Abstract
The analysis of the relationships between population density and habitat features is important to evaluate the ecological needs of a species, its potential impact on ecosystems and its interspecific interactions. We analysed the spatial variation of roe deer Capreolus capreolus and fallow deer Dama dama densities in a Mediterranean area in summer 2007 and winter 2007/2008. Previous research has shown that fallow deer can actively displace and exclude roe deer from natural feeding sites. Here we show that both species have the greatest densities in ecotone habitats between wood and open fields (abandoned olive groves and pastures), but with contrasting geographic patterns. The fallow deer showed the greatest densities in the central northern part of the study area near to local historical release sites. The densities of roe deer were great where fallow deer were rare and low where fallow deer were abundant. Spatial overlap was great at the habitat scale, indicating a high potential for competition, but was low at the plot scale, suggesting that partitioning of space occurred at a fine scale. Supporting great numbers of deer, the ecotone areas are crucial for the management of ecosystems. We suggest that roe deer avoid areas with great densities of fallow deer and that interspecific interference from the latter affects the density and distribution of the former both at a fine and at a large scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatiotemporal variation of densities of wild populations is an important parameter to evaluate their ecological needs (Apps et al. 2004) and dynamics (Kruuk et al. 1999; Forchammer et al. 2002; Angulo et al. 2007), as well as their potential impacts on ecosystems (Putman and Moore 1998; Stockton et al. 2005). A comparison of densities of species living in sympatry can help in understanding interspecific interactions (Latham et al. 1997; Forsyth and Hickling 1998).
In ungulates, patterns of density and resource use of species living in sympatry have been analysed (e.g. Jenkins and Wright 1988; Putman 1996; Arsenault and Owen-Smith 2008). Some studies suggested resource partitioning (e.g. Jenkins and Wright 1988; Arsenault and Owen-Smith 2008), but overlap can be great (Putman 1996; Bertolino et al. 2009). Most of these studies often identified only the potential for competition (Putman 1996). Linking knowledge on density and habitat relationships to information on behavioural interactions could help in understanding the patterns of density and distribution of species in sympatry (Anthony and Smith 1977), but relevant information is scarce.
Because of its large distribution range, in sintopy with those of other ungulate taxa, the roe deer Capreolus capreolus is a useful model species to study density–habitat relationships as well as interactions with sympatric ungulates (Andersen et al. 1998; Latham 1999). The roe deer is a medium–small-sized cervid (20–30 kg; Andersen et al. 1998) well adapted to the woodfields ecotone as well as to the early succession stages of wood (Gill et al. 1996; Putman 1996; Mysterud et al. 1999). This deer relies on highly nutritious vegetation for survival and reproduction (mating: July–August; births: April–May; Hofmann 1989; Andersen et al. 2000). Habitat selection occurs when high-quality food and vegetation cover are available (Duncan et al. 1998; Tufto et al. 1996). The density of roe deer appears to be negatively affected by that of other cervid species (red deer Cervus elaphus, Latham et al. 1997; barking deer Muntiacus reevesi, Hemami et al. 2005; fallow deer Dama dama, Focardi et al. 2006).
The roe deer shares part of its present distribution range with the fallow deer, a medium-sized cervid (males, 55–85 kg; females, 35–55 kg, in Italy; Boitani et al. 2003), introduced to Europe from Asia Minor, after the Neolithic (Nowak 1991). This deer is well adapted to open habitats (fields, meadows, open wood; Batcheler 1960; Apollonio et al. 1998) and can use highly energetic food as well as fibrous vegetation (Hofmann 1989). In captivity, the fallow deer has been reported to be intolerant of the red deer (Bartoš et al. 1996) and of the spotted deer Axis axis (McGhee and Baccus 2006).
Relationships between roe and fallow deer have been object of debate (Batcheler 1960; Putman 1996; Bartoš et al. 2002; Focardi et al. 2006; Ferretti et al. 2008). A partial overlap in the use of resources has been shown (Putman 1996), suggesting the potential for exploitation competition. Inverse numerical trends have also been recorded (Batcheler 1960; Putman and Sharma 1987; Focardi et al. 2006), which may be an effect of independent and inverse reactions to a third factor (Putman 1996; e.g. contrasting reactions to succession changes of wood: Batcheler 1960). Great densities of fallow deer may reduce the habitat quality for roe deer, leading the latter to a smaller body size, larger home ranges and a population decrease (Focardi et al. 2006). Ferretti et al. (2008) showed that fallow deer can actively exclude roe deer from natural feeding sites even through direct aggression. It is unclear whether behavioural interference by fallow deer could affect the density of roe at a large spatial scale.
The aims of our study were: (1) to assess the spatial variation of densities of roe and fallow deer in summer and in winter in an area where active behavioural interference has been shown from the latter to the former (Ferretti et al. 2008; Ferretti et al. in press) and (2) to analyse habitat relationships and spatial overlap between roe and fallow deer.
Methods
Study area
Our study was carried out in the central southern part (6,260 ha) of the Maremma Regional Park (MRP, Central Italy; 42°39′ N, 11°05′ E). The local climate is Mediterranean. Vegetation is composed mainly by Mediterranean sclerophyllic scrubwood (58%) of three main wood types (Mencagli and Stefanini 2008): oakwood, with the prevalence of holm oak Quercus ilex trees with a height > 7 m; scrubwood, with the prevalence of holm oak and strawberry tree Arbutus unedo, with a height of <7 m; garigue, with bushes (mainly holm oak, rosemary Rosmarinus officinalis, juniper Juniperus spp., rockrose Cistus spp.), with a height of <2 m. Other habitats are pinewood (10%, mainly domestic pine Pinus pinea), abandoned olive groves and pastures (partially re-colonized by wood species and bushes, 15%), set-aside grassland (4%) and crops (12%, mainly cereals and sunflower). Evergreen, deciduous and half-woody plants as well as wild forbs and wild graminoids are the most used plant categories by roe deer in our study area (Minder 2006) and preliminary information suggests considerable diet overlap with the fallow deer in scrubwood (Minder in prep.). The availability of food resources for ungulates is generally lower in the scrubwood than in open habitats (Minder 2006).
The local ungulate community includes wild boar Sus scrofa, free-ranging cattle and horses, which are locally and irregularly moved through some pastures and part of pinewood. Wild boar density did not influence roe deer presence in sampling plots (Ferretti et al. in press). A study on behavioural interactions between roe deer, fallow deer and wild boar showed that both deer species were rarely displaced by wild boar (roe deer: 22% of 74 interspecific encounters; fallow deer: 13% of 61 interspecific encounters; Ferretti et al. in press). Wild boar density is expected not to affect the densities of our study species. Avoidance of livestock by fallow deer occurred only once in 16 observations and mixed groups were repeatedly seen in other pastures within the MRP (Ferretti et al. in press). No encounter was recorded between roe deer and livestock (Ferretti et al. in press). The predators are the wolf Canis lupus and the red fox Vulpes vulpes (the latter being a potential predator of young fawns only; Aanes and Andersen 1996). The territory size of wolves is generally more than 100 km2 (Fuller et al. 2003), suggesting that they use all of our study area (about 63 ha). No study has been published on the local diet of the wolf, but preliminary observations suggest that the fallow deer appears to be the most used prey (Manghi et al. 2006). The red fox is widespread in all our study area, using both wood and open habitats (Cavallini and Lovari 1994). Its diet includes mainly juniper berries and insects while large mammals are used mainly during the cold season (Cavallini and Lovari 1994). A recent study confirmed a small use of roe deer during their fawning season: roe deer hairs were present in only two scats out of 205 (Coneybeer 2003).
Density estimates
Deer densities were estimated through pellet group counts in summer 2007 (June–August) and winter 2007/2008 (January–March). We used the faecal accumulation rate technique, which is based on the counts of pellet groups accumulated in sample areas during a defined time interval (Mayle et al. 1999). This technique is considered more reliable than faecal standing crop counts (Mayle et al. 1999; Campbell et al. 2004) because it does not imply the use of pellet group decay rates, variable across habitats (in our same study area: Massei et al. 1998), to calculate densities.
There were 196 circular sampling plots (5-m radius) placed through a stratified sampling design (Gregoire and Valentine 2008). We assessed strata according to the main habitat categories: Mediterranean scrubwood, pinewood, abandoned olive groves and pastures, set-aside grassland and crops, as well as local features (see below; Mayle et al. 1999) and differences in deer densities detected through preliminary pellet group counts (Sforzi 2004). Because of that, we divided Mediterranean scrubwood, abandoned olive groves and pastures, as well as crops, in two strata each, respectively, in the north and in the south of our study area, in relation to local deer densities (Sforzi 2004; Table 1). In larger strata (north/south Med. scrubwood and pinewood), we adopted a two-stage strategy. In the first stage, the strata were partitioned into spatial units of different sizes (determined on the basis of natural or man-made edges), easily recognizable on the vegetation map as well as in the field. A sample of units was selected through a sequential (draw-by-draw) sampling with inclusion probabilities proportional to unit size and avoiding the selection of contiguous units. The use of inclusion probabilities proportional to size was adopted to handle the presence of units with different sizes (Skalski 1994). With equal-sized units, the population variance simply measures the spatial variation of abundance across the area of interest; with different-sized units, the population variance is inflated by the size variation with detrimental effects in the subsequent estimation (Skalski 1994). In turn, the selection of contiguous units was avoided since adjacent units were more alike than units that were far apart, thus giving poor contribution to sample information (Barabesi et al. 1997). In the second stage, plots were placed within selected units by means of tessellation stratified sampling (Cordy and Thompson 1995), also referred to as unaligned systematic sampling (EPA 2002), to provide a more even distribution of plots over the units. We allocated a number of plots to strata proportionally to their size, and seven plots were assigned to each unit. In smaller strata (north/south abandoned olive groves, north/south cultivated fields and set-aside grassland), the first phase was avoided and plots were placed directly on the stratum through the same scheme adopted within the spatial units. The methodological details and theoretical justifications regarding this sampling scheme are given in Fattorini et al. (in press), where unbiased estimators of abundance and conservative estimators of sampling variances are provided for each stratum and for the whole study area. The reliability of this sampling strategy was assessed through nine sampling seasons and through the comparison with direct counts in open areas, which revealed estimates stable between near seasons and agreement among estimates obtained through different methods (Fattorini et al. in press).
On the vegetation map of the MRP (Mencagli and Stefanini 2008), geographic coordinates were assigned to the centre of each selected plot through the ArcView GIS 3.2 ESRI © software. A portable GPS Garmin Etrex was used to find plots in the field. During a first survey, we carefully searched plots for any pellet group, which were later removed from the plot. We marked the centre of each plot through a peg or a tape. Local landmarks were noted down to help plot detection in subsequent surveys. A second survey was conducted after 40 days (according to the local decay rate of deer pellet groups: Massei et al. 1998; Minder 2006) to count pellet groups (more than five pellets, Mayle et al. 1999) in the plots. Pellets of fallow and roe deer were recognized out of shape and size: the former defecates cylindrical pellets, usually with a pointed end and slightly concave at the other, whereas the latter makes small, elongated pellets, rounded at both ends (Mayle et al. 1999). For fallow deer, we used a defecation rate of 25 pellet groups/day (data from our same study area: Massei and Genov 1998). For roe deer, local information on defecation rate was lacking. Researches on different deer species indicated little variation of defecation rates between different habitats or geographic areas (e.g. fallow deer: Stubbe and Goretzki 1991 in Massei and Genov 1998; Mayle et al. 1996; Massei and Genov 1998; red deer C. elaphus: Mitchell and McCowan 1984; Bruno 1996; white-tailed deer Odocoileus virginianus: Rogers 1987; Sawyer et al. 1990). We used an estimated value of 20 pellet groups/day (i.e. an average between two sites with different environmental conditions: Mitchell et al. 1985), recommended for roe deer (Ratcliffe and Mayle 1992; Mayle et al. 1999; see also Fattorini et al. in press; Ferretti et al. in press). Defecation rate could vary across seasons since severe variations in climactic conditions (e.g. temperatures ranging from −35°C to 35°C: Rogers 1987) may determine the changes in the feeding activity of deer. Accordingly, the use of a single defecation rate for both winter and summer surveys may constitute a source of bias. Even if we could not exclude an across-season variability of defecation in MRP, the variation is expected to be moderate owing to the moderate climactic changes occurring in our study area (mean monthly temperatures range from 7°C to 24°C and snow is absent).
Habitat relationships
Habitat niche breadths of both deer species were investigated descriptively through the Hurlbert standardized index (H; Krebs 1999) from data collected through pellet group counting surveys in summer and in winter. This index considers differences in availability across habitats, is sensible to the presence of rare habitats and ranges from 0 to 1 (Krebs 1999). We considered the following habitat types: oakwood, scrubwood, garigue (i.e. the three wood types composing Mediterranean scrubwood strata; Mencagli and Stefanini 2008), pinewood, abandoned olive groves and pastures, set-aside grassland, cultivated fields. Considering M habitats, the availability of each of them, denoted by π i (i = 1,…,M), was calculated through the ratio of the total size of the sampling plots fallen in the habitat to the total plot size. Defining O i the proportion of pellet groups found in the i-th habitat with respect to the total amount of pellet group found in the survey, the index turns out to be (Krebs 1999):
where \( B = 1/\sum\limits_{{i = 1}}^M {(o_i^2/{\pi_i})} \) and \( {\pi_{{\min }}} = \mathop{{\min }}\limits_i \,{\pi_i} \). In each season, spatial overlap was assessed through the Pianka index (Pianka 1974). This index ranges between 0 (no overlap) and 1 (complete overlap). Overlap can depend on the spatial scale adopted (Hemami et al. 2004): we calculated spatial overlap at two scales. At the habitat scale, overlap was assessed through the formula (Pianka 1974):
where O iF and O iR denote the proportion of fallow deer and roe deer pellet groups in the i-th habitat, respectively. The same index was also assessed at plot scale:
where n denotes the total number of plots and O iF and O iR denote the proportion of fallow and roe deer pellet groups in the i-th plot, respectively.
Results
Densities
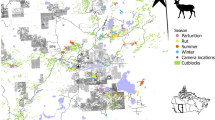
In both seasons, the greatest densities of fallow deer were found in the north abandoned olive groves and pastures (more than 45 individuals/100 ha; Figs. 1a and 2). By contrast, densities were much lower in the corresponding habitats in the south (less than ten individuals/100 ha; Figs. 1a and 2). Densities were intermediate in the set-aside grassland (18–36 individuals/100 ha) and in scrubwood (ten to 20 individuals/100 ha), being 1.3 and 2.1 times greater in the north than in the south in summer and in winter, respectively (Figs. 1a and 2). Densities were low in the pinewood (eight to 12 individuals/100 ha) and in the south cultivated fields (less than seven individuals/100 ha), whereas fallow deer were not found in the cultivated fields in the north (Fig. 1a).
Roe deer showed greater densities in the south than in the north (Fig. 2). Densities were the greatest in the south abandoned olive groves and pastures, as well as in Mediterranean scrubwood (20–34 individuals/100 ha; Figs. 1b and 2). In the corresponding habitats in the north, densities were more than three times lower than in the southern ones (less than eight individuals/100 ha; Figs. 1b and 2). Densities showed medium to low values in the set-aside grassland and in the cultivated fields (four to 12 individuals/100 ha; Fig. 1b). Roe deer were not found in the pinewood (Fig. 1b).
Differences in densities between northern and southern strata did not depend on differences in the proportion of plots sampled in the wood types of Mediterranean scrubwood (oakwood, scrubwood, garigue), which was similar between strata (G-test: G adj = 2.499, df = 2, P = 0.287).
Habitat relationships
The habitat niche breadth of fallow deer was 31% (summer) and 64% (winter) broader than that of roe deer (Fig. 3a). At the habitat scale, the spatial overlap was great (Pianka index = 0.79 in summer, 0.82 in winter; Fig. 3b). At the plot scale, the spatial overlap was 5.3 (summer) and 4.3 (winter) smaller than that measured at the habitat scale (Pianka index = 0.15 in summer, 0.19 in winter; Fig. 3b).
Discussion
The abundance of pellet groups in a habitat may not be a function of the number of individuals or of time spent in that place because defecation rates may depend on activity (Collins and Urness 1981; Mitchell et al. 1983). On the other hand, pellet group counts can help in estimating the relative habitat use in comparison with other methods (radio-tracking: Loft and Kie 1988; Edge and Marcum 1989; direct observation: Leopold et al. 1984) and have been used from several decades in various contexts (Neff 1968; Putman 1984; Latham et al. 1997; Hemami et al. 2005).
The greatest densities of both the roe and the fallow deer were found in the abandoned olive groves and pastures, although a contrasting pattern was recorded between northern and southern strata (Figs. 1 and 2). These habitats, at the boundary between wood and open fields, are mostly covered by bushes, interspersed with pastures, providing food and cover to deer species. Ecotone areas are an optimal habitat for deer, determining high reproductive success (Miyashita et al. 2008; McLoughlin et al. 2007) and great densities (Wahlström and Kjellander 1995; Hemami et al. 2005).
The fallow deer reached its greatest densities in the central northern part of our study area, whereas the roe deer showed the opposite pattern (Figs. 1 and 2). This pattern should not depend on differences in habitat between northern and southern strata because the proportion of plots sampled in the wood types of Mediterranean scrubwood was not significantly different. As to the fallow deer, this pattern may be explained because of historical reasons: in MRP, fallow deer were introduced to the northern part of our study area. The last release of individuals dates back to the mid 1960s of the twentieth century when, presumably, less than 30–40 fallow deer inhabited the park (G. Anselmi, MRP Agency, personal communication). Most likely, fallow deer have colonized our northern strata before the southern ones. In 2001–2003, more than 15–20 individuals/100 ha and less than five to six individuals/100 ha were estimated in the northern and in the southern scrubwood, respectively (Sforzi 2004), whereas 16–22 individuals/100 ha and ten to 12 individuals/100 ha were estimated in 2006–2008 (Fattorini et al. in press; this study). The large standard errors of estimates in 2001–2003 and the variations in the sampling design in 2006–2008 (Fattorini et al. in press) complicate comparisons between the periods but, apparently, fallow deer numbers have increased in the southern strata.
As to roe deer, the most used plants in their diet in the scrubwood (Q. ilex, A. unedo, R. officinalis; Minder 2006) are largely present both in the north and in the south of our study area but in the pinewood (Minder 2006; Mencagli and Stefanini 2008) where this deer was not detected. In this habitat, the understorey is composed mainly by species of which few are used or avoided by this deer: mastic Pistacia lentiscus, juniper (both Juniperus oxycedrus and Juniperus phoenicea) and myrtle Myrtus communis (Minder 2006; Mencagli and Stefanini 2008). In north scrubwood and abandoned olive groves, the relative scarcity of roe deer should not depend on the lower availability of palatable species than in the southern strata. Most likely, in these habitats, fallow deer have been living at densities greater than 15–20 individuals/100 ha from at least one decade (Sforzi 2004). The former is expected to have a broader diet than roe deer (Hofmann 1989) and could also eat plants selected by the latter (Putman 1996). In the Mediterranean scrubwood, food offer is low (Minder 2006) and even moderate densities of fallow deer may have a negative effect on densities of roe deer. Accordingly, especially in summer, medium to high densities of fallow deer had a strong negative effect on the probability of roe deer presence in sampling plots even considering the effects of habitat (Ferretti et al. in press). We suggest that, in the north scrubwood and abandoned olive groves, medium to high densities of the former could determine low densities of the latter. Fallow deer may have not completed the colonization of the southern part of the MRP, which could contribute to explain the greater densities of roe deer in the southern strata.
Other studies showed inverse numerical trends between populations of these deer species with a decrease of roe and an increase of fallow deer numbers (Putman and Sharma 1987; Focardi et al. 2006). A similar pattern has been recently recorded also in our study area, with an increase in both the number of fallow deer sightings in open areas and fallow deer density and an opposite trend for the roe deer between 2006 and 2009 (Ferretti et al. in press). In our study area, active behavioural interference by fallow to roe deer has been documented, with the former being dominant over the latter in about 80% interspecific encounters, suggesting that fallow displace roe deer at natural feeding sites (Ferretti et al. in press; Ferretti in press). Interference was greater on roe deer females in spring (Ferretti et al. in press), i.e. when pregnancy and lactation occur and a reduced food intake can affect reproductive success (Pettorelli et al. 2005; McLoughlin et al. 2007).
The fallow deer showed a broader habitat niche in comparison to the roe deer (Fig. 3a). This could be due to a different foraging behaviour: the roe deer usually selects vegetation poor in fibre and rich in cell content, whereas the fallow deer can exploit food rich in fibre as well as high-quality vegetation (Hofmann 1989). Alternatively, this pattern may be explained by behavioural interference (Ferretti et al. 2008): roe deer may contract their distribution in areas with great densities of fallow to reduce the probability of interspecific encounters. Spatial overlap between our study species was high at the habitat scale, suggesting a great potential for competition, but decreased markedly at the plot scale (Fig. 3b), suggesting that partitioning of space occurs at a finer resolution. Similar results were also found in sympatric roe deer and muntjac in a forest of Great Britain (Hemami et al. 2004). In our study area, the probability of presence of roe deer was low in areas with great densities of fallow deer (Ferretti et al. in press). Our results suggest that behavioural interference by fallow deer may affect the small-scale distribution of roe, which avoids areas with great densities of the former.
Densities of livestock can negatively affect those of wild ungulates through the depletion of food resources (Baldi et al. 2001; Madhusudan 2004; Mishra et al. 2004). Interspecific negative effects of densities have been suggested also for wild ungulates (Putman 1996; Forsyth and Hickling 1998; Focardi et al. 2006), although the mechanisms of interactions are unclear. Our data suggest that the density of the superior competitor negatively affects density, habitat use and distribution of the inferior competitor. This negative effect is probably determined by behavioural avoidance of the former by the latter (Ferretti et al. 2008; Ferretti et al. in press). Wild ungulates are often elusive species and behavioural interactions are difficult to observe. Their role in determining ecological relationships between large herbivores has not been assessed but for anecdotal records (Anthony and Smith 1977; Forsyth 1997). We suggest that behavioural interactions could have an important role in shaping relationships between sympatric ungulates, leading to changes on density, habitat use and distribution of competing species both at fine and at large scales.
References
Aanes R, Andersen R (1996) The effects of sex, time of birth, and habitat on the vulnerability of roe deer fawns to red fox predation. Can J Zool 74:1857–1865
Andersen R, Duncan P, Linnell JDC (1998) The European roe deer: the biology of success. Scandinavian University, Oslo
Andersen R, Gaillard JM, Linnell JDC, Duncan P (2000) Factors affecting maternal care in an income breeder, the European roe deer. J Anim Ecol 69:672–682
Angulo E, Roemer GW, Berek L, Gascoigne J, Courchamp F (2007) Double Allee effects and extinctions in the island fox. Cons Biol 21:1082–1091
Anthony RG, Smith NS (1977) Ecological relationships between mule deer and white-tailed deer in Southeastern Arizona. Ecol Monogr 47:255–277
Apollonio M, Focardi S, Toso S, Nacci L (1998) Habitat selection and group formation pattern of fallow deer Dama dama in a submediterranean environment. Ecography 21:225–234
Apps CM, McLellan B, Woods JG, Proctor MF (2004) Estimating grizzly bear distribution and abundance relative to habitat and human influence. J Wildl Manage 68:138–152
Arsenault R, Owen-Smith N (2008) Resource partitioning by grass height among grazing ungulates does not follow body size relation. Oikos 117:1711–1717
Baldi R, Albon SD, Elston DA (2001) Guanacos and sheep: evidence for continuing competition in arid Patagonia. Oecologia 129:561–570
Barabesi L, Fattorini L, Ridolfi G (1997) Two-phase surveys of elusive populations. In: Proceedings of the Statistic Canada Symposium 97: new direction in surveys and censuses. Statistic Canada, Ottawa, pp 285–287
Bartoš L, Vankova D, Siler J, Losos S (1996) Fallow deer tactic to compete over food with red deer. Aggr Behav 22:375–385
Bartoš L, Vankova D, Miller KV, Siler J (2002) Interspecific competition between white-tailed, fallow, red and roe deer. J Wildl Manage 66:522–527
Batcheler CL (1960) A study of the relations between roe, red and fallow deer, with special reference to Drummond Hill Forest, Scotland. J Anim Ecol 29:375–384
Bertolino S, di Montezemolo NC, Bassano B (2009) Food–niche relationships within a guild of Alpine ungulates including an introduced species. J Zool 277:63–69
Boitani L, Lovari S, Vigna Taglianti A (2003) Fauna d’Italia, vol. XXXVIII, Mammalia III, Carnivora—Artiodactyla. Calderini Ed., Bologna
Bruno E (1996) Habitat selection and feeding ecology of red (Cervus elaphus L.) and roe (Capreolus capreolus L.) deer in the Central Apennines, Italy. Ph.D. thesis, University of Aberdeen, Aberdeen
Campbell D, Swanson GM, Sales J (2004) Comparing the precision and cost-effectiveness of faecal pellet group count methods. J Appl Ecol 41:1185–1196
Collins WB, Urness PJ (1981) Habitat preferences of mule deer as rated by pellet-group distributions. J Wildl Manage 45:969–972
Cavallini P, Lovari S (1994) Home range, habitat selection and activity of the red fox in a Mediterranean coastal ecotone. Acta Theriol 39:279–287
Coneybeer R (2003) An investigation into the diet of the red fox at Maremma Natural Park, Grosseto, Central Italy. M.Sc. thesis, March–June 2003, University of Swansea, Swansea
Cordy CB, Thompson CM (1995) An application of deterministic variogram to design-based variance estimation. Math Geology 27:173–205
Duncan P, Tixier H, Hoffman RR, Lechner-Doll M (1998) Feeding strategies and the physiology of digestion in roe deer. In: Andersen R, Duncan P, Linnell JDC (eds) The European roe deer: the biology of success. Scandinavian University, Oslo, pp 91–116
Edge WD, Marcum CL (1989) Determining elk distribution with pellet-group and telemetry techniques. J Wildl Manage 53:621–624
EPA (2002) Guidance for choosing a sampling design for environmental data collection. EPA QA/G-5S. US Environmental Protection Agency, Washington, DC
Fattorini L, Ferretti F, Pisani C, Sforzi A (in press) Two-stage estimation of ungulate abundance in Mediterranean areas using pellet group count. Environ Ecol Stat
Ferretti F (in press) Interspecific aggression between fallow and roe deer. Ethol Ecol Evol
Ferretti F, Sforzi A, Lovari S (2008) Intolerance amongst deer species at feeding: roe deer are uneasy banqueters. Behav Process 78:487–491
Ferretti F, Sforzi A, Lovari S (in press) Behavioural interference between ungulate species: roe are not on velvet with fallow deer. Behav Ecol Sociobiol
Focardi S, Aragno P, Montanaro P, Riga F (2006) Inter-specific competition from fallow deer Dama dama reduces habitat quality for the Italian roe deer Capreolus capreolus italicus. Ecography 29:407–417
Forchammer MC, Post E, Stenseth NC, Boertmann DM (2002) Long-term responses in arctic ungulate dynamics to changes in climatic and trophic processes. Popul Ecol 44:113–120
Forsyth DM (1997) Ecology and management of Himalayan thar and sympatric chamois in the Southern Alps, New Zealand. Ph.D. thesis, University of Lincoln, Lincoln
Forsyth DM, Hickling GJ (1998) Increasing Himalayan tahr and decreasing chamois densities in the eastern Southern Alps, New Zealand: evidence for interspecific competition. Oecologia 113:377–382
Fuller TK, Mech LD, Cochrane JF (2003) Wolf population dynamics. In: Mech LD, Boitani L (eds) Wolves. Behavior, ecology and conservation. The University of Chicago Press, Chicago, pp 161–191
Gill RMA, Johnson AL, Francis A, Hiscocks K, Peace AJ (1996) Changes in roe deer (Capreolus capreolus L.) population density in response to forest habitat succession. For Ecol Manage 88:31–41
Gregoire TG, Valentine HT (2008) Sampling strategies for natural resources and the environment. Chapman & Hall, New York
Hemami MR, Watkinson AR, Dolman PM (2004) Habitat selection by sympatric muntjac (Muntiacus reevesi) and roe deer (Capreolus capreolus) in a lowland commercial pine forest. For Ecol Manage 194:49–60
Hemami MR, Watkinson AR, Dolman PM (2005) Population densities and habitat associations of introduced muntjac Muntiacus reevesi and native roe deer Capreolus capreolus in a lowland pine forest. For Ecol Manage 215:224–238
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78:443–457
Krebs CJ (1999) Ecological methodology. Addison Wesley Longman, USA
Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE (1999) Population density affects sex ratio variation in red deer. Nature 399:459–461
Jenkins KJ, Wright RG (1988) Resource partitioning and competition among cervids in northern rocky mountains. J Appl Ecol 25:11–24
Latham J (1999) Interspecific interactions of ungulates in European forests: an overview. For Ecol Manage 120:13–21
Latham J, Staines BW, Gorman ML (1997) Correlations of red (Cervus elaphus) and roe (Capreolus capreolus) deer densities in Scottish forests with environmental variables. J Zool 242:681–704
Leopold BD, Krausman PR, Hervert JJ (1984) Comment: the pellet-group census technique as an indicator of relative habitat use. Wild Soc Bull 12:325–326
Loft ER, Kie JG (1988) Comparison of pellet-group and radio triangulation methods for assessing deer habitat use. J Wildl Manage 52:524–527
Madhusudan MD (2004) Recovery of wild large herbivores following livestock decline in a tropical Indian wildlife reserve. J Appl Ecol 41:858–869
Manghi L, Ciucci P, Boitani L (2006) Progetto per la ricerca e il monitoraggio del lupo (Canis lupus) nel Parco Regionale della Maremma. Relazione delle attività svolte, risultati e proposte di ricerca (Febbraio–Aprile, unpublished report). Ente Parco Regionale della Maremma, Alberese Italy
Massei G, Genov P (1998) Fallow deer (Dama dama) winter defecation rate in a Mediterranean area. J Zool 245:209–210
Massei G, Bacon P, Genov P (1998) Fallow deer and wild boar pellet group disappearance in a Mediterranean area. J Wildl Manage 62:1086–1094
Mayle BA, Doney J, Lazarus G, Peace AJ, Smith DE (1996) Fallow deer (Dama dama) defecation rates and its use in determining population size. Supplementi alle Ricerche di Biologia della Selvaggina 25:63–78
Mayle BA, Peace AJ, Gill RMA (1999) How many deer? A field guide to estimating deer population size. Forestry Commission field book no. 18, Edinburgh
McGhee JD, Baccus JT (2006) Behavioural interactions between axis and fallow deer at high-value food patches. Southwest Nat 51:358–367
McLoughlin PD, Gaillard J-M, Boyce MS, Bonenfant C, Messier F, Duncan P, Delorme D, Moorter B, Said S, Klein F (2007) Lifetime reproductive and composition of the home range in a large herbivore. Ecology 88:3192–3201
Mencagli M, Stefanini P (2008) Carta della vegetazione per il Piano del Parco. Ente Parco Regionale della Maremma, Alberese, Italy
Minder I (2006) Adaptive parameters of the diet of roe deer in a coastal Mediterranean area. Ph.D. thesis. University of Siena, Siena
Mishra C, Van Wieren SE, Ketner P, Heitkong IMA, Prins HT (2004) Competition between domestic livestock and wild bharal Pseudois nayaur in the Indian Trans-Himalaya. J Appl Ecol 41:344–354
Mitchell B, McCowan D (1984) The defecation frequencies of red deer in different habitats. Institute of Terrestrial Ecology annual report 1983. ITE, Cambridge, pp 15–17
Mitchell B, McCowan D, Campbell D (1983) Faecal depositions as indicators of site use by red deer. A Rep Inst Terr Ecol 1982:85–87
Mitchell B, Rowe JJ, Ratcliffe PRR, Hinge M (1985) Defecation frequency in roe deer (Capreolus capreolus) in relation to the accumulation rates of faecal deposits. J Zool 207:1–7
Miyashita T, Suzuki M, Ando D, Fujita G, Ochiai K, Asada M (2008) Forest edge creates small-scale variation in reproductive rate of sika deer. Popul Ecol 50:111–120
Mysterud A, Larsen PK, Ims RA, Østbye E (1999) Habitat selection by roe deer and sheep: does habitat ranking reflect resource availability? Can J Zool 77:776–783
Neff DJ (1968) The pellet group count technique for big game trend, census and distribution: a review. J Wildl Manage 32:597–614
Nowak RM (1991) Walker’s mammals of the world, II, 5th edn. Johns Hopkins University, Baltimore, p1629
Pettorelli N, Gaillard JM, Yoccoz NG, Duncan P, Maillard D, Delorme D, Van Laere G, Toïgo C (2005) The response of fawn survival to changes in habitat quality varies according to cohort quality and spatial scale. J Anim Ecol 74:972–981
Pianka ER (1974) The structure of lizard communities. Annu Rev Ecol Syst 4:53–74.
Putman RJ (1984) Facts from faeces. Mamm Rev 14:79–97
Putman RJ (1996) Competition and resource partitioning in temperate ungulate assemblies. Chapman and Hall, London
Putman RJ, Moore NP (1998) Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mamm Rev 28:141–164
Putman RJ, Sharma SK (1987) Long term changes in new forest deer populations and correlated environmental change. Symp Zool Soc Lond 58:167–179
Ratcliffe PR, Mayle BA (1992) Roe deer biology and management. Forestry Commission bulletin no. 105, London
Rogers LL (1987) Seasonal changes in defecation rates of free-ranging white-tailed deer. J Wildl Manage 51:330–333
Sawyer TG, Marchington RL, MacLentz W (1990) Defecation rates of female white-tailed deer in Georgia. Wildl Soc Bull 18:16–18
Sforzi A (2004) Stima dell’abbondanza di cervidi per mezzo del pellet group count in ambiente mediterraneo. Ph.D. thesis, University of Siena, Siena
Skalski JR (1994) Estimating wildlife population based on incomplete area surveys. Wild Soc Bull 22:192–203
Stockton SA, Allombert S, Gaston AJ, Martin JL (2005) A natural experiment on the effects of high deer densities on the native flora of coastal temperate forests. Biol Conserv 126:118–128
Stubbe C, Goretzki J (1991) Hohe und Bedeutung der Defakationsrate beim Damwild zur Ermittlung der Wilddichte. Z Jadgwiss 37:273–277
Tufto J, Andersen R, Linnell JDC (1996) Habitat use and ecological correlates of home range size in a small cervid: the roe deer. J Anim Ecol 65:715–724
Wahlstrom LK, Kjellander P (1995) Ideal free distribution and natal dispersal in female roe deer. Oecologia 103:302–308
Acknowledgements
We are indebted to S. Lovari, who stimulated us to carry out this work and gave helpful suggestion throughout all stages, from its planning to the revision of drafts. We thank the MRP Agency, the Azienda Agricola Regionale di Alberese and the landowners who provided the permission to work in their lands. We are indebted to C. Pisani, who helped in planning the sampling design and carried out the density estimates. We thank the park wardens and the administrative personnel of the MRP, as well as L. Varaglioti, for logistic support. G. Sammuri encouraged us throughout our work. I. Minder kindly helped with the references. G. Anselmi and E. Fanton gave important information on the presence of fallow deer in the MRP. Funding was provided by the Maremma Regional Park Agency to F. Ferretti.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Ferretti, F., Bertoldi, G., Sforzi, A. et al. Roe and fallow deer: are they compatible neighbours?. Eur J Wildl Res 57, 775–783 (2011). https://doi.org/10.1007/s10344-010-0487-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-010-0487-5