Abstract
Conflicts between the conservation of large vertebrates and the use of biological resources are common and affect many species and activities. The vast majority of the Portuguese fish farms are located in estuarine protected areas and production of marine fish species is done mostly in semi-intensive regimes and with the imposition of some restrictive rules. This exposed form of production is prone to predation by wild animals. The Eurasian otter Lutra lutra is widely distributed in Portugal and this combination of factors has led to a conflict scenario between fish farming and the conservation of otters. This study aimed to determine the influence of landscape factors in otter damages in fish farms of Sado estuary (SW Portugal) and the usefulness of visiting rates and otter diet as surrogates for damage assessment. The impact of otter predation (damage) in each fish farm was assessed by combining the percentage of consumption of reared species with otter daily food consumption, daily visitation rates, and number of otters visiting each fish farm. Landscape descriptors were recorded in the field and several landscape metrics were calculated using a geographic information system. Multiple linear regressions were used to identify key landscape factors for damage and for damage surrogates. Distance to streams and refuge cover areas were identified as key landscape features to damages. This information can be used as a management tool in landscape planning, specifically in estuaries with fish-farming activity, as high-risk farms can be identified and selectively protected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conflicts between the conservation of large vertebrates and the use of biological resources are common and affect many species and activities. The use of fish by humans and animals has set the stage for conflict. Many large fish predators (e.g., seals, dolphins, otters, cormorants, and herons) compete with humans by eating species of interest to man (Silva et al. 2002; Carss 2003; Kranz et al. 2009; Brotons et al. 2008) including reared fish (Skarén 1990; Trindade 1991; Kloskowski 2000, 2005a; Lekuona 2002; Carss 2003; Opačak et al. 2004; Quick et al. 2004; Freitas et al. 2007; Kranz et al. 2009). Harbor seals Phoca vitulina and gray seals Halichoerus grypus were reported to predate on 81% of the marine salmon farms in Scotland (Quick et al. 2004) and the impact of cormorants Phalacrocorax carbo and gray herons Ardea cinerea was estimated to be 53% and 11%, respectively, of the annual fish-farming yield in the Arcachon Bay of southwest France (Lekuona 2002). Damage assessments are mostly based on diet results or stakeholder information (Kloskowski 2005a, b; Lekuona 2002; Quick et al. 2004; Opačak et al. 2004), but more complex approaches combining species ecology and numbers, landscape ecology, and stakeholders perception have also been pursued (Carss 2003; Kranz et al. 2009). For example, for cormorant damage, large-scale landscape approaches have identified the key factors involved. Cormorant conflicts on a pan-European scale showed similar distribution patterns: low altitudes (<500 m); wide (10–50 m) lower and middle river stretches; and access to shallow (<50 m deep) inshore waters in coastal areas. Overall, most conflict cases were reported in nutrient-rich (i.e., eutrophic) waters, particularly in freshwater aquaculture ponds, lakes, and coastal areas, supporting the idea that cormorant distribution is, in part at least, determined by the nutrient status of these waters (Carss 2003). Eurasian otters Lutra lutra and managed fisheries in Central Europe, mostly carp Cyprinus carpio production, is another example of a widely studied conflict, focusing on otter diet (Gossow and Kranz 1998; Kloskowski 2000, 2005b; Adámek et al. 2003; Lanszki and Molnár 2003; Jacobsen 2005; Poledník 2005), damage assessment (Bodner 1995a; Gossow and Kranz 1998; Kloskowski 2005b; Poledník 2005; Kranz et al. 2009), damage prevention (Bodner 1995b; Gossow and Kranz 1998; Leblanc 2003; Kranz et al. 2009), and compensation schemes (Gossow and Kranz 1998; Schwerdtner and Gruber 2007; Kranz et al. 2009). This type of biological and socio-economic information is critical for resource management, conflict resolution, and species conservation because it allows a wise application of mitigation, compensation, and species protection measures.
For a quarter of century, fish farming has been the world’s fastest-growing food production sector, sustaining an annual growth rate of 8.8% since 1970 (FAO 2007, http://www.fao.org/newsroom/en/news/2007/1000701/index.html). Aquaculture has also been financially encouraged by the European Union Structural Policy as a way to reduce pressure upon wild stocks. As a result, this economic activity grew significantly in Portugal in the early 1990s (INE 2007). The vast majority of Portuguese fish farms are located in estuarine areas and production is a mixture between traditional and modern methods. The rearing of marine fish species is done in extensive or semi-intensive regimes by recovering old salt production ponds and using the tidal cycles to circulate water in the tanks but it also includes pre-timed mechanical feeders and electric devices to keep water oxygen and temperature levels. Most estuaries where production occurs are included in the national network of protected areas due to their high conservation value (ICN 2004, http://www.icn.pt); in 2001, 56% of all marine fish farms in Portugal were located in the Tejo and Sado river estuaries (INE 2002), both classified as nature reserves. This has resulted in the imposition of some restrictions on fish-farming management (e.g., fencing of property is prohibited).
This exposed form of production is prone to predation by wild animals and so, in 1990, a questionnaire was sent by the Portuguese Institute for Nature Conservation (Trindade 1991) to fish farmers, with the intent of determining the role of predation on fish losses. Response rate was relatively low (29%) but revealed the emerging conflict with fish-eating predators. The Eurasian otter was the most commonly mentioned predator, causing damages all year-round. Some fish farmers perceived the species as a pest. However, producers could not quantify their losses and nearby farms reported contrasting damages. Similar results were obtained by Santos -Reis et al. (2007) who found that, overall, 40% of the marine and trout farms were visited by otters and that fish farmers used preventive methods, including dogs, net, and electric fencing but also illegal trapping, shooting, and poisoning. The Eurasian otter is widely distributed in Portugal and is listed in the Portuguese Red Data Book of Terrestrial Vertebrates in the “Least Concern” category (Cabral et al. 2005). Nevertheless, in Europe, the species is still a matter of concern and therefore considered “Near Threatened” (IUCN 2006).
This combination of factors: extensive fish production, estuaries as protected areas, production restrictive rules, otter nationwide distribution, the use of illegal predation preventive measures, and otter conservation status has led to a conflict scenario between fish farming and the conservation of otters. Furthermore, escalating pressures are expected in the future due to the continuous depletion of marine fish stocks and the desire to increase fish-farming production.
We conducted this study to determine the influence of landscape factors on otter visiting rates and fish losses at fish farms. We also assessed the usefulness of visiting rates and/or otter diet as a surrogate for damage assessment. We expected that key landscape features might be used as a management tool in landscape planning, specifically in estuaries with fish-farming activity, to identify and protect high-risk farms.
Materials and methods
Study area
The river Sado estuary was selected as study area. An important part of this estuary is used for fish farming, salt production, and agriculture, especially rice plantations. Fish farms in the Sado estuary are managed semi-intensively and primarily involve four marine fish species—gilthead sea bream Sparus aurata, European sea bass Dicentrarchus labrax, Senegal sole Solea senegalensis, and common sole Solea solea (INE 2002, 2007). Although this region has a strong urban and industrial influence, viz., Setúbal, a densely populated city with its associated industrial complex, a large part of the estuary (2.3 km2) is classified as a nature reserve (Sado Estuary Nature Reserve—38° 27’ 34.09’’ N, 8° 45’ 39.44’’ W). The area is highly valuable as a wintering, nesting, and feeding zone for migratory birds and is classified as a Special Protection Area (EC Birds Directive) and a Ramsar Site and included in the national list of Natura 2000 Sites, in part because of the presence of otters. The protection statute of the estuary imposes some restrictions on land use and management practices are enforced to preserve habitats and species (e.g., fencing and construction on wetlands is not allowed).
Otters are common in the estuary (Trindade et al. 1998; Sales-Luís et al. submitted manuscript) and produced fish species are part of their diet (Freitas et al. 2007). Damages are reported by local fish farmers as significant and form the basis of an escalating conflict (Freitas et al. 2007).
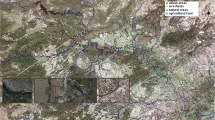
For this study, 14 fish farms were selected (Fig. 1), varying in size, number of ponds, and stocking levels (Table 1). Their distribution is clustered, a common pattern in estuaries and particularly in the study area.
Field data sampling
Surveys of each of the fish farms outside perimeter (enclosing all ponds) were conducted weekly from July 2003 to June 2004 to assess otter visiting rates to the fish farms and to collect spraints for otter diet analysis. Every sign of otter presence (e.g., spraints and footprints) was recorded for a posteriori mapping of marking points. In each survey, all spraints were collected and labeled and footprints were destroyed. Spraints were classified as fresh (from the previous night) or old (up to 1 week old). Old spraints can be determined by their dried surface and solid consistency, whereas fresh spraints from the previous day still have a wet and soft consistency (Mason and Macdonald 1987). Bank vegetation, bank structure, hinterland use, alternative (non-reared) prey availability (empirical assessment based on the hinterland—e.g., estuary vs dense marsh—and on the existence and prey-carrying capacity of close by freshwater sources), and existence of preventive measures (wire mesh or electric fences, dogs) were recorded in each fish farm for use as descriptive factors in our landscape analyses (Table 2).
Additionally, we conducted surveys in the 2004 winter to collect fresh spraints for molecular analyses. We surveyed the fish farm perimeters for eight consecutive days at sunrise to assure that sample collection was performed within 15 h of deposition. Spraints were stored in dimethyl sulfoxide (dimethyl sulfoxide 20%, NaCl 6 M) and then frozen at −20°C until DNA extraction. This molecular approach was used to assess the number of otters visiting each fish farm (Dallas et al. 2003; Arrendal et al. 2004; Selkoe and Toonen 2006).
Data analyses
Otter visiting rates
Weekly otter visiting rate (VR) were calculated for each fish farm (VR = number of positive surveys for otter presence / total number of surveys × 100). Daily otter visiting rates (VRMLE) were estimated using the spraint categories described earlier (fresh or old) and a maximum likelihood function estimate developed by Gruber et al. (2008) that gave us a measurement of the probability of otter visit per day.
Otter diet
Prey remains obtained from otter spraints were identified using a reference collection. Whenever possible, identification was made to species level and minimum number of individuals consumed was calculated through matching of paired diagnostic bony/skeletal structures. Size of prey consumed by otters was assessed using regression equations that allow estimating length and weight based on the dimensions of non-digested remains (for details, see Freitas et al. 2007).
Otter diet results were calculated as percentage of occurrence (PO = number of individuals of a particular prey item in all spraints / the sum of individuals of all prey items × 100) and as percentage of biomass, i.e., weight consumed (PB = biomass of a particular species in all spraints / the sum of biomass of all species × 100). Size of fish consumed at each fish farm in each season was compared to prey availability in the estuary (fish size and abundances sampled using a beam trawl, in sites close to fish farms) and in the fish farms (sampling inside the ponds with a drag net and fish farmers’ stocking information) to assess the origin of the prey captured by the otter (fish farm vs estuary) and to estimate otter predation (PO and PB of reared fish) in each fish farm (for details, see Freitas et al. 2007).
Number of otters
Fecal DNA extraction was done using the Qiagen extraction kit (QIAamp® DNA Stool Mini Kit; Bradley et al. 2000; Frantz et al. 2003). The presence of otter mitochondrial DNA (mtDNA) in fecal samples was verified by amplification of a Carnivora-specific cytochrome b fragment (Hansen and Jacobsen 1999) that was digested by the restriction enzyme BspLI. Only samples that successfully amplify mtDNA were used to amplify the microsatellite loci. Five microsatellites (Lut-701, Lut-733, Lut-782, Lut-832, and Lut-914—Dallas and Piertney 1998; Dallas et al. 1999) were used for individual typing. The primers were fluorescently labeled and polymerase chain reaction (PCR) products of microsatellite loci were genotyped on ABI 310 sequence analyzer (Applied Biosystems), with a fluorescently labeled size standard (Rox 350).
To reduce the errors of mistyping, each locus was amplified by PCR at least four times following the multiple-tubes approach (Taberlet et al. 1996; Gagneux et al. 1997).
Only genotypes that achieved 95% estimated probability of reliability (software RELIOTYPE—Miller et al. 2002) were accepted. As the same individual can be sampled more than once (several spraints from the same otter), software GIMLET (Valiére 2002) was used to group data and identify individual genotypes. The typing process was calibrated using a genetic profile of the species obtained from 20 individuals found dead (mostly road kills) in southern Portugal (Beja et al. 2004) and stored at the “biological samples databank” of the Centro de Biologia Ambiental—Carnivore Conservation Research Group (Lisbon). The rate of genotyping success was assessed by dividing the number of fecal samples yielding reliable genotypes by the number of samples yielding a successful DNA extraction (Prigioni et al. 2006).
Damage assessment
The impact of otter predation in each fish farm represents the sum of the losses of each of the three reared species (sole, sea bream, sea bass). Results are expressed in kilogram of lost produced fish (D_kg) and in economic loss (D_Euros). D_kg was assessed by multiplying: (1) the PB of each of the three produced species (sea bream PBSa; sea bass PBDl; and soles PBSsp); (2) the daily food consumption of an adult Iberian otter (0.75 kg/day—J. Ruiz-Olmo personal communication using captive individuals.); (3) VRMLE to each fish farm; (4) 365 days; and (5) the respective number of otters visiting the fish farm (Table 2). The economic loss was calculated by multiplying the D_kg of each fish species by its price on the local market (Table 2). An overall value, that is, the losses of the three produced species, was then summed for each fish farm.
Landscape descriptors
In addition to the descriptors recorded in the field (bank vegetation and structure, hinterland, preventive measures, alternative prey), several landscape metrics (distances, areas in buffers) were calculated using a geographic information system containing digitized military charts (1:25,000—IGEOE—Portuguese Military Institute), aerial photographs, global positioning system field information, and several constructed information layers, specifically a theme with all existing fish farms, freshwater pools, rivers, streams, brooks, and ditches (Table 2).
Statistical analyses
Spatial variation in otter visiting rates, consumption of reared species in terms of occurrences (CPsp_PO) and biomass (CPsp_PB), and damages in terms of fish (D_kg) and economic loss (D_Euros) were assessed using standard chi-squared tests (Zar 1999). The association between these variables was tested using Spearman correlation coefficients (Zar 1999).
VR, VRMLE, CPsp_PO, and CPsp_PB were considered potential surrogates for otter damage and used as dependent variables. Correlation results to these variables were used to select landscape descriptors for further analyses. Normality of all selected variables was tested using the Shapiro–Wilks statistic (w statistic; Zar 1999), and, when needed, variables were transformed to provide the best w statistic value. Selection among correlated explanatory variables to include in the regression analyses was done according to their relation to the dependent variable. Multiple linear regression (backwards stepwise; Sokal and Rohlf 1995) was used to identify key landscape factors for damage and for damage surrogates.
All statistical calculations were performed using SPSS 15 software.
Results
Otter visiting rates
Weekly otter visiting rates (Table 3) were very high, with half of the fish farms showing VR values above 0.8 (80% of the weekly surveys were positive for otter presence) and only two values below 0.5. The probability of daily visits by otters, although also relatively high, best represents the highly significant spatial variability (Table 3; VR—χ 2 = 84.0; p < 0.0001; VRMLE—χ 2 = 165.2.0; p < 0.0001).
Otter diet
Fish were the main component of otter diet at the studied fish farms, representing 87% of occurrences and 96% of consumed biomass. Although otters seem to concentrate on wild fish species (PO = 56%—freshwater and other marine fish species), 31% of occurrences corresponded to reared species (CPsp_PO) and the importance of produced fish species increased when biomass was considered (CPsp_PB = 61%), although a significant spatial variation was observed (CPsp_PO: χ 2 = 127.9; p < 0.0001; CPsp_PB: χ 2 = 125.15; p < 0.0001; for detailed results, see Freitas et al. 2007; Santos-Reis et al. 2009).
Otter abundance
Sampling undertook to specifically address the number of otters visiting the studied fish farms resulted in the collection of 72 fresh spraints, varying from one to 21 in each fish farm. At four fish farms (E3, P4, P10, P14), it was not possible to infer the number of otters visiting the fish farm because, due to access problems, daily surveys were not conducted. Otter DNA was extracted from 62 (86%) fecal samples and, of these, 49 (47%) were successfully typed for at least three loci. Fourteen different genotypes, corresponding to as many otters, were identified. The molecular analysis revealed that the minimum number of otters visiting each of the studied fish farms varied between one and seven individuals (Table 3).
Damage assessment
The yearly impact of otter predation (D_kg, D_Euros) was significantly different across fish farms (χ 2 > 1,500; p < 0.0001) and ranged from irrelevant (12 kg, 150€) to fairly high (434 kg, 5,775.52€; Table 4) with an average of 100 kg and 1,685.00€ per year. Additionally, some farms may have similar damages in weight consumed but very different economic losses (P2 and P6) or vice versa (P6 and P16) due to differences in the market prices of each produced species.
Key landscape features for damages
Because weekly and daily otter visiting rates and consumption of reared species in occurrences and biomass were highly correlated (Table 5), for the follow-up analyses, we first selected the response variables with higher correlation to the explanatory variables. These were VRMLE and CPsp_PB. Moreover, otter daily visiting rates best represent the variability between fish farms and consumption of reared species in biomass more realistically defines otter consumption in terms of size of fish (which in turn influences losses to fish farm; Freitas et al. 2007).
Contrary to the expectations, no clear relation was found between visiting rates (VRMLE) and the consumption of the stocked species (CPsp_PB; Table 5).
The landscape descriptors with the highest associations (r > 0.6; p < 0.03; α = 13) to CPsp_PB were: distance to running fresh waters [ditches (−); streams and brooks (+)]; distance to refuge cover areas (+); area of fish farms (+); area of villages (+); natural bank structure (+); and alternative prey (−) (Tables 6, 7, and 8). Nevertheless, the best model for key landscape features only included the positive influence of distance to the nearest refuge cover areas and the negative influence of the distance to the nearest ditch:
The landscape descriptors most associated to otter visiting rates (VRMLE) were semi-natural bank structure (+), alternative prey (+), area of refuge cover area in a 1-km buffer around the fish farm (+), and preventive measures (−), but none of these associations were significant (r > 0.4; p < 0.25; α = 13; Tables 6, 7, and 8). Additionally, preventive measures could not be normalized and so, although it is the variable with the highest negative correlation to VRMLE, it was excluded from the analyses. As a result, the best model for explaining otter visiting rates is significant but weak and includes the positive influence of nearby refuge cover areas:
Most probably because the formula for calculating “real” damages (see Methods -Damage assessment and Table 2) uses both VRMLE and CPspPB, the landscape descriptors significantly associated to the calculated damages (D_kg; D_€) were a mixture of the ones associated to those surrogate variables when considered independently: bank structure [natural (-), semi-natural (+) and artificial (-)]; preventive measures (-); distance to ditches (-); and width of the nearest stream (-)(Tables 6, 7, and 8). Both expressions of damage (kg and euros) revealed similar explanatory models, being the one using D_kg as dependent variable the strongest and including the positive influence of semi-natural bank structure and of nearby brooks and the negative influence of ditches:
Discussion
Estuaries are highly productive with high levels of biodiversity, including otters. Their conservation role has been recognized, benefiting, nowadays, often from the classification of protected areas. Additionally, these areas have attracted many important economic activities that generate significant income and employment (e.g., as salt producers). Currently, they have a high potential as semi-intensive fish-farming areas that may produce fish and reduce pressure over the depleted sea stocks. Safeguarding and managing these estuaries depend therefore on the ability to find solutions that reconcile both nature conservation and sustainable economic development. Fish farming, and specifically semi-intensive fish farming, may be reconcilable with conservation if some precautionary management measures are taken in terms of sustainable development of the farming activities.
The issue of predation on fish farms in the Sado estuary is bipolarizing. On the one hand, fish farmers are aware of the existence of otter predation and privately use whatever means they can to keep otters out (including illegal killing). On the other hand, managers of the Sado estuary nature reserve, aware of the predation as well as of the weakness of the monitoring system for the control of illegal actions, still forbid the use of any mitigation measures to prevent predation. The nature reserve has legal jurisdiction over licensing, land use change, and construction of fences and effluents, and hence conservation decisions can impact fish-farming activity in the Sado estuary. There are no instruments in place to ensure the attainment of the objectives of maintaining viable species population and sustainable economic activities (Freitas et al. 2007; Santos-Reis et al. 2009).
Damage assessment is a laborious task, depending on difficult-to-obtain ecological data (e.g., predator diet and average consumption per day, number of predators, and number of their visits to production site). In our approach, the impact of otter predation in each fish farm, in each year, was assessed by combining the percentage of consumption of reared species with otter daily food consumption, daily visiting rates, and number of otters visiting each fish farm. With this elaborate and complete approach, we wanted to identify not only the real damages but also the best surrogate measure so that the issuing of mitigation or compensation measures permits could be based on an effective and easily obtained measure of suffered damage or predation risk.
Produced fish species in the Sado estuary are undoubtedly an important otter food source and otters are feeding in the fish farms, as nearly 65% of the studied fish farms are being damaged in some way by otter predation; nevertheless, otters are also feeding in the estuary and in the surrounding freshwater bodies (Freitas et al. 2007). Visiting rates were quite high and the fact that there were significant spatial differences, both in the consumption of the reared species and otter visiting rates, enhanced the probability of existence of important explanatory landscape variables that were pinpointed by the regression models. Both dependent variables were influenced, although on different levels, by refuge cover areas, alternative prey, streams, ditches, and bank structure. Surprisingly, visiting rates did not reflect predation upon stocks and shared landscape descriptors frequently had opposite effects. A possible explanation to why visiting rates do not correlate with consumption of reared species is that otters may regularly visit fish farms, not to predate, but to scent mark. That is, some fish farms might be important territory sites, but not as feeding areas, and are therefore territorially marked but not heavily predated. This rationalization is supported by the results: fish farms located in areas with high alternative food availability and refuge (typically near streams), even when being highly visited by otters (VRMLE positively influenced by nearby refuge cover areas), had a diet less based on produced fish (CPsp_PB positively influenced by the distance to streams and refuge cover areas). This contrasting result revealed, and helped explain, key landscape factors to damages in general. Thus, damages are higher in fish farms further away from water lines of some importance (streams and brooks), especially if the fish farm is close to ditches that may act like “roads” for otters, but which alone do not have enough carrying capacity.
Moreover, the existence of preventive measures (fences, electric fences, dogs) influenced otter damages by reducing its impact. Mitigation methods, such as ordinary and electric fencing, are available and fairly efficient when correctly applied.
Estuaries are complex and productive systems and that usually makes any management task very difficult to keep simple. Being so, and although being the easiest to assess, otter visiting rates to fish farms were not correlated to the predation of reared fish species and, therefore, not a very accurate surrogate for damages (the fish farm with the lowest level of predation was the second most frequently visited by otters—P2). Nevertheless, it was highly correlated to the number of otters visiting each fish farm which also greatly influences damages. Even so, it seems necessary to look at otter diet as the best surrogate to otter damages.
Meanwhile, the perceived high damages (by the fish farmers) and the prohibition of use of most mitigation measures (by the nature reserve) contribute to enlarge species aversion and may lead to an increase in illegal killing. If fish farming is to continue to rise in importance in the future, as some stakeholders believe, impacts on the socio-economic context might start to gain importance leading to an urgent call for the development of measures to deal with this type of conflicts.
Scientific results proved that the impact of otters is not important at the study area level but varies from one fish farm to another and may be of significance at the farm level (Freitas et al. 2007; Santos-Reis et al. 2009). This suggests that different solutions should be recommended for each fish farm, such as to promote the use of mitigation measures in those farms where damages are high, no matter how they are perceived, and to invest in educational activities on those in which damages are not significant but may be perceived as such (Fig. 2). The design of a predation risk map of Sado estuary, if possible involving not only the otter but other fish-eating predators, would be informative to fish farmers and the nature reserve administration, helping to decide about the adequate mitigation measures or to give guidelines during site selection for the installation of new fish-farming facilities.
References
Adámek Z, Kortan D, Lepic P, Andreji J (2003) Impacts of otter (Lutra lutra) predation on fishponds: a study of fish remains at ponds in the Czech Republic. Aquacult Int 11:389–396. doi:10.1023/A:1025787330366
Arrendal J, Walker CW, Sundqvist AK, Hellborg L, Vilá C (2004) Genetic evaluation of an otter translocation program. Conserv Genet 5:79–88. doi:10.1023/B:COGE.0000014059.49606.dd
Beja P, Cancela L, Mira-Silva S, Cruz C (2004) Monitorização de lontras costeiras Lutra lutra no Parque Natural do Sudoeste Alentejano e Costa Vicentina. Relatório Final. Centro de Ciências do Mar da Universidade do Algarve & Transgás Atlântico, SA
Bodner M (1995a) Fish loss in Austrian fish-ponds as a result of otter (Lutra lutra L.) predation. IUCN Otter Specialist Group Bull 12:3–10
Bodner M (1995b) Otters and fish-farming: preliminary experiences of a WWF project in Austria. Hystrix 7(1–2):223–228
Bradley BJ, Boesch C, Vigilant (2000) Identification and redesign of human microsatellite markers for genotyping wild chimpanzee (Pan troglodytes verus) and gorilla (Gorilla gorilla gorilla) DNA from faeces. Conserv Genet 1:289–292. doi:10.1023/A:1011514007741
Brotons JM, Grau AM, Rendell L (2008) Estimating the impact of interactions between bottlenose dolphins and artisanal fisheries around the Balearic Islands. Mar Mamm Sci 24:112–127. doi:10.1111/j.1748-7692.2007.00164.x
Cabral MJ (coord.), Almeida J, Almeida PR, Dellinger T, Ferrand de Almeida N, Oliveira ME, Palmeirim JM, Queiroz AL, Rogado L, Santos-Reis M (eds.) (2005) Livro Vermelho dos Vertebrados de Portugal. Instituto da Conservação da Natureza, Lisboa
Carss DN (2003) Reducing the conflict between cormorants and fisheries on a pan-European scale (REDCAFE).Vol. 1, pan-European overview. Report of a Concerted Action of European Union. Centre for Ecology and Hydrology, Banchory
Dallas JF, Piertney SB (1998) Microsatellite primers for the Eurasian otter. Mol Ecol 7(9):1247–1263. doi:10.1046/j.1365-294x.1998.00406.x
Dallas JF, Bacon PJ, Carss DN, Conroy JWH, Green R, Jefferies DJ, Kruuk H, Marshall F, Piertney SB, Racey PA (1999) Genetic diversity in the Eurasian otter, Lutra lutra, in Scotland. Evidence from microsatellite polymorphism. Biol J Linn Soc Lond 68:73–86. doi:10.1111/j.1095-8312.1999.tb01159.x
Dallas JF, Coxon KE, Sykes T, Chanin PRF, Marshall F, Carss DN, Bacon PJ, Piertney SB, Racey PA (2003) Similar estimates of population genetic composition and sex ratio derived from carcasses and faeces of Eurasian otter Lutra lutra. Mol Ecol 12:275–282. doi:10.1046/j.1365-294X.2003.01712.x
Frantz AC, Pope LC, Carpenter PJ, Roper TJ, Wilson GJ, Delahay RJ, Burke T (2003) Reliable microsatellite genotyping of the Eurasian badger (Meles meles) using faecal DNA. Mol Ecol 12:1649–1661. doi:10.1046/j.1365-294X.2003.01848.x
Freitas D, Gomes J, Sales-Luís T, Madruga L, Marques C, Baptista G, Rosalino LM, Antunes P, Santos R, Santos-Reis M (2007) Otters and fish farms in the Sado estuary: ecological and socio-economic basis of a conflict. Hydrobiologia 587:51–62. doi:10.1007/s10750-007-0693-7
Gagneux P, Boesch C, Woodruff DS (1997) Microsatellite scoring errors associated with non-invasive genotyping based on nuclear DNA amplified from shed hair. Mol Ecol 6(9):861–868. doi:10.1111/j.1365-294X.1997.tb00140.x
Gossow H, Kranz A (eds) (1998) Otters and fish farms. Boku—Reports on Wildlife Research and Game Management, 14. Institute of Wildlife Biology and Game Management, Vein
Gruber B, Reineking B, Calabrese JM, Kranz A, Poledníková K, Poledník L, Klenke R, Valentin A, Henle K (2008) A new method for estimating visitation rates of cryptic animals via repeated surveys of indirect signs. J Appl Ecol 45(2):728–735. doi:10.1111/j.1365-2664.2007.01406.x
Hansen MM, Jacobsen L (1999) Identification of mustelid species: otter (Lutra lutra), American mink (Mustela vison) and polecat (Mustela putorius), by analysis of DNA from faecal samples. J Zool (Lond) 247(2):177–181. doi:10.1017/S0952836999002058
INE (2002) Statistics on fisheries 2001. Instituto Nacional de Estatística, Lisboa http://www.ine.pt
INE (2007) Statistics on fisheries 2006. Instituto Nacional de Estatística e Direcção Geral das Pescas e Aquicultura, Lisboa http://www.ine.pt
IUCN (2006) 2006 IUCN red list of threatened species. http://www.iucnredlist.org
Jacobsen L (2005) Otter (Lutra lutra) predation on stocked brown trout (Salmo trutta) in two Danish lowland rivers. Ecol Freshwat Fish 14:59–68. doi:10.1111/j.1600-0633.2004.00076.x
Kloskowski J (2000) Selective predation by otters Lutra lutra on common carp Cyprinus carpio at farmed fisheries. Mammalia 64(3):287–294. doi:10.1111/j.1365-3083.2006.01813.x
Kloskowski J (2005a) Otter Lutra lutra damage at farmed fisheries in southeastern Poland, I: an interview survey. Wildl Biol 11(3):201–206. doi:10.2981/0909-6396(2005)11[201:OLLDAF]2.0.CO;2
Kloskowski J (2005b) Otter Lutra lutra damage at farmed fisheries in southeastern Poland, II: exploitation of common carp Cyprinus carpio. Wildl Biol 11(3):257–261. doi:10.2981/0909-6396(2005)11[257:OLLDAF]2.0.CO;2
Kranz A, Jepsen N, Klenke R, Ring I, Rauschmayer F, Henle K (eds) (2009) In: Human wildlife conflicts in Europe—fisheries and fish-eating vertebrates as a model case. Springer, Berlin
Lanszki J, Molnár T (2003) Diet of otters living in three different habitats in Hungary. Folia Zool (Brno) 52(4):378–388
Leblanc F (2003) Protecting fish farms from predation by Eurasian otter (Lutra lutra) in the Limousin region of central France: first results. IUCN Otter Specialist Group Bull 20(1):45–48
Lekuona JM (2002) Food intake, feeding behaviour and stock losses of cormorants, Phalacrocorax carbo, and grey herons, Ardea cinerea, at a fish farm in Arcachon Bay (Southwest France) during breeding and non-breeding season. Folia Zool (Brno) 51(1):23–34
Mason CF, Macdonald SM (1987) The use of spraints for surveying otter Lutra lutra populations: an evaluation. Biol Conserv 41:167–177. doi:10.1016/0006-3207(87)90100-5
Miller CR, Joyce P, Waits LP (2002) Assessing allelic dropout and genotype reliability using maximum likelihood. Genetics 160:357–366
Opačak A, Florijančić T, Horvat D, Ozimec S, Bodakoš D (2004) Diet spectrum of great cormorants (Phalacrocorax carbo sinensis L.) at the Donji Miholjac carp fishponds in eastern Croatia. Eur J Wildl Res 50:173–178. doi:10.1007/s10344-004-0059-7
Poledník L (2005) Otters (Lutra lutra L.) and fishponds in the Czech Republic: interactions and consequences. Ph.D. thesis, Department of zoology and anthropology, Faculty of Science, Palacky University Olomouc, Czech Republic
Prigioni C, Remonti L, Balestrieri A, Sgrosso S, Priore G, Mucci N, Randi E (2006) Estimation of European otter (Lutra lutra) population size by fecal DNA typing in Southern Italy. J Mammal 87(5):855–858. doi:10.1644/05-MAMM-A-294R1.1
Quick NJ, Middlemas SJ, Armstrong JD (2004) A survey of antipredator controls at marine salmon farms in Scotland. Aquaculture 230:169–180. doi:10.1016/S0044-8486(03)00428-9
Santos-Reis M, Freitas D, Sales-Luís T (2007) Man-otter conflict in Portuguese fisheries: introducing a comprehensive approach. In: Conroy JWH, Gutleb J, Ruiz-Olmo J, Yoxon GM (eds) Proceedings of the European Otter Survival Fund. Journal of the International Otter Survival Fund No 2
Santos-Reis M, Santos R, Antunes P, Sales-Luís T, Gomes J, Freitas D, Madruga L (2009) Reconciliation of the conflict between otters and fish farmers: lessons learned from Sado Estuary in Portugal. In: Kranz A, Jepsen N, Klenke R, Ring I, Rauschmayer F, Henle K (eds) Human wildlife conflicts in Europe—fisheries and fish-eating vertebrates as a model case. Springer, Berlin
Schwerdtner K, Gruber B (2007) A conceptual framework for damage compensation schemes. Biol Conserv 134(3):354–360. doi:10.1016/j.biocon.2006.08.010
Selkoe KA, Toonen RJ (2006) Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett 9:615–629. doi:10.1111/j.1461-0248.2006.00889.x
Silva MA, Feio R, Prieto R, Goncalves JM, Santos RS (2002) Interactions between cetaceans and the tuna fishery in the Azores. Mar Mamm Sci 18(4):893–901. doi:10.1111/j.1748-7692.2002.tb01080.x
Skarén U (1990) Fish farming and otters in Finland. IUCN Otter Specialist Group Bull 5:28–34
Sokal R, Rohlf F (1995) Biometry. 3rd edn. Freeman, New York
Taberlet P, Sally G, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24(16):3189–3194. doi:10.1093/nar/24.16.3189
Trindade A (1991) Fish farming and otters in Portugal. IUCN Otter Specialist Group Bull 6:7–9
Trindade A, Farinha N, Florêncio E (1998). A distribuição da lontra Lutra lutra em Portugal: situação em 1995. Estudos de Biologia e Conservação da Natureza do Instituto para a Conservação da Natureza, 28. Instituto para a Conservação da Natureza, Lisboa
Valiére N (2002) GIMLET: a computer program for analysing genetic individual identification data. Mol Ecol 2(3):377–379. doi:10.1046/j.1471-8278.2002.00228.x
Zar J (1999) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Acknowledgements
This study was conducted with the financial support of the European Union (contract number EVK2-CT-2002-00142-FRAP) and the Fundação para a Ciência e a Tecnologia (FCT, SFRH/BD/5163/2001).
The molecular spraint analysis was done by Dr. Sara Mira at the Centro de Ciências do Mar, Universidade do Algarve.
The authors wish to thank the socio-economic FRAP project partners from the Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa and the colleagues who helped in the fieldwork: Carla Marques, Luís Miguel Rosalino, Hugo Matos, Ana Rita Alves, Joana Caixinha, and Gonçalo Trindade.
The authors are grateful to Dr John A Bissonette and Dr. Reinhard Klenke for the insightful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Lutz
This study was conducted with the financial support of the European Union (contract number EVK2-CT-2002-00142-FRAP) and the Fundação para a Ciência e a Tecnologia (grant number SFRH/BD/5163/2001).
Rights and permissions
About this article
Cite this article
Sales-Luís, T., Freitas, D. & Santos-Reis, M. Key landscape factors for Eurasian otter Lutra lutra visiting rates and fish loss in estuarine fish farms. Eur J Wildl Res 55, 345–355 (2009). https://doi.org/10.1007/s10344-009-0250-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-009-0250-y