Abstract
We examined the spatial structure and socio-biology of a native wild population of Eurasian otters (Lutra lutra) on mesotrophic rivers in a mild temperate climate. Radio-tracking of 20 individuals revealed exclusive intra-sexual adult home-ranges. Adult female home-ranges (7.5 km, SD = 1.5 km, n = 7) were inversely related to river width (\(R_{{\text{adj}}}^2 = 0.68\), F 6 = 13.5, P = 0.014) and so appeared to be based on food resources. The aquatic area within adult male home-ranges (30.2 ha, SD = 9.5 ha, n = 5) was greater than that within adult female’s (16.8 ha, SD = 7.0 ha) (t 10 = 2.437, P = 0.035), though this result is inconclusive because some males were tracked on oligotrophic rivers. One adult male expanded its range from 10.2 km to 19.3 km within 5 days of the death of the neighbouring male, suggesting that male home-ranges were heavily influenced by conspecifics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In spite of its high priority for conservation (CITES 1979; Council of Europe 1979, EU Habitats Directive 1992; IUCN 2006), the socio-biology of the Eurasian otter (Lutra lutra) remains largely unknown (Chanin 2003; Kruuk 2006). On only two occasions have researchers identified the spatial structure of a native otter population (Erlinge 1967; Kruuk and Moorhouse 1991). Adult male territories were mate-based (Erlinge 1967; Kruuk and Moorhouse 1991) and heavily influenced by topography and the occurrence of other dog otters (Erlinge 1967). Female spatial structure appeared to rely on resource distribution with exclusive communal ranges in a coastal area of heterogeneous resources (Kruuk and Moorhouse 1991) and exclusive individual territories in a highly productive freshwater area (Erlinge 1967). Therefore, both studies were consistent with the resource dispersion hypothesis and the classical mustelid model (Powell 1979; MacDonald 1983).
Otters in the freshwater system studied by Erlinge (1967) occupied large annual ranges of around 20–30 km. Equally large home-ranges were consistently observed in other studies of native freshwater otter populations involving small samples of dispersed individuals (e.g. Green et al. 1984; Durbin 1993, 1996a, b; Kruuk et al. 1993; Kruuk 2006; Poledník 2005). Translocated or reintroduced populations have also been monitored following their release and were again found to occupy similarly large ranges, even where river systems were highly productive (Jefferies et al. 1986; Saavedra 2002).
Otters in freshwater systems appear characterised by large and fairly constant linear spatial requirements. Nevertheless, rich lowland rivers have received little attention in north-western Europe (Chanin 2003), although higher densities were observed in such systems in Mediterranean areas (Ruiz-Olmo 2001). The classical mustelid model predicts female home-ranges based on food resources (Powell 1979; Johnson et al. 2000) that should, therefore, be shorter on more productive rivers and on wider sections of autochtonous systems. We aimed to identify the spatial structure of otter populations in a simple mesotrophic system to augment the limited knowledge of otter socio-biology and to facilitate the interpretation of fragmented data or data from complex environments.
Materials and methods
Study area
In contrast with most other European populations (Macdonald and Mason 1994), Irish otters remained widespread during the twentieth century (Chapman and Chapman 1982; Lunnon and Reynolds 1991; Bailey and Rochford 2006). Ireland has a mild, temperate, oceanic climate. In the east and south-east, where this study was conducted, mean monthly temperature varies from 5–15°C, mean monthly rainfall from 50–70 mm, and snow persists past 0900 h in lowland areas for 5–6 days per year (Met Éireann-Irish Meteorological Service 2006). Three alkaline, calcium-rich rivers were chosen for this study; the River Boyne, the River Liffey and the King’s River (Fig. 1). The Boyne and King’s are mesotrophic (0.04–0.06 mg orthophosphate per liter) (EPA 2006). The fish communities are dominated by brown trout (Salmo trutta) and salmon (Salmo salar), but include pike (Esox lucius), perch (Perca fluvialtilis), eel (Anquilla anquilla), stickleback (Gasterosteus aculeatus), and minnow (Phoxinus phoxinus), with crayfish (Austropotamobius pallipes) also present (Reynolds 1998; Demers et al. 2005). The section of the Liffey within the study area was oligotrophic (0.01 mg orthophosphate per liter [EPA 2006]) and was again dominated by salmonids and crayfish (W. Champ, Eastern Fisheries Board, Dublin—unpublished report).
Biotelemetry
We captured otters with alarmed leghold traps and fitted them with radio-transmitters in the field. Details of the trapping and tagging program have been described in detail elsewhere (Ó Néill et al. 2007, 2008). The trapping technique yielded a remarkably high trapping rate of 8.4 trap-nights per otter, or 1.7 (SD = 0.9) nights per capture at successful sites. Initially, we fitted 13 individuals with externally mounted radio-transmitters (TW-5 high power twin cell tags with two 10–28 cells, Biotrack, Peterborough, UK), 11 with harnesses (Mitchell-Jones et al. 1984) and two with glue. Highly variable and generally short retention of these transmitters led to the use of intra-peritoneal implants on a further 15 individuals (TW-5 high power twin cell with 1/2 AA, Biotrack, Peterborough, UK).
We captured and radio-tracked 12 otters on a 30-km stretch of the King’s River. We estimated maturity based on genitalia and body size. One young male was tracked in 2005, while the remainder were tracked concurrently in 2006, although the tag failed almost immediately for one larger adult male (AM3) (Fig. 3a). Only one animal escaped from a trap in this study area and we determined from its prints that it was a small sub-adult. We continued to trap the King’s River study area for 21 unsuccessful trap-nights following the last capture. Based on the efficiency of the trapping technique, we were confident that all individuals commonly using the area were trapped. We captured 13 otters on the River Boyne but the data-set was fragmented by escapes, radio-transmitter failures and the death of adult females AF6 and AF7 (Fig. 3b, c). Finally, we captured six otters on the River Liffey, but equipment failures and the death of one adult male again fragmented the data-set.
Receiving equipment consisted of a Sika® receiver and a flexible three-element Yagi® antenna (Biotrack). Owing to safety concerns, surveying was largely limited to daylight hours following the procedure of Melquist and Hornocker (1983). Individuals with external radio-transmitters were tracked to source daily as the expected retention time was short, whereas individuals with implants were located once or twice a week. No more than one location per day was included in the analysis of home-ranges.
Home-range analysis
We calculated Kernel density contours (95%) with least squares cross-validation (LSCV) smoothing from the distribution of fixes for each individual (Arc View 3.2, Animal Movement SA v.2.04 beta) (Seaman and Powell 1996; Gorman et al. 2006). Least squares cross-validation smoothing can result in excessive fragmentation of kernels and underestimate (discontinuous) linear home-ranges (Blundell et al. 2001). Nevertheless, we used LSCV smoothing because we did not observe much fragmentation of continuous riverine home-ranges, and because fixed-kernels with reference smoothing (Blundell et al. 2001) extended too far beyond the apparent boundaries of territories. We measured home-ranges as the length of river or lake-shore bounded by the 95% kernel density contour (Blundell et al. 2001) because the contours included a high proportion of unused terrestrial habitat. We estimated the average channel width of the river section contained within each home-range from three evenly spaced measurements per kilometer made at straight sections of watercourse. We calculated home-range aquatic area as the product of the watercourse width and the home-range length.
It is important that any home-range estimate remains stable with increasing numbers of radio locations (Aebischer et al. 1993). Individual home-ranges were accepted as stable when five successive partial home-range estimates varied by less than 5%. To establish the minimum number of fixes required to estimate home-ranges for the population, we calculated the difference between successive partial home-ranges for each individual i.e. the difference between the home-range calculated from seven fixes and that calculated from eight fixes etc. The differences were normalised as a proportion of the final home-range. Stability was satisfactory where the mean absolute difference between successive partial home-ranges approached 0 with a standard deviation less than 0.05, leading to 95% confidence intervals of 10% around the home-range estimates. After accepting a home-range as stable, we corrected any fragmentation by including intervening stretches between fragments in the final home-range estimate.
Results
Home-range stability
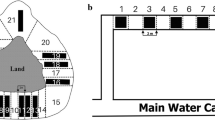
For those animals with at least 15 fixes, excluding lactating females that required extended or intensive monitoring, we found that home-range estimates became stable after 13 locations (n = 16) (Fig. 2). We accepted home-range estimates for 20 animals as stable (Table 1). Various equipment failures limited the usefulness of the data from the other otters. Fluctuating signals indicated that otters were active on 21% (SD = 6.8%) of occasions (358 fixes, n = 20). Based on an average of 14.1 (SD = 4.0) fixes where the otter was inactive, we recorded 7.0 (SD = 1.9) separate resting sites each (n = 20). This is a conservative estimate based on a limited number of fixes and no distinction of nearby resting sites (<80 m apart).
The stability of home-range estimates (95% kernel density) for 14 otters as the number of radio-locations is increased. Points are plotted as the mean absolute difference between the proportions of each final home-range (HR i .) plotted by successive partial home-ranges (HR i ,j − HR i ,j−1) or \(\frac{1}{n}\sum\limits_1^n {\left( {\frac{{\left| {HR_{i,j} - HR_{i,j - 1} } \right|}}{{HR_i }}} \right)} \) Stability was acceptable when the mean difference approached 0 with a standard deviation below 0.05 (giving 95% confidence intervals around the home-range estimate of ±10%)
Female social structure
The King’s River data-set allowed us to determine the spacing pattern of family groups consisting of mother and offspring (Fig. 3a). Crucially, this data-set showed adult females occupying exclusive intra-sexual home-ranges. The ranges of advanced dependent or newly independent offspring (SAF3, SAF4, YAF1, SAM8) approximated the resident adult female home-ranges (AF2, AF3, AF8). Although the fragmented data-set for the Boyne is not suitable for determining spacing patterns on its own, the home-range boundaries of adult and sub-adult females are not inconsistent with evenly spaced exclusive female home-ranges (Fig. 3b, c).
The spatial structure of otter populations on a the King’s River (7–20 m wide), b the lower River Boyne (30–40 m wide), and c the middle River Boyne (20–25 m wide). Thick lines represent simplified water-courses. Lines without stoppers indicate incomplete home-ranges based on less than 13 fixes. Individuals are classed as adult, (A), young-adult (YA) or sub-adult (SA) and as males (M) or females (F). Family range boundaries are indicated with a broken line. *YAM2 was tracked 11 months prior to the others in the King’s River data-set
Observations of pre-dispersal range restriction supported exclusive intra-sexual home-ranges by suggesting that mature or maturing individuals were not tolerated within the resident adult female’s home-range. Juveniles were initially limited to a range spanning 1–2 km of the maternal range (n = 2). Dependent sub-adults covered the maternal range in the company of their mother (n = 5). Finally, just before dispersing, females became restricted to the edge of the resident adult female’s home-range (n = 3) (Fig. 4). When we caught YAF1, an adolescent or young adult female, the larger resident adult female was lactating. YAF1 covered the full home-range of the resident adult female AF2 but seemed to avoid the area surrounding the holt containing AF2’s cubs (based on 18 fixes over 32 days). YAF1’s range eventually became confined to the edge of AF2’s home-range (based on 11 fixes over 43 days) before we lost contact, probably because of dispersal. The genetic relationship between YAF1 and AF2 was unknown. A similar pattern was observed for sub-adult female SAF3 who was almost certainly AF3’s daughter. Initially, we located her in the company of AF3 on eight of 12 occasions covering a period of 50 days. We then found her alone on six consecutive occasions over a period of 30 days while continuing to use AF3’s full home-range. Finally, we found her within a small area at the edge AF3’s home-range on four occasions over 12 days before losing contact, probably following dispersal. We also observed this pattern of range restriction in a sub-adult female where the resident adult female died. We saw SAF5 following a slightly larger otter on two occasions. Twenty-five days following her capture, we found the fresh cadaver of an adult female from within SAF5’s home-range. SAF5 continued to cover her home-range as before until day 30 (18 fixes in total) when she became confined to a resting-site 200 m away from the main river. She remained at this location for 7 days (five fixes) before dispersing downstream (five further fixes). SAF5’s cadaver was eventually retrieved as a road-traffic victim on day 52. We consider it likely that the range restriction and subsequent dispersal were caused by a new adult female entering and occupying AF3’s home-range.
The behaviour of otters immediately prior to dispersal. The contours represent 95% kernel density contours for the period indicated. SAF5 was forced to disperse following the death of the resident adult female on day 25. On day 30, she suddenly became confined to a holt 200 m away from the main river. Seven days later, she dispersed downstream and was ultimately recovered as a road traffic victim on day 52. When we caught YAF1, the resident adult female was already lactating. YAF1 was apparently tolerated within the resident’s territory but not in the core area. YAF1’s range gradually declined until she eventually became confined almost exclusively to the edge of the territory. SAF3 was semi-independent of her mother when captured. She gradually became fully independent and used the full range of her mother before becoming confined to a small area at the extreme edge of her mother’s range
Further support for exclusive, intra-sexual territoriality was provided by the trapping data. Traps remained within every captured otter’s home-range for 10–15 days. The extremely high efficiency of the traps meant that we were confident that resident animals were either captured or had escaped from traps. Gradually moving the traps upstream resulted in otters being caught as soon as the traps entered their home-ranges. Sixteen of the 20 otters whose home-ranges we identified were caught towards the edges of their home-ranges. Adult females represented 15 of 36 captures overall. We plotted home-ranges for six adult females and none of the 12 additional animals caught within these ranges were conspecific.
The spatial structure of female home-ranges appears to be determined by the resident adult female. Therefore we used only the home-ranges of the adult females, or a well-developed resident sub-adult where data was lacking for the resident adult female, to estimate the relationship between home-range size and river width (AF1, AF2, AF3, AF4, AF5, AF8 and SAF5). We found that their home-ranges were inversely related to river width (7.5 km, SD = 1.5 km, n = 7, \(R_{{\text{adj}}}^2 = 0.68\), F 6 = 13.5, P = 0.014) (Fig. 5). In terms of aquatic area, adult female home-ranges averaged 16.8 ha (SD = 7.0).
Male social structure
Adult males were tracked on both mesotrophic (n = 3) and oligotrophic (n = 2) rivers whereas the females were all tracked on mesotrophic rivers. The adult male ranges (30.2 ha [SD = 9.5 ha], 13.2 km [SD = 5.3 km], n = 5) were quite variable and appeared to be influenced by the presence of conspecifics. Two animals occupying adjacent home-ranges, AM6 and AM5, were tracked on the Liffey. When AM6 was killed in an illegal snare, AM5 expanded his range from 10.2 km (based on 16 fixes over 80 days) to 19.3 km within 5 days (based on three additional fixes). Adult male home-range aquatic area was larger than female’s (Levene’s F = 0.63, t 10 = 2.437, P = 0.035).
Discussion
Methodological issues
The quality of our data may be challenged in two ways: we tracked animals over relatively short periods averaging less than 3 months and we based our analysis of home-ranges on daytime fixes. We are confident from our analysis of home-range stability that we identified the area ‘normally traversed by the animals’ (Burt 1943) for the periods studied. We expect that the otters would disperse or otherwise change their home-ranges if social or environmental conditions warranted. Therefore, a longer term study might identify a larger range consisting of several temporally distinct home-ranges. In this sense, the data should be viewed as a snapshot of otter space use over a few months. Seasonality in precipitation and temperature is remarkably moderate in the study area and we suspect that ranging behaviour should not display great temporal variability. We tracked animals in all life-stages; lactating females, non-lactating females with cubs, adult females without cubs, adult males and juvenile and sub-adult individuals. Therefore, we believe that the data describes the ranging behaviour of otters in the study area in general, although there is certainly scope for longer term study.
Health and safety considerations limited surveying to the daytime. Daytime locations have been used to analyse ranging behaviour in nocturnal North American River otters (Melquist and Hornocker 1983) and re-introduced Eurasian otters (Sjöåsen 1997) but it is possible that unrecorded nocturnal movements were more expansive. The many resting sites used by the otters should represent a reasonable proxy for the extent of foraging movements if cover or disturbance are not limiting. Our subjective assessment of cover and disturbance in the study area was that they were at favourable levels and indeed otters were frequently active during the daytime. We trapped intensively along the main rivers and did not catch animals outside of the area later identified as their home-ranges, suggesting that excursive nocturnal movements were not common. If unrecorded foraging on small tributaries was common, we would expect great variation in the home-ranges arising from the unequal distribution of such tributaries. Furthermore, Green et al. (1984) recorded many resting sites on small tributaries and we should have found the otters there also. If the otters were moving up tributaries at night we would expect the neighbours on the tributaries to make reciprocal nocturnal movements down onto the main river and consequently to have been caught in the traps. This did not occur. We conclude that the methodology was sufficient to identify the areas that were of importance to our study animals in the study area. Studies that calculated home-ranges from continuous tracking data and hence included the full extent of excursive movements may give rise to more precise home-range estimates than ours (e.g. Green et al. 1984; Kruuk et al. 1993; Durbin 1996b).
Biological significance
Male home-ranges appeared to be intra-sexually exclusive to some degree but we did not track enough neighbouring pairs to determine the degree of overlap. Males had larger home-ranges than females though this result is confounded by two males being tracked on an oligotrophic river. We observed remarkably rapid expansion of one male’s home-range following the removal of a neighbouring conspecific. Adult male home-ranges (7–19 km, n = 5) were of a similar size to those recorded in a Swedish freshwater environment (10–21 km, n = 8) (Erlinge 1967) and in a Scottish coastal environment (7–19 km, n = 5) (Kruuk and Moorhouse 1991).
Adult females occupied intra-sexually exclusive territories that were inversely related to river width. Females shortened the length of their home-ranges as the width of the foraging habitat increased and this relationship suggests that the home-ranges were food-resource-based as predicted by the classical model of mustelid social organisation (Powell 1979; Johnson et al. 2000). In a productive freshwater environment, Erlinge (1967) observed females ranging over annual ranges that were several times larger than those we recorded. However, these females seasonally switched from 2–3 km radius home-ranges consisting of lakes and streams, to stream-only home-ranges averaging 5 km of stream length when lakes froze. Following Burt’s (1943) definition of home-ranges, these otters can be regarded as having occupied two home-ranges each year, both of which were of a similar size to those identified in our study.
Home-ranges averaging 18.6 km (SD = 3.5 km, SE = 1.1 km) were recorded for females in Scottish oligotrophic river systems (0.0–0.02 mg/l orthophosphate) (n = 10) (Green et al. 1984; Kruuk et al. 1993; Durbin 1996a, b; Kruuk 2006). As expected for food-based ranges in a less productive environment, these home-ranges were much larger than the 7.6 km (SD = 1.1 km, SE = 0.6 km) we observed on mesotrophic rivers (0.04–0.06 mg orthophosphate per liter). The low resources in the riverine part of the oligotrophic habitat were highlighted in one study by frequent crossings of watersheds, the maintenance of exclusive cores of non-riverine habitat, and just 14–29% of home-range area consisting of riverine habitat (Green et al. 1984). Interestingly, the values for the Scottish female home-ranges were unrelated to river width (Green et al. 1984; Kruuk et al. 1993; Durbin 1996b) and appeared to arise from otters selecting a length of terrestrial-riverine interface (Durbin 1996b). We feel that the lack of relationship with river width in the Scottish data reflects the origin of the river’s productivity. In allochtonous, oligotrophic systems fish productivity relies primarily on terrestrial input and hence the terrestrial–riverine interface. A given length of a wide stream has an equal amount of this interface as a narrow stream, resulting in a sharp decrease in the density of aquatic biomass on wider rivers as observed by Kruuk et al. (1993). In such systems otter home-ranges include enough of this interface (channel length) to supply their needs, and females concentrate on small streams where biomass density is greater (Kruuk 2006). Conversely, as in-stream productivity increases and rivers become autochtonous, biomass density is expected to be more constant as the river widens, leading to home-ranges inversely related to river width. Hence, the relationship between female home-ranges and river width across mesotrophic and oligotrophic systems is consistent with food-resource-based home-ranges.
The trapping results also support the importance of trophic status. Trapping rates on the oligotrophic river Liffey averaged 25.0 trap-nights/otter (n = 6), while on mesotrophic rivers they averaged just 5.1 (n = 30). The importance of the trophic status of fresh-waters for otters has also been demonstrated by higher densities of otters in rich rivers in Spain and by re-introduced Swedish otters focussing almost exclusively on freshwaters with orthophosphate levels above 0.02 mg/l (Sjöåsen 1997; Ruiz-Olmo 2001).
Recently translocated populations on resource-rich systems displayed far greater and more variable ranges than the native populations we studied, averaging 30 km and varying from 3–85 km (Saavedra 2002; Jefferies et al. 1986). Saavedra (2002) recorded a female occupying a 5-km home-range for several months around parturition, although she ranged over 85 km during the year following her release. The static territory was similar to those observed in this study and by Erlinge (1967). In an under-saturated system, there is less incentive to maintain a static territory, except when burdened with non-mobile young. In light of the results, and our interpretation of Erlinge’s (1967) results, data from unstable under-saturated systems are inappropriate for estimating otter spatial requirements.
Most previous studies of established otter populations were carried out on oligotrophic or complex systems. We observed higher otter densities in simple mesotrophic river systems. While for these systems, rivers supported greater female densities than streams, the length of their home-ranges became roughly constant as rivers widened beyond 15 m. For an autotrophic system this suggests that otters could not efficiently exploit the additional mid-stream habitat available.
An understanding of habitat-specific population density is often considered critical to conservation planning (EU Habitats Directive 1992). This understanding has proven elusive for otters owing in large part to difficulties associated with their capture or observation (Kruuk 2006). As a consequence, conservation planning has relied heavily on qualitative scientific advice. Estimates of otter populations have been attempted occasionally (Harris et al. 1995; Heggberget 1995; Prigioni et al. 2007) but are often based on dubious assumptions e.g. equating sprainting intensity with otter density (Kruuk 2006). The current study has improved this situation in two ways. Firstly, it has shown that adult female otters occupy exclusive home-ranges in lowland mesotrophic rivers in Northwestern Europe. This understanding of spatial structure facilitates the interpretation of the relatively large body of work for that region that has determined individual home-ranges without determining spatial patterns. Adult females appear to be the dominant unit in determining overall otter density, and as such are the most appropriate life-class for use in extrapolating habitat specific density data over large areas. The study has also determined otter spatial requirements in rich lowland rivers and increased the information available for relating otter density to habitat.
References
Aebischer NJ, Robertson PA, Kenward RA (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325 doi:10.2307/1940062
Bailey M, Rochford J (2006) Otter Survey of Ireland 2004/2005. National Parks and Wildlife Service, Irish Wildlife Manuals 23. Dublin, Ireland
Blundell GM, Maier JA, Debevec EM (2001) Linear home ranges; effects of smoothing, sample size, and autocorrelation on kernel estimates. Ecol Monogr 71:469–489
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammal 24:346–352 doi:10.2307/1374834
Chanin P (2003) Monitoring the otter. Conserving Natura 2000 Rivers Monitoring Series 10. English Nature, Peterborough
Chapman PJ, Chapman LL (1982) Otter survey of Ireland—Suirbhé dobharchú na hÉireann 1980–1981. Vincent Wildlife Trust, London
CITES (1979) Convention on International Trade in Endangered Species of Wild Flora and Fauna. <http://www.cites.or org/ >. Accessed 30 March 2007
Council of Europe (1979) Convention on the Conservation of European Wildlife and Natural Habitats. CETS 104. Bern, Switzerland
Demers A, Lucey J, McGarrigle ML, Reynolds JD (2005) The distribution of the white-clawed crayfish (Austropotamobius pallipes) in Ireland. Proc R Ir Soc 105B:65–69
Durbin L (1993) Food and habitat utilization of Otters (Lutra lutra L.) in a riparian habitat in the River Don in North-East Scotalnd. Dissertation, University of Aberdeen, Aberdeen, UK
Durbin LS (1996a) Some changes in the habitat use of a free-ranging female otter Lutra lutra during breeding. J Zool (Lond) 240:761–764
Durbin L (1996b) Individual differences in spatial utilisation of a river-system by otters (Lutra lutra). Acta Theriol (Warsz) 41:137–147
EPA (2006) Environmental Protection Agency of Ireland—water quality data. <http://www.epa.ie> Accessed 1 December 2006
Erlinge S (1967) Home range of the otter (Lutra lutra) in Southern Sweden. Oikos 18:186–209 doi:10.2307/3565098
Habitats Directive EU (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild flora and fauna. Off J L 206:7
Gorman TA, Erb JD, McMillan BR, Martin DJ (2006) Space use and sociality of river otters (Lontra canadensis) in Minnesota. J Mammal 87:740–747 doi:10.1644/05-MAMM-A-337R1.1
Green J, Green R, Jefferies DJ (1984) A radio-tracking survey of otters (Lutra lutra) on a Perthshire river system. Lutra 27:85–145
Harris S, Morris P, Wray P, Yalden D (1995) A review of British mammals: population estimates and conservation status of British mammals other than cetaceans. Joint Nature Conservancy Council, Peterborough
Heggberget TM (1995) Food resources and feeding ecology of marine feeding otters (Lutra lutra). In: Skjoldal HR, Hopkins C, Erikstad KE, Leinas HP (eds) Ecology of fjords and coastal waters. Elsevier Science, London, pp 609–618
IUCN (2006) International Union for the Conservation of Nature and natural resources: 2006 Red List of Threatened Species. <www.iucnredlist.org>. Accessed 30 March 2007
Jefferies DJ, Wayre P, Jessop RM, Mitchell-Jones AJ (1986) Reinforcing the native otter (Lutra lutra) population in East Anglia. Mammal Rev 16:65–79 doi:10.1111/j.1365-2907.1986.tb00023.x
Johnson DDP, MacDonald DW, Dickman AJ (2000) An analysis and review of models of the sociobiology of the Mustelidae. Mammal Rev 30:171–196 doi:10.1046/j.1365-2907.2000.00066.x
Kruuk H (2006) Otters—ecology, behaviour, and conservation. Oxford University Press, Oxford
Kruuk H, Moorhouse A (1991) The spatial organization of otters (Lutra lutra) in Shetland. J Zool (Lond) 224:41–57
Kruuk H, Carss DN, Conroy JW, Durbin L (1993) Otter (Lutra lutra) numbers and fish productivity in rivers in north-east Scotland. Symp Zool Soc Lond 65:171–191
Lunnon RM, Reynolds JD (1991) Distribution of the otter (Lutra lutra) in Ireland and its value as an indicator of habitat quality. In: Jeffrey DW, Madden B (eds) Bio-indicators and environmental management. Academic, London, pp 435–443
Macdonald DW (1983) The ecology of carnivore social behaviour. Nature 301:379–384 doi:10.1038/301379a0
Macdonald SM, Mason CF (1994) Status and conservation needs of the otter (Lutra lutra) in the western Palearctic. Nature Environment 67, Council of Europe, Strasbourg
Melquist WE, Hornocker MG (1983) Ecology of river otters in West Central Idaho. Wildl Monogr 83:1–60
Met Éireann-Irish Meteorological Service (2006) Thirty year climatic averages. http//www.meteireann.ie/. Accessed on 1 December 2006
Mitchell-Jones AJ, Jefferies DJ, Twelves J, Green J, Green R (1984) A practical system of tracking otters using radiotelemetry and Zn65. Lutra 27:71–74
Ó Néill L, de Jongh A, Ozoliņš J, de Jong T, Rochford J (2007) Minimising leghold trapping trauma for otters with mobile phone technology. J Wild Man 7:2776–2780
Ó Néill L, Wilson P, de Jongh A, de Jong T, Rochford J (2008) Field techniques for handling, anaesthetising and fitting radio-transmitters to Eurasian otters (Lutra lutra). Eur J Wildl Res 54(4):681–687 doi:10.1007/s10344-008-0196-5
Poledník L (2005) Otters (Lutra lutra) and fishponds in the Czech Republic: interactions and consequences. Dissertation, Palacky University Olomouc, Czech Republic
Powell RA (1979) Mustelid spacing patterns: variations on a theme by Mustela. Zeit Tier-psychol 50:153–165
Prigioni C, Balestrieri A, Remonti L (2007) Decline and recovery in otter Lutra lutra populations in Italy. Mammal Rev 37:71–79 doi:10.1111/j.1365-2907.2007.00105.x
Reynolds JD (1998) Ireland’s freshwaters. Marine Institute, Dublin
Ruiz-Olmo J (2001) Pla de conseració de la llúdriga a Catalunya: biologia i conservació. Documents dels Quaderns de Medi Ambient no. 6. Catalunya, Spain, pp 119–140 [In Catalan, summary and legends in English]
Saavedra D (2002) Reintroduction of the Eurasian otter (Lutra lutra) in Muga and Fluvià basins (north-eastern Spain). Dissertation, Departament de Ciències Ambientals, Universitat de Girona, Spain
Seaman DE, Powell RA (1996) An evaluation of the accuracy of kernel density estimators for home-range analysis. Ecology 77:2075–2085 doi:10.2307/2265701
Sjöåsen T (1997) Movements and establishment of reintroduced European otters (Lutra lutra). J Appl Ecol 34:1070–1080 doi:10.2307/2405295
Acknowledgements
We thank P. Wilson, Tj. de Jong, E. Ó Néill, D. Ó hÓgáin, L. Nesterko, and L. Pembroke for their help with the fieldwork. For helpful comments throughout the study and in the preparation of this manuscript, we thank H. Kruuk, R. Macdonald, K. Irvine, N. Marples, and L. Finnegan. Thanks also to F. Marnell, P. Stafford, and the staff and students of the Department of Zoology TCD for their support and advice throughout the study. This work was funded by the Irish Research Council for Science Engineering and Technology, the National Parks and Wildlife Service, and the Dutch Otterstation Foundation. The experiments presented in this paper comply with the Irish law.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Kierdorf
Rights and permissions
About this article
Cite this article
Ó Néill, L., Veldhuizen, T., de Jongh, A. et al. Ranging behaviour and socio-biology of Eurasian otters (Lutra lutra) on lowland mesotrophic river systems. Eur J Wildl Res 55, 363–370 (2009). https://doi.org/10.1007/s10344-009-0252-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-009-0252-9