 2 years (Anonymous 2010).
A combination of traditional disease-tracing investigation, laboratory diagnostic, and molecular typing of strains is needed to understand the epidemiology of bTB, providing valuable insight into the importance of different hosts in the maintenance and spread of the infection (Aranaz et al. 2004). Geographical Information System (GIS) enables the incorporation and management of spatial data, such as game hunting and farm geographical location, and the evaluation of disease distribution by means of geostatistical analysis (Goovaerts 1997; Hoef et al. 2001; Schröder 2006; Hengl 2007), constituting an important tool to understand the epidemiology of wildlife diseases (Goodchild and Haining 2004).
The overall objective of this study was to characterize bTB infection in wild boar and red deer populations in one of the most important areas of large game hunting activity in Portugal, the Idanha-a-Nova County, and investigate the spatial pattern of the disease based on field and molecular data.
Material and methods
Area of study
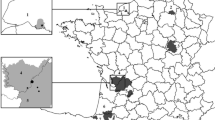
The study was developed in Idanha-a-Nova County, located in central-east Portugal (lat 39° 55′N; long 7º 14'W) in a plateau region with an area of 1412.7 km2 with a large borderline with Spain defined by the river Erges to the east and the river Tagus to the south (Fig. 1). This county has approximately 20% of its area dedicated to agriculture, supporting considerable domestic animal production (cattle, sheep, and goat), mainly based on outdoor extensive production (16.9%). Idanha-a-Nova is also considered one of the largest hunting areas in Portugal covering 502 km2 (Anonymous 2009). Much of this vast area is simultaneously used for livestock production and wild game hunting where domestic and wild animals may share pastures and water resources. During the hunting season from November 2008 to February 2009, 18 organized hunting campaigns (sampling plots) were surveyed in Idanha-a-Nova in six hunting areas (A–F; Fig. 1).
Map of Portugal showing the Idanha-a-Nova County position. The Idanha-a-Nova map presents the geographical distribution of sampling sites (1 to 18) within the analyzed hunting areas (A to F). The open and fenced hunting areas are distinguished in the map by means of continuous or discontinuous circle line. Forestry and shrub are shown as gray shadow
Animal sampling
From the total of the large game hunted in Idanha-a-Nova during the 2008–2009 hunting season, 85% (471 animals, 132 wild boar and 339 red deer) were analyzed. After each hunting campaign, the harvested animals were brought to a collection point, geographically mapped by means of a Global Positioning System (GPS) receiver and examined in loco (European Regulation 853/2004). Briefly, the in loco examination included the incision of the mandibular, prescapular, pulmonary, and mesenteric lymph nodes and the inspection of the kidney, heart, liver, lung, and intestine. Due to field work constraints, red deer heads were not examined.
Bovine tuberculosis-compatible lesions (bTBCL) were identified and classified according to the lesion type (with and without calcification) and location. Samples from bTBCL were collected for histopathology and bacteriological analysis.
Histopathology analysis
Tissue samples were fixed in 10% neutral buffered formalin and processed in hemotoxylin and eosin, stained by the Ziehl–Neelsen procedure, and examined for the presence of acid-fast resistant bacilli according to OIE (Organización Internacional des Epizooties) Manual (OIE 2004).
Bacterial isolation and identification
Samples were processed and decontaminated according to OIE Manual standard procedures (OIE 2004), and bacterial isolation was performed by inoculation in BACTEC 9000 liquid medium and in the solid media Stonebrink, Lowenstein–Jensen, Lowenstein–Jensen with thiophen-2-carboxylic acid hydrazide, and Lowenstein–Jensen with pyruvate. The isolates were identified by an in-house polymerase chain reaction (PCR)–restriction endonuclease analysis system, based on gyrB gene amplification followed by hydrolysis with RsaI and SacII restriction enzymes according to Niemann et al. (2000).
Spoligotyping
Spoligotyping, based on the Direct Repeat (DR) region, has been considered a first-line molecular typing method for M. bovis (Kamerbeek et al. 1997; Kremer et al. 1999), with international databases (http://www.mbovis.org and http://www.pasteur-guadeloupe.fr/tb) being available for comparison between laboratories (Duarte et al. 2008). PCR targeting the DR region and hybridization of PCR products was performed according to Van Embden et al. (2000). Mycobacterium tuberculosis H37Ra-ATCC 25177 and M. bovis ATCC 19015 reference strains were used as controls in each hybridization assay. The obtained patterns (a series of 43-digit binary numbers) were compared using the database www.mbovis.org, and a spoligotype identification number was attributed.
Clustering analysis
Clustering of M. bovis strains was performed based on spoligotyping results. For each strain, the presence or absence of each of the 43 oligonucleotides was recorded as 1 (presence) or 0 (absence). The obtained patterns were used to construct a 43-digit binary matrix representing the eight obtained spoligotypes and an UPGMA (unweighted pair group method with arithmetic mean)-based tree (Dice coefficient) by using NTSYS 2.0 software.
GIS project
A GIS project (ArcGis 9.2) was created in order to map hunting sampling plots, to analyze landscape and land cover associated to each hunting area, and to calculate maps for bTB prevalence distribution within Idanha-a-Nova County. As data source, a CORINE Land Cover map (CLC_06PT, IGP, 2009, www.igp.pt), topographic plans and contours (ww.igeoe.pt), and GPS to record positions were used. The data collected during field work, such as number and species of hunted animals and tuberculosis-compatible lesions, were assigned to each GPS-recorded sampling position and used to update GIS project. In a second stage, geostatistical calculation was performed by means of the inverse distance weighted method, in order to create continuous maps for bTB prevalence (Goovaerts 1997; Hoef et al. 2001; Schröder 2006; Hengl 2007), using Geostatistical Analyst 2.0 for ArcGIS 9.2. For geostatistical data analysis, inverse distance weighted interpolation process was used, powered by 2, overall sampling plot locations throughout the County (n = 18). In a third stage, facility areas (ESRI 2010) around each of the 18 sampling plot locations were calculated in order to assign bTB prevalence to main hunting areas and to analyze the relationship between land cover and disease prevalence. A radius of 3,500 m was used because this distance is the one corresponding to the hunting game edge where hunters stand.
Statistical procedures
For bTB (based on M. bovis isolation) prevalence analysis, a binomial distribution approach was used. Differences in the bTB means prevalence for wild boar and red deer, within and between hunting areas, were calculated by means of z test for comparison of proportions in independent samples (Daniel 1987; Snedecor and Cochran 1995), as presented in the following equations:
Where:
- n :
-
Number of hunted animals
- p :
-
Proportion of hunted animals with tuberculosis
- q :
-
Proportion of hunted animals with no tuberculosis = 1 − p
- \( t_n^{{0.05}} \) :
-
Student’s t value for 95% confidence and n samples
Where:
- Cp = z :
-
Is a comparison of proportions in independent samples in a 2 × 2 table, using a normal z curve approach
- p :
-
Proportion of animals with tuberculosis in both hunting areas
- q :
-
Proportion of animals with no tuberculosis in both hunting areas = 1 − p
- n1:
-
Number of hunted animals in hunting area 1
- n2:
-
Number of hunted animals in hunting area 2
- p1:
-
Proportion of animals with tuberculosis in hunting area 1
- p2:
-
Proportion of animals with tuberculosis in hunting area 2
A linear regression analysis and a Pearson correlation test were applied in order to study the association between wild boar and red deer bTB prevalence, as well to analyze the association between bTB prevalence and percentage of land cover, both for wild boar and red deer.
Results
Bovine tuberculosis-compatible lesions
From the 471 animals hunted and examined in loco, 69 (14.7%) presented bTBCL. These lesions were mainly observed in wild boar (22%, 29/132) while only 11.8% (40/339) of hunted red deer presented bTBCL. Bovine tuberculosis lesions in wild boar were mainly found in the mandibular lymph nodes (72.4%, 21/29) and, to a lesser extent, in mesenteric lymph nodes (27.5%, 8/29). From the 40 affected red deer, bTBCL were almost equally found in mesenteric lymph nodes (22/40, 55%) and lungs (23/40, 57.5%). In red deer, 12.5% of the surveyed animals presented bTBCL in two different organs, in contrast with wild boar that only presented single lesions. In wild boar, bTBCL were mainly calcified (21/29, 72.4%), while in red deer the majority of lesions were non-calcified (36/40, 90%).
Histopathology analysis, bacteriological isolation, and molecular identification
Due to field work constrains, only 67 (27 from wild boar and 40 from red deer) bTBCL animals were sampled for laboratory diagnostic confirmation by histopathology and bacteriological analysis. In 56 samples (56/471, 11.9%), the existence of bTB was confirmed by bacteriological isolation and identification. From 27 wild boar samples, 22 were positive in microscopic examination while, in 21, M. bovis was isolated by culture in selective media. In the 40 red deer samples, 39 were positive in microscopic examination, and 35 M. bovis isolates were obtained (Table 1). Based on bacteriological results, the prevalence of bTB in wild boar was 15.9% (21/132) and in red deer, 10.3% (35/339), as presented in Table 2.
In three cases, the suspicion of bTB, by macroscopic compatible lesions, was not confirmed by culture. Those included a Mycobacterium avium complex infection in mandibular lymph node from wild boar, an interstitial pneumonia with lymphoid tissue hyperplasia in wild boar lung and an actinogranulomatosis infection in mesenteric lymph node from red deer.
GIS and statistical analysis
Results from facility areas around hunting areas intersection over CORINE Land Cover map enabled the characterization of those hunting areas, as presented in Table 3.
Idanha-a-Nova County is limited at the south by river Tagus and at east by river Erges (Fig. 1) which confined animals’ movements in these directions. However, due to continuous forestry landscape, animals can easily move from A to C. Hunting areas D and E have a large percentage of agro-forestry area and are surrounded by agricultural lands, which makes red deer movement difficult from or to other areas but does not limit that of wild boar. Hunting area F is different from the other areas, which is limited at the south by a hilly area and is a unique hunting area which has forest stands of pine trees.
In relation to bTB prevalence, calculated on the basis of M. bovis isolation, wild boar presented consistently a higher infection rate than the red deer (Table 2). However, the difference is not significant at the hunting area level, except in the southern B area where bTB prevalence in wild boar is higher than in red deer (z = 2.703; p value < 0.05) as presented in Tables 4 and 5.
Particular results (Tables 4 and 5) were obtained for hunting areas B and F that are fenced areas (partially damaged) belonging to the same company and with equivalent game-management system. For both animal species, hunting area B presented higher values of bTB prevalence (up to 72.7% in wild boar and 34.8% in red deer) in relation to the total areas, these differences being statistically significant (wild boar z = 4.026, p value < 0.001; red deer z = 3.223, p value < 0.01). Opposite is hunting area F presenting lower values of bTB prevalence in relation to the total area, with these differences being statistically significant (wild boar z = −2.311; p value < 0.05; red deer z = −2.711; p value < 0.05).
When wild boar and red deer bTB prevalence within hunting areas are analyzed together (Fig. 2a), the relationship is not significant due to absence of disease for red deer in areas D and F. However, when wild boar and red deer bTB prevalence is compared in hunting areas A, B, and C (Fig. 2b), the association is significantly positive (r = 0.999; p value < 0.001).
Regression analysis between: wild boar and red deer bTB prevalence in all hunting areas (a) and just in A, B, and C hunting areas (b); wild boar and red deer bTB prevalence and agro-forestry percentage of land cover (c, d); wild boar and red deer bTB prevalence and sparse forestry and new plantation percentage of land cover (e, f)
Regression analysis was performed over the prevalence of bTB and land cover characteristics, and the results point out a positive association between wild boar bTB prevalence and agro-forestry areas (Fig. 2c; r = 0.9914; p value < 0.01) and a negative association between wild boar bTB prevalence and space forested areas and new plantation areas (Fig. 2d; r = −0.868; p value < 0.05). Non-significant association was calculated between red deer bTB and land cover (Fig. 2e, f).
Geostatistical analyses were performed in order to create a continuous disease intensity map for both wild boar and red deer, which enables the investigation of the spatial pattern of the disease and analysis of bTB spread direction throughout Idanha-a-Nova County, as presented in Fig. 3a, b.
As Fig. 3a, b shows, bTB prevalence is more intense at the south part of the county and presents a spread trend on a south-west direction for both animal species, this tendency being more notorious for wild boar.
Spoligotyping
Among the 56 M. bovis isolates, 48 (27 from red deer and 21 from wild boar) were randomly spoligotyped, and eight different patterns were identified (Table 6). The prevalence of each spoligotype varied from 2.1% to 25%. Spoligotypes SB1264 (25%), SB01195 (20.8%), SB0122 (16.7%), and SB1174 (14.6%) were the most prevalent, representing 77% of all identified spoligotypes in this study (Table 6). Four spoligotypes (SB0122, SB0140, SB0265, and SB1314) were exclusive to red deer while four spoligotypes (SB0121, SB1174, SB1195, and SB1264) were common to both red deer and wild boar. The M. bovis profile SB1314 was produced by a single M. bovis isolate from red deer. A dendogram based on the spoligotyping data was generated using UPGMA and the Dice similarity index (Fig. 4). All spoligotypes were closely associated, clustering within a similarity index of approximately 0.9. One major group could be defined using a cut-off of approximately 0.95 that clustered five spoligotypes, including three out of the four most prevalent ones (Fig. 4).
Discussion
Results from this study indicate the presence of bTB in wild boar and red deer, the two most significant wild ungulates known to occur in Idanha-a-Nova. The gold standard diagnostic method of bacteriological isolation and identification confirmed the presence of bTB in 83.6% of bTBCL (Table 1). In 16.4% of the bTB culture-negative samples, other agents were responsible for causing lesions.
Bacteriological results of bTBCL samples revealed the prevalence of bTB in 15.9% (21/132) of wild boar and 10.3% (35/339) in red deer (Table 2). These values are high when compared with data obtained in Sierra de San Pedro (Extremadura, Spain), situated only 50 km from Idanha-a-Nova, where the prevalence of bTB lesions was approximately 7.7% in wild boar and 2.91% in red deer (Mendoza et al. 2006). The results obtained in this study are within the range of values for wild boar reported by Santos et al. (2009), with a prevalence of 6.3% (2/32) in an area to the north of the study area and of 22.2% (4/18) in an area to the south. These results are not directly comparable since Santos et al. (2009) estimated bTB prevalence by isolating M. bovis from wild boar samples with and without lesions. This approach may have increased the prevalence value compared with the values obtained in our study where only bTBCL samples were analyzed. Both Mendoza et al. (2006)) and Martín-Hernando et al. (2007)) have referred to the limited accuracy of macroscopic inspection in detecting infected animals, which excludes infected specimens that do not have apparent lesions. This was confirmed by Santos et al. (2009) where M. bovis was isolated from specimens of wild boar with no visible lesions (5/18; 27.7%). Martín-Hernando et al. (2007)) also demonstrated that macroscopic inspection failed to detect 17.32% of the infected animals.
The bTB lesions found in wild boar were mainly present in the mandibular lymph nodes, in agreement with the findings of Vicente et al. (2006), Martín-Hernando et al. (2007), and Santos et al. (2009). In this present study, bTB lesions were also found in the mesenteric lymph nodes, but pulmonary lesions were never found. No histopathology analyses were done on lung samples which, according to Martín-Hernando et al. (2007), could have helped to find possible macroscopically invisible lesions. The apparent exclusive presence of bTB in the mandibular lymph nodes of wild boar supports the hypothesis of infection by ingestion of contaminated food, water, offal, or carrion. The latter source of infection, providing a large dose of infective microorganisms via the alimentary route, is important in wild boar due to its natural foraging feeding habits. The wild boar bTB lesions observed during our survey were mostly calcified (72.4%) which is in accordance with the results previously reported by Zanella et al. (2008) and Santos et al. (2009). All observed wild boar bTB lesions were single and less severe than red deer lesions where the majority of organs had non-calcified lesions (90%), and bTB lesions were mostly found in mesenteric lymph nodes and lung, confirming aerogenous and ingestion routes of M. bovis (Serraino et al. 1999). The pattern of observed bTB lesions indicated that the disease pattern was more serious in red deer than in wild boar. These results concur with those of Zanella et al. (2008) in France but not with the results found by Vicente et al. (2006) in Spain. The results of the present study also indicate that the transmission of M. bovis from wild boar to red deer is unlikely due to the infrequent occurrence of pulmonary and generalized disease and to the high occurrence of calcified lesions in wild boar.
Since the hunting bag in this study was representative of the large game population in Idanha-a-Nova County and the shot process used is a quite random type of hunting, it was possible to compare the distribution of bTB between large game populations throughout this county. In this study, wild boar presented consistently a higher infection rate than the red deer (Table 2). However, the difference was only significant in southern B area where bTB prevalence in wild boar was higher than in red deer (Tables 4 and 5). As presented in Fig. 3, bTB prevalence was more intense in the southern area of Idanha a Nova but appears to be spreading a south-west in both animal species, in particular, the wild boar. Results indicate that, in the near future, northern areas of Idanha a Nova will be more heavily affected by the disease if no bTB control strategies are implemented.
A significant positive association was observed (Fig. 2b) between wild boar and red deer bTB prevalence in the main affected areas (A, B, and C). Analysis of continuous disease intensity maps and the land characteristics of the main affected areas indicated that animal movement between the hunting areas A and C were relatively unrestricted, facilitating the spread of the disease (Fig. 3 and Table 3). Animals (mainly wild boar) from area B, where the disease was particularly prevalent, can cross damaged fences and pass to areas A and C. Timely reparation of fences in area B would restrict animal movement and help prevent the spread of bTB into peripheral areas.
We found that two fenced hunting areas (B and F) belonging to the same company and with the same game-management system had different results. The northern F area had a significantly lower bTB prevalence value (Tables 4 and 5 indicating that factors besides management practices may be responsible for bTB distribution and transmission in these areas. These factors should be analyzed in further studies in order to develop and implement appropriate control strategies. We observed that northern hunting area (F) was more isolated with less animal movement, due to the presence of a cliff in the south, which limited animal access from surrounding hunting areas. A regression analysis performed between bTB prevalence (Table 4) and land cover characteristics (Table 3) indicated a positive association (Fig. 2c) between wild boar bTB prevalence and agro-forestry areas (largest percentage in hunting area B and smallest in hunting area F) which are strictly associated with human activity where cattle is breed in large open areas. The results also indicated a negative association between wild boar bTB prevalence, sparsely forested areas, and new plantation areas (smallest percentage in hunting area B and largest in hunting area F), where vegetation is rare and bare soil dominates landscape. Due to absence of red deer in hunting area E and the absence of disease in hunting areas D and E, the relationship between bTB prevalence and land cover is not significant, although it follows the same trend than for wild boar (Fig. 2d, f). These differences in landscape may partially explain the geographical pattern of bTB observed in this study; however, further studies are needed to identify and evaluate other factors that influence the occurrence of bTB in large game in Idanha-a-Nova.
Results indicate that several factors may contribute to the significantly higher prevalence of bTB in hunting areas A and B rather than in the area (F) situated further north, where wild boar presented low levels of the disease (Tables 2 and 4). These results also suggest that wild boar may play an important role in the emergence of new disease foci in distant areas that infected red deer cannot reach.
Spoligotyping of 48 M. bovis isolates from red deer and wild boar revealed eight different closely related spoligotypes. Cluster analysis identified three groups of spoligotypes (Fig. 3). The first and largest group (group I) comprised five spoligotypes, four of which were derived from spoligotype SB0121 by the absence of one spacer and the fifth by the absence of two spacers. Spoligotype SB0121 is the most frequent type occurring in cattle from Portugal and Spain (Duarte et al. 2008; Aranaz et al. 2004; Parra et al. 2005). Group I also included the two most frequent spoligotypes found in this study, SB1264 and SB1195. These two spoligotypes were the most widely distributed, occurring in all hunting areas except areas D and E (SB1264) and areas C, E, and F (SB1195, Table 6). Hunting area A had the highest number of M. bovis isolates and the highest level of spoligotype diversity; all the eight spoligotypes were reported. All spoligotypes were also detected in area B, with the exception of spoligotype SB1314 which occurred exclusively in area A. Four of the eight spoligotyping patterns identified were exclusive to red deer (SB0122, SB0140, SB0265, SB1314), while the remaining four patterns (SB0121, SB1174, SB1195, SB1264) were common to both wild boar and red deer, indicating a possible common source of infection (Table 6). Red deer isolates had greater spoligotype diversity, generating all the spoligotypes identified in this study. According to Corner (2006), the presence of exclusive spoligotypes in one animal species may indicate an important role as a bTB maintenance host. The most prevalent spoligotype SB1264 (25%), reported for the first time in Portuguese wildlife in this study, was already referred by Aranaz et al. (2004) in Doñana National Park (South Spain) in wild boar, red deer, fallow deer, cattle, and Iberian lynx.
Cattle, sheep, and goat are often present in hunting areas throughout Idanha-a-Nova, and domestic and wild animals occasionally share pastures and water resources. In bTB-infected cattle from Idanha-a-Nova, the most frequent spoligotypes detected between 2008 and 2010 were SB0121 (28.6%), SB1090 (23.8%), and SB0119 (19.1%; unpublished data), corresponding to 71.4% of the spoligotype patterns identified in cattle from that region. Spoligotype SB0121 was the only type common to both cattle and large game in this study, identified in 12.5% of bTB-infected game animals. The spoligotypes SB1090 and SB0119 belong to a group of seven closely related spoligotypes identified in Portugal (Matos et al. 2010) that differ from the SB0121 pattern by the absence of a single spacer. Although our results indicate possible transmission between domestic and wild animal species, additional sources of M. bovis infection in cattle should be considered.
Our results highlight the potential importance of wild boar and red deer in the continuance of bTB in East-Central Portugal. This area should be the focus of special veterinary measures to improve surveillance control and apply strategies to reduce spread of the disease. The information gathered in the GIS project created specifically for this study enables information to be updated on a regular basis and is an essential tool for the assessing bTB progress and improvement of disease control schemes in Portugal.
References
Anonymous (2009) Lazer/Caça e Pesca. http://www.cm-idanhanova.pt/lazer/caca_pesca.html [accessed 25 November 2009]
Anonymous (2010) Tuberculose bovina: relatório técnico. Direcção Geral de Veterinária, DGV, Portugal.
Aranaz A, Juan L, Montero N, Sánchez C, Galka M, Delso C, Álvarez J, Romero B, Bezos J, Vela A, Briones V, Mateos A, Dominguez L (2004) Bovine tuberculosis (Mycobacteria bovis) in wildlife in Spain. J Clin Microbiol 42(6):2602–2608
Bollo E, Ferroglio E, Dini V, Mignone W, Biolatti B, Rossi L (2000) Detection of Mycobacterium tuberculosis complex in lymph nodes of wild boar (Sus scrofa) by a target-amplified test system. J Vet Med B 47:337–342
Corner LAL (2006) The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: How to assess the risk. Vet Microbiol 112:303–312
Daniel WW (1987) Biostatistics: A Foundation for Analysis in the Health Sciences. Wiley, Canada, p 734
Duarte EL, Domingos M, Amado A, Botelho A (2008) Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet Microbiol 130(3–4):415–421
ESRI (2010) http://www.esri.com/software/arcgis/extensions/networkanalyst
European Regulation (EC) Nº 853/2004 of the European Parliament and of the Council of 29 April 2004. (2004) Laying down specific hygiene rules for food of animal origin. Official Journal of the European Union L 139/55
Goodchild MF, Haining RP (2004) GIS and spatial data analysis: covering perspectives. Pap Reg Sci 83:363–385
Goovaerts P (1997) Geoestatistics for Natural Resources Evaluation. Oxford University Press, New York
Gortázar C, Ferroglio E, Höfle U, Frölich K, Vicente J (2007) Diseases shared between wildlife and livestock: a European perspective. Eur J Wildl Res 53:241–256
Hengl T (2007) A Practical guide to Geoestatistical Mapping of Environmental Variables. European Commission, Joint Research Center–Institute for Environment and Sustainability, Office for Official Publications oh the European Communities, Luxembourg
Hoef JM, Johnston K, Lucas N, Krivoruchko K (2001) Using ArcGIS Geostatistical Analyst. ESRI, USA
Kamerbeek J, Schouls L, Kolk A, Van Agterveld M, Van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, Van Embden J (1997) Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol 35:907–914
Kremer K, Van Soolingen D, Frothingham R, Haas WH, Hermans PWM, Martin C, Palittapongarnpim P, Plikaytis BB, Riley LW, Yakrus MA, Musser JM, Van Embden JDA (1999) Comparison of methods based on different molecular epidemiological markers for typing Mycobacterium tuberculosis complex strains: interlaboratory study power and reproducibility. J Clin Microbiol 37:2607–2618
Martín-Hernando MP, Hofle U, Vicente J, Ruiz-Fons F, Vidal D, Barral M, Garrido JM, de la Fuente J, Gortázar C (2007) Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis 87:360–367
Matos F, Cunha MV, Canto A, Albuquerque T, Amado A, Botelho A (2010) Snapshot of Mycobacterium bovis and Mycobacterium caprae infections in livestock in an area with a low incidence of bovine tuberculosis. J Clin Microbiol 48:4337–4339
Mendoza JH, Parra A, Tato A, Alonso JM, Rey JM, Peña J, Garcia Sánchez A, Larrasa J, Teixidó J, Manzano G, Cerrato R, Pereira G, Fernandez-Llario P, Hermoso de Mendoza M (2006) Bovine tuberculosis in wild boar (Sus scrofa), red deer (Cervus elaphus) and cattle (Bos taurus) in a Mediterranean ecosystem (1992–2004). Prev Vet Med 74:239–247
Niemann S, Harmsen D, Rusch-Gerdes S, Richter E (2000) Differentiation of clinical Mycobacterium tuberculosis complex isolates by gyr B DNA sequence polymorphism analysis. J Clin Microbiol 38:3231–3234
OIE (2004) Manual of diagnostic tests and vaccines for terrestrial animals, 5th edn. Word Organisation for Animal Health-OIE, Paris
Parra A, Larrasa J, Garcia A, Alonso JM, Hermoso de Mendoza J (2005) Molecular epidemiology of bovine tuberculosis in wild animals in Spain: a first approach to risk factor analysis. Vet Microbiol 110(3–4):293–300
Phillips CJ, Foster CR, Morris PA, Teverson R (2003) The transmission of Mycobacterium bovis infection to cattle. Res Vet Sci 74:1–15
Santos N, Correia-Neves M, Ghebremichael S, Kallenius G, Svenson SB, Almeida V (2009) Epidemiology of Mycobacterium bovis infection in wild boar (Sus scrofa) from Portugal. J Wildl Dis 45(4):1048–1061
Schröder W (2006) GIS, geostatistics, metada banking, and tree-based models for data analysis and mapping in environmental monitoring and epidemiology. Int J Med Microbiol 296(1):23–36
Serraino A, Marche IG, Sanguine V, Rossi MC, Zanoni RG, Catozzi L, Bandera A, Dini W, Mignone W, Franze IF, Gori A (1999) Monitoring of transmission of tuberculosis between wild boar and cattle: genotypical analysis, of strains by molecular epidemiology techniques. J Clin Microbiol 37:2766–2771
Snedecor GW, Cochran WG (1995) Statistical Methods, 8th edn. Iowa State University Press, AMES, p 503
Van Embden JDA, Van Gorkom T, Kremer K, Jansen R, Vander Zeijst BAM, Schouls LM (2000) Genetic variation and evolutionary origin of the Direct Repeat locus of Mycobacterium tuberculosis complex bacteria. J Bacteriol 182:2393–2401
Vicente J, Höfle U, Garrido JM, Fernández-De-Mera IG, Acevedo P, Juste R, Barral M, Gortázar C (2006) Wild boar and red deer display high prevalences of tuberculosis-like lesions in Spain. Vet Res 37:107–119
Zanella G, Duvauchelle JH, Moutou ML, Durand B (2008) Patterns of lesions of bovine tuberculosis in wild red deer and wild boar. Vet Rec 163(2):43–47
Acknowledgments
The authors would like to thank to Dr. António Manteigas, from Veterinary Services of Castelo-Branco–DIVCB, for all the help provided during the development of this study. Thanks are also due to the anonymous referee for valuable comments to the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Vieira-Pinto, M., Alberto, J., Aranha, J. et al. Combined evaluation of bovine tuberculosis in wild boar (Sus scrofa) and red deer (Cervus elaphus) from Central-East Portugal. Eur J Wildl Res 57, 1189–1201 (2011). https://doi.org/10.1007/s10344-011-0532-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-011-0532-z