Abstract
Models of herbivore diversification rely heavily on adaptations that reflect the nutritional quality of foods consumed. In particular, browsers and grazers are expected to show dichotomous adaptations to deal with high quality (concentrate) browse-based and poor quality grass-based diets, respectively. In this study, we test the widespread assumption that browse represents a higher quality food source than grass. We analyzed plants from a South African savanna, collected over one dry and one wet season across several habitat types, for percent nitrogen (%N), neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) to compare variations in nutritional value of different food types. Results show consistently higher %N and lower NDF and ADF of tree foliage and forbs compared to monocots, but the former have consistently higher ADL, implying a higher fiber digestibility in grass compared with browse. Some fruit species have a high NDF and ADL content, implying poorer nutritional value than is commonly assumed. Our findings are in agreement with several other studies depicting relatively poor digestibility of browse (tree foliage and fruit) compared to grass. Reference to browse as high quality foods is therefore misleading, and models of herbivory that rest on this assumption require revision. The more efficient fiber digestibility recorded in grazers compared to browsers cannot be treated as an adaptation to poor quality diets, but rather to maximize benefits of higher fiber digestibility of grass. Spatio-seasonal variations in plant nutritional seem to reflect seasonal and spatial diet changes expected for grazers and intermediate (mixed) feeders. We propose that future studies require further detail on variations in diet, diet quality, and digestive efficiency to properly understand mechanisms of adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant nutritional composition provides the basis for most models of herbivore performance, niche compartmentalization, and evolutionary adaptation. Among ungulates, this refers principally to differences in cell soluble/cell wall ratios (often expressed as protein/fiber ratios) between browse (dicots) and grass. Oral and dental morphology, gut anatomy, digestive physiology, body size, and social behavior have all been related to intake and processing of these two food groups (Hofmann and Stewart 1972; Jarman 1974; Demment and Van Soest 1985; McNaughton and Georgiadis 1986; Hofmann 1989; Owen-Smith 1982, 1997; Van Wieren 1996; Clauss and Lechner-Doll 2001; du Toit 2003; Perez-Barberia et al. 2004). However, many assumptions of plant food quality do not focus on the different components of plant cell walls, which more correctly determine digestibility and diet quality than do overall fiber concentrations.
While polysaccharides such as cellulose, hemicellulose, and pectin are at least largely digestible by microbial fermentation, the lignin in plant cell walls is assumed to be indigestible, hence, plant fiber digestibility is expected to decrease with increased lignification rather than with total cell wall concentration (see Robbins 1993; Van Soest 1994; Meissner et al. 1999). It is often assumed, however, that the higher protein/fiber ratios of browse compared to grass imply a higher nutritive value in the former (e.g., Jarman 1974; Demment and Van Soest 1985; Hofmann 1989; Gagnon and Chew 2000). Indeed, in his landmark description of anatomical and presumed physiological characteristics of ruminants, Hofmann (1989) classified browsers as “concentrate selectors” and grazers as “bulk/roughage feeders.” Several studies, however, point to browse as a poorer quality resource because the higher lignin content of dicotyledonous plant leaves means that dry matter digestibility (DMD) of these plants is lower than that of grasses (Gordon and Illius 1994; Van Wieren 1996; Illius 1997; Owen-Smith 1997; Meissner et al. 1999; Hummel et al. 2006). The same can be said for fruits, which are commonly regarded as high quality foods, but these may in fact be poorly digestible, comprising relatively large concentrations of fiber (Demment and Van Soest 1985; Molloy and Hart 2002; Shipley and Felicetti 2002).
The complexity of plant biochemical properties as a determinant of food quality for herbivores may also be amplified by seasonal and regional changes in cell soluble and cell wall composition, reflecting changes in factors including rainfall, temperature, and geology (Minson 1990; Ellery et al. 1995; Meissner et al. 1999). Very few studies, however, have focused on regional and seasonal differences among a broad range of plant food groups, hence, spatiotemporal comparisons are usually restricted to collation of data from a variety of literature sources representing diverse time scales (see Meissner et al. 1999). A standard method to analyze plant cell walls that can be applied across a variety of environments is detergent analysis (Van Soest and Wine 1967). This method yields the neutral detergent fiber (NDF; hemicellulose, cellulose, and lignin), acid detergent fiber (ADF; cellulose and lignin), and acid detergent lignin (ADL) fractions of plant cell walls. Thus, while incomplete in that certain components are not detected, detergent analysis provides a useful measure to compare fiber digestibility across different plant types (Demment and Van Soest 1985; Robbins 1993; Van Soest 1994).
Although a number of studies have reviewed differences in plant cell soluble and cell wall composition, oversimplified concepts of plant food quality are still common in the literature. In this paper, we test the validity of the assumption that browse is a higher quality food source than grass, using percent nitrogen (%N, a reflection of crude protein content), NDF, ADF, and ADL of plants from South African semiarid savannas. We present data for tree foliage, forbs, fruit, grass, and sedges, facilitating comparison across a range of food groups. Our dataset includes plants collected from a variety of landscapes in the Kruger National Park and Hans Merensky Nature Reserve, over one dry and one wet season, to determine whether observed trends persist across multiple taxonomic habitat and seasonal scales.
Materials and methods
Study area
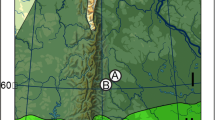
Kruger National Park is a large (∼2,000,000 ha) semiarid savanna situated in the northeast of South Africa between the latitudes 22°20′ and 25°32′ S and the longitudes 30°53′ and 32°02′ E (Fig. 1). The park forms part of the “lowveld” savanna, lying at ∼300 m above sea level on average, although this varies from 900 m in the southwest to 200 m in the east. The spatial mosaic of landscapes in Kruger Park represent a wide range of contrasting habitats differing in available resources. The physical characteristics of the park’s various ecotypes have been studied well and are reviewed in Venter et al. (2003). The geological succession and associated vegetational landscapes can be simplistically reduced along a longitudinal gradient, with relatively nutrient-poor granites associated with woodland savannas in the west while nutrient-rich basalts give rise to open grasslands in the east (Venter et al. 2003; Fig. 1). There is a marked difference in the vegetation of the northern regions compared to the south; the region to the north of the centrally located Olifants River is dominated by broad-leaved Colophospermum mopane savanna, while the south is dominated by fine-leaved Acacia and Combretum savanna (Venter et al. 2003). Based on these geological and vegetational differences, the landscapes of Kruger Park can be broadly categorized as northern and southern granites and basalts, respectively. The densely wooded, partially mountainous region of Punda Maria in the far northern sector, underlain by sandveld, is treated here as a separate habitat type. Hans Merensky Nature Reserve is a reserve of ∼5,000 ha situated ∼100 km to the west of Kruger Park in the Tzaneen District of the Limpopo Province (23°39′ S, 30°40′ E). Vegetation is predominantly C. mopane woodland savanna on a relatively flat granitic topography (428 to 542 m above sea level).
Simplified geological map of Kruger National Park and outline of Hans Merensky Nature Reserve, showing the longitudinal granite/basalt division of Kruger, and location and number allocated to vegetation sampling sites (white circles) with visual descriptions of site type in parentheses. KP Kruger Park, NBP Northern Basalt Plains, PM Punda Maria, HM Hans Merensky
The Kruger Park and Hans Merensky form part of the temperate summer rainfall region of South Africa. Rainfall is highly seasonal, falling mainly during the austral summer between October and March. Mean annual rainfall varies between 500 and 700 mm in the southern regions of Kruger Park (and on Hans Merensky) to between 300 and 500 mm in the northern Kruger Park (Venter et al. 2003). Rainfall during the study period (June 2004 to May 2005) was more than 200 mm lower than the long-term annual mean for the northern Kruger Park and Hans Merensky (Shingwedzi: 229 mm, Punda Maria: 268 mm, and Hans Merensky: 272 mm), slightly lower than the mean on southern basalts (Lower Sabie: 412 mm) and fairly high on southern granites (Skukuza: 780 mm). Daily maximum temperatures vary from 30 to 35°C in the summer and from 25 to 30°C in winter, although temperatures in the southern regions of Kruger Park and at Hans Merensky are slightly lower than that of northern Kruger.
Materials
The study was carried out from June 2004 to May 2005, although the majority of vegetation samples were collected in July 2004 (representing the dry season) and January 2005 (wet season). Circular sampling transects, ∼10 m in diameter, were established on northern and southern granites and basalts, respectively, at Punda Maria and on Hans Merensky. Sampling sites were chosen by visual discretion to represent the dominant vegetation composition for each landscape, as described in Venter et al. (2003) (see Fig. 1). The transect on southern basalts (site KP1) represents Acacia nigrescens savanna on open grassland, on southern granites (KP4) vegetation is Combretum woodland, and on northern granites (KP7) C. mopane woodland on sandy soil with sparse grass cover. Three sampling sites were established within each of the other landscapes, i.e., northern basalts (NBP), Punda Maria (PM), and Hans Merensky (HM), so that comparisons could be made across subtler habitat scales. NBP1 and NBP3 represent the dominant open grassland habitat of the NBP landscape, with NBP3 situated within 50 m of an artificial waterhole. NBP2 is a more closed, wooded site on the landscape. The undulating topography at PM allowed us to sample at an open woodland site with thick grass cover (PM2), in dense woodland on one of the mountain slopes (PM2), and within a very dense, closed canopy woodland (PM3). On Hans Merensky, we established an open grassland site (HM1), a woodland site dominated by C. mopane and Grewia spp. (HM2), and in a dense riparian zone along one of the streams that are tributary to the Letaba River to the north of the reserve (HM3).
Samples of tree foliage and grass (leaves and stems) were collected to represent the most prominent species occurring within each transect. Three to five specimens of each species were obtained per site, and pooled for analysis. Other samples collected for comparison included forbs, fruit, and sedges, which were obtained at random localities within the study areas when available. All forbs collected were small woody shrublets. Fruits represented both pods and fleshy drupes; in the case of drupes bearing large kernels, such as Strychnos madagascariensis and Sclerocarya birrea, only the fleshy parts were retained for analysis.
Analytical techniques
Plant samples were oven-dried at 60°C for 24 h, and mill-ground into a homogenous powder through a 1-mm sieve. Percent N was determined by combustion in an automated Elemental Analyzer (Carlo Erba, Milan, Italy), and measurement of the resultant N2 gas on a Finnigan MAT 252 or DELTA XP Mass Spectrometer via a continuous flow-through inlet system (Conflo). Cell wall constituents were determined by exposure to neutral detergent and acid detergent solutions in an ANKOM 220 Fiber Analyzer for 75 and 60 min to yield NDF and ADF fractions, respectively. Lignin (ADL) was determined by exposure of the ADF fraction to 72% (by weight) H2SO4 for 4 h; NDF, ADF, and ADL fractions are presented in terms of dry matter (DM), correcting for ash residue after furnace combustion at 525°C.
One of the main difficulties with interpreting these data arises in assessing the interactions between cell wall components to estimate potential fiber digestibility. Ideally, testing differential digestibilities requires in vivo measurements, but controlled feeding experiments with the variety of diets used in this study, representing similar spatiotemporal resolutions, are clearly impractical, especially for the diversity of ungulates found across the savanna. Indeed, the constraint common to all studies of ungulate nutritional physiology is the small number of taxa for which reliable data are available (see Clauss et al. 2006). Many previous approaches have relied on ADF as a proxy for digestibility, but this fraction is in fact weakly correlated with DMD, explaining only between 1 and 20% of the variation therein (e.g., Moore and Coleman 2001). Lignin content is more consistently correlated with overall digestibility of fiber (Jung and Deetz 1993; Van Soest 1994). Jung et al. (1997) showed that ADL concentration explained between ∼50 and 87% of the variation in DMD and NDF digestibility of legumes and grass. Thus, approximates of fiber digestibility based on ADL/NDF ratios provide a better method for diet quality, the higher this ratio the lower the cell wall digestibility (CWD) of the food (e.g., Perez-Barberia et al. 2004). To make direct comparisons, i.e., on a similar scale, between CWD from ADL/NDF ratios with other approaches (see below), we used the following equation:
While ADL/NDF ratios likely express the relative forage value of a plant, these do not provide a measure for what actually takes place within the digestive tract. Mould and Robbins (1982) developed regression models for predicting DMD from NDF and ADL based on digestion trials of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) fed a variety of browse and grass food types. They proposed that DMD can be predicted from:
where NDS represents neutral detergent solubles (100−%NDF) and A lignin + cutin (A is represented by ADL). We compare results of Eq. 1 with those based on Mould and Robbins’ (1982) models to guide interpretations of fiber digestibility.
Comparisons between dependant variables for tree foliage, forbs, and grass were carried out using one-way analysis of variance and Tukey’s honest significant difference (HSD) post hoc test for significant differences (P level 0.05). For comparisons with other food groups represented by smaller sample sizes (fruits and sedges), we used the nonparametric Mann–Whitney U test. Regional and seasonal differences in nutritional content of tree foliage and grass were compared between dry (April to September, principally June) and wet (October to March, principally January) seasons and across the six major landscapes included in this study (southern and northern granites and basalts, Punda Maria, and Hans Merensky). For these comparisons, we used Tukey’s HSD to test for significant seasonal and landscape differences within and between these food groups.
Results
Percent N, NDF, ADF, and ADL varied widely between different tree and grass species (Table 1). Percent N of tree foliage varied from 1.4% in Euclea divinorum to 3.1% in Philonoptera violacea. Grass %N ranged from 0.6% in Sporobolus africanus to 1.3% in Bothriochloa sp. and Panicum maximum. Fibrous components of tree foliage ranged in NDF from 21.3% (Vitex sp.) to 59.4% (P. violacea), ADF from 13.2% (Vitex sp.) to 40.6% (P. violacea), and ADL from 5.7% (Combretum apiculatum) to 19.7% (P. violacea). Grass ranges were narrower, NDF varying from 60.7% (Bothriochloa spp.) to 78.5% (S. africanus), ADF from 29.4% (Bothriochloa spp.) to 39.0% (S. africanus), and ADL from 3.3% (Panicum coloratum) to 5.3% (Bothriochloa sp.). Despite the variation across species, there were large differences in nutritional content between tree foliage and grass; tree foliage maintained higher N and ADL content and lower NDF and ADF than grasses (P < 0.0001 for all variables).
Percent N of forbs was similar to that of tree foliage (P = 0.40), but forbs had higher NDF (P < 0.0001) and ADF (P < 0.0001) and lower ADL (P < 0.05) (Table 1). However, there appeared to be a seasonal effect; mean N content of forbs shifted from 1.3 ± 0.3% (n = 14) in the dry season to 2.5 ± 0.7% (n = 17) in the wet, and consequently forbs had lower %N than tree foliage during the dry season (P < 0.0001). It is interesting to note that forb ADF was not significantly different to that of grass (P = 0.16), but forbs had higher %N (P < 0.0001) and ADL (P < 0.0001) and lower NDF (P < 0.0001). Fruit had similar %N compared with leaves (Mann–Whitney P = 0.24), but higher mean NDF (P < 0.01) and ADF (P < 0.001). Within fruits, no differences were observed between values for species enclosed in pods compared with fleshy drupes (P = 0.67 for %N, 0.46 for NDF, 0.29 for ADF, and 0.25 for ADL), and the range for each group overlapped considerably. Sedge %N and ADF values were similar to those for grasses (P = 0.98 and 0.14, respectively), but sedges had lower NDF and ADL (P < 0.05 and 0.01, respectively).
Calibrations of fiber digestibility
As a result of differences in NDF and ADL content of tree foliage, forbs, and grass, these three food groups differed significantly in estimated potential CWD (Eq. 1) (P < 0.0001; Fig. 2). The highest CWD was calculated for grass and sedges due to their lower ADL and higher NDF content, followed by forbs, while tree foliage had the lowest CWD among these plant groups. DMD based on regression models of Mould and Robbins (1982) of tree foliage and forbs appear to be similar (P = 0.94 and 0.85 for Eqs. 2 and 3, respectively), but DMD of both remain significantly lower than that of grass (P < 0.0001 for both models).
Estimated fiber digestibilities of different plant food types, comparing results from three calibrations to calculate digestibility; the CWD index is determined from proportions of ADL to NDF (Eq. 1), DMD is based on published regressions obtained from in vivo digestibility trials using elk and white-tailed deer (NDF, ADL, and NDS, Eqs. 2 and 3; from Mould and Robbins 1982)
Between tree foliage and fruit, the CWD index (Eq. 1) suggests higher fiber digestibility of fruit (P < 0.01), but DMD calculated from the cervid regressions was similar for both food types (P = 0.10 and 0.09 for Eqs. 2 and 3, respectively). Fiber digestibility of grass and sedges show no differences using Eq. 1 (P = 0.17), but results of Eqs. 2 and 3 suggest higher DMD in sedges (P < 0.01 in both cases).
Species differences in fiber digestibility among tree foliage, fruit, and grasses are plotted in Fig. 3 as CWD (Eq. 1) vs DMD from deer regressions of Mould and Robbins (1982) (Eq. 3). The graph shows that higher CWD of grass compared to the browse food groups is consistent for all species (>90%), and is in fact higher than the maximum derived for any tree species (for leaves C. apiculatum = 81.6%, for fruit S. birrea subsp. caffra = 89.0%). These results also suggest substantially more variation among tree foliage than grass; whereas CWD for all grass species was >90%, far lower CWD was estimated for several tree species (minimum for leaves was 58.2% in Dichrostachys cinerea and 59.1% in E. divinorum). Similarly, DMD for grasses ranged from only 72.8% in Aristida spp. to 83.6% in P. coloratum, whereas for tree foliage values varied from 40.4% in P. violacea to 82.6% in Vitex sp. Estimated fiber digestibility of fruits showed no overall difference between fleshy drupes and pods (Fig. 2; P = 0.67, 0.29, and 0.34 for Eqs. 1, 2, and 3, respectively), as was the case for %N and fiber data in general (see Table 1). However, interspecific variation among fruits was even larger than that observed for tree foliage; minimum CWD of fruits was 50.2% in Rhoicissus tomentosa, and DMD of Afzelia quanzensis was only 10.1% owing to the high NDF (85.2%) and ADL (23.9%) content of pods of this species.
Species differences in fiber digestibility based on calibrations of cell wall (CWD, Eq. 1) and DMD (as determined from regressions based on white-tailed deer, Eq. 3; Mould and Robbins 1982) among browse (tree foliage and fruit) and grass. Aq Afzelia quanzensis, Ari Aristida spp., Bg Bauhinia galpini, Bs Bolusanthus speciosus, Bot Bothriochloa spp., Ca Combretum apiculatum, Cc Cenchrus ciliaris, Ch Combretum hereroense, Ci Combretum imberbe, Cm Colophospermum mopane, Dc Dichrostachys cinerea, De Digitaria eriantha, Dm Diospyros mespiliformis, Ec Eragrostis curvula, Era Eragrostis spp., Ed Euclea divinorum, Fi Ficus sp., Gr Grewia spp., Op Ozoroa paniculosa, Pa Peltophorum africanum, Pc Panicum coloratum, Pm Panicum maximum, Pv Philonoptera violacea, Rt Rhoicissus tomentosa, Sa Sporobolus africanus, Sb Sclerocarya birrea, Sm Strychnos madagascariensis, Sol Solanum sp., Tt Themeda triandra, Um Urochloa mosambicensis, Vit Vitex sp
Seasonal and regional changes
Differences in nutritional content of tree foliage and grasses are displayed in Fig. 4. The graph shows that higher %N and ADL, and lower NDF and ADF, of tree foliage compared with grass was maintained across all regional and seasonal scales (P < 0.0001). However, both these food groups showed significant seasonal changes in nutritional content. NDF, ADF, and ADL of tree leaves increased from the dry (means ± 1 SD = 35.9 ± 7.5%, 24.2 ± 4.8%, and 10.5 ± 3.6%, respectively, n = 35) to the wet season (40.3 ± 10.0%, 28.3 ± 7.2%, and 13.6 ± 6.2%, respectively, n = 35; P < 0.05), although %N of leaves remained relatively unchanged (2.1 ± 0.5% in the dry and 2.2 ± 0.5% in the wet season; P = 0.38). Grasses, by contrast, increased in %N from the dry to the wet season (0.9 ± 0.3%, n = 38 and 1.2 ± 0.5, n = 33, respectively; P < 0.001), but decreased in NDF (71.9 ± 3.8% in the dry and 63.9 ± 7.6% in the wet season) and ADF (38.3 ± 2.9% in the dry and 34.0 ± 4.5% in the wet season) over this time (P < 0.0001). ADL of grass was similar in both seasons (5.1 ± 1.3% and 4.7 ± 2.7%, respectively; P = 0.44). In terms of fiber digestibility, CWD (Eq. 1) remained higher for grass than tree foliage during both seasons (P < 0.0001). However, based on DMD calibrated from regressions for elk and deer (Eqs. 2 and 3), fiber digestibility of tree foliage decreased significantly from the dry to the wet season (P < 0.05 in both instances) while grasses did not change (P = 0.68 and 0.61, respectively). Consequently, the high DMD inferred for grass (Fig. 2) compared to tree foliage only persisted in the wet season (P < 0.0001), but not in the dry (P = 0.29 and 0.76 for results of Eqs. 2 and 3, respectively).
Seasonal (dry vs wet) and regional differences in %N (presented ×101 for scale), NDF, ADF, and ADL of tree foliage and grass. Open circles represent variations in the CWD index (Eq. 1), and filled circles DMD based on regressions for white-tailed deer (Mould and Robbins 1982; Eq. 3). PM Punda Maria, NB northern basalts, NG northern granites, SB southern basalts, SG southern granites, HM Hans Merensky
There were no differences in foliar %N, NDF, ADF, or ADL between the six landscapes included in this study (P = 0.86, 0.40, 0.5, and 0.46, respectively; Fig. 4), nor did tree foliage show any significant landscape variation in fiber digestibility (P = 0.50, 0.35, and 0.35 for Eqs. 1, 2, and 3, respectively). However, within sampling sites on northern basalts, Punda Maria, and Hans Merensky, %N and ADL of tree foliage did vary (P < 0.05 and 0.001, respectively). The highest %N for leaves was recorded at NBP3 (waterhole site; mean = 3.0 ± 0.5%, n = 4) and the lowest at PM1 (open woodland site; 1.8 ± 0.7%, n = 4); ADL was highest at NBP3 (18.9 ± 7.3, n = 4) and lowest at HM3 (riparian site; 7.5 ± 2.7%, n = 4). Fiber digestibility of tree foliage appeared therefore to be highest in the riparian habitat and lowest at the waterhole site. Grass fibers were also similar across landscapes (P > 0.16 in all cases), but grass %N did show significant landscape variations (P < 0.01) with the highest values recorded for southern granites (1.3 ± 0.4%, n = 9) and the lowest on northern basalts (0.8 ± 0.3%, n = 20). Within sampling sites, the highest %N for grass was recorded for PM3 (open woodland site at Punda Maria; 1.5 ± 0.7%, n = 5).
Discussion
Data presented in this study are consistent with available literature, especially predictions for higher %N and ADL, and lower NDF and ADF, of dicots compared to monocots. Species-level differences are also consistent with previously published data. For instance, relatively high %N was previously reported for P. violacea (= Lonchocarpus capassa; du Toit 2003), and E. divinorum had relatively low %N and CWD, consistent with expectations that this species is avoided by browsers such as kudu Tragelaphus strepsiceros (Owen-Smith and Cooper 1989). Percent N and fiber data for grasses are also within the range of previously reported values for grass forages in the Timbavati on the western boundary of Kruger Park (see Meissner et al. 1996; Bodenstein et al. 2000). In terms of relative nutritional quality, these data suggest higher fiber digestibility in monocots compared with dicots, which is in accord with predictions based on higher NDF and lower ADL content of the former (Owen-Smith 1997; Meissner et al. 1999), and with data from in vitro digestibility trials (Van Wieren 1996; Hummel et al. 2006).
While browse foods do contain higher proportions of cell solubles (reflected by %N in the current study), this finding cannot be assumed to be a reflection of high quality food compared with grass. Nonnutritive cell solubles may further reduce browse quality, especially secondary compounds such as condensed tannins that are known to precipitate proteins through the herbivore digestive tract (Cooper and Owen-Smith 1985; Robbins et al. 1987; Hay and van Hoven 1988). A number of studies showed that some grass species do contain tannins and other secondary compounds, including cyanogens and flavonoids, that reduce forage quality (e.g., Georgiadis and McNaughton 1988; Chesselet et al. 1992; Meissner et al. 1999), but these generally occur in larger proportions in woody plants (e.g., Meissner et al. 1999). Higher concentrations of secondary compounds and nonprotein nitrogen also means that the higher %N of browse compared with grass does not necessarily reflect higher crude protein content in the former (Milton and Dintzis 1981). Because of the lower fiber digestibility of browse and the effects of a tannin-rich diet, some authors have even suggested that overall metabolizable energy yields of browse and grass are similar or even lower among browse (Illius 1997; Owen-Smith 1997).
Fruits, too, cannot be regarded as necessarily high quality foods. Many savanna fruit species consist of a high proportion of structural cell wall, especially the NDF fraction, which can be as high as 70% (or 80% in the case of A. quanzensis pods). High NDF content of fruits is congruent with recent studies that report NDF values as high as 60 to 70% among forest species (Molloy and Hart 2002; Shipley and Felicetti 2002). Demment and Van Soest (1985) suggested that fruit hulls have a higher fibrous component than the fleshy portions, but in our study, despite large differences across species, fruits enclosed in pods were not consistently more fibrous than fleshy drupes. Some species of fruit nevertheless appear to offer nutritive food sources (e.g., marula S. birrea subsp. caffra), but species such as R. tomentosa and S. madagascariensis, which are both often eaten by ungulates and other mammals (e.g., Skinner and Smithers 1990; van Wyk et al. 2000), consist of >50% NDF, and R. tomentosa is very highly lignified (>25% ADL). Obviously, the data presented in this study exclude nonstructural carbohydrates (such as sugars) in fruits that may increase overall value of the food, as might their relatively high %N. In a review on the ecological attributes of browsing and grazing ruminants, Owen-Smith (1997) expressed that fruits are also rich in digestion-inhibiting secondary compounds. Thus, even the high %N recorded for some fruits in the current study does not necessarily imply high nutritional quality (see also Milton and Dintzis 1981).
Given the congruence with previous predictions (e.g., Van Wieren 1996; Owen-Smith 1997; Hummel et al. 2006), our findings are not necessarily surprising, but these data do demonstrate problems that may arise from oversimplifying plant biochemical components to characterize herbivore food quality. Owen-Smith (1997) remarked that reference to browse as high quality “concentrate” foods is misleading, an assertion supported by our data and findings of other studies (e.g., Van Wieren 1996; Hummel et al. 2006). Models of herbivore differentiation that assume browse to be a higher quality resource than grass therefore require (and have received) some revision.
Hofmann’s (1989) characterization of anatomical adaptations of browsers and grazers was previously criticized because the dichotomy invoked does not account for the full scale of dietary variation that exists among ruminants (and other ungulates), the physiological mechanisms for these differences were not studied directly, and because other morphophysiological constraints, especially body size, might be more important drivers of evolutionary adaptation (reviewed in Ditchkoff 2000). Bodmer (1990) and Gagnon and Chew (2000), for example, both suggest that frugivory should be treated as a separate trophic guild to browsing and grazing. Available evidence for high fiber of fruit compared with foliage and forbs supports this possibility and highlights the effects that body size may elicit; the high NDF of fruits may explain why predominantly frugivorous duikers Cephalophus spp. appear to have unusually high retention times and achieve high NDF digestibilities given their small body size (Wenninger and Shipley 2000; Shipley and Felicetti 2002; Perez-Barberia et al. 2004).
Despite limitations to his model, Hofmann’s prediction that grazers should achieve longer rumen retention times and hence increased fiber digestion efficiencies than browsers was demonstrated empirically and with the use of statistical models (Owen-Smith 1988; Van Wieren 1996; Perez-Barberia et al. 2004). The higher fiber digestibility of grass compared with browse implies that evolution of longer rumen retention times in grazers does not necessarily represent an adaptation to extract energy from poor quality foods as suggested by Hofmann (1989), but rather to facilitate complete digestion of fiber. However, some studies have suggested there is no relationship between feeding style and fiber digestion efficiency among ungulates, based on statistical models for digesta kinetics (Gordon and Illius 1994) and empirical experiments (Robbins et al. 1995). One possible explanation for the apparent contradiction here is that fiber digestibility and overall nutritional quality of the diet may not be the primary determinant for achieving differential retention times. Alternative hypotheses for the evolution of longer rumen retention in grazers were put forward based on differences in particle sizes into which browse and grass are broken down in the digestive tract, differences in fluid dissociation between these food types, and perhaps most importantly, grass stratification and formation of a fibrous raft in the rumen (Owen-Smith 1982; Clauss and Lechner-Doll 2001; Clauss et al. 2002, 2003, 2006). Thus, if at all, differences in physical, rather than biochemical, properties of grass and browse may have provided the basis for diversification of ungulate feeding styles and associated stomach physiology (see Clauss et al. 2003). It will be interesting for future studies to compare the physicomechanical properties of fruit in the ruminant digestive tract to those of foliage and grass.
Perhaps the biggest constraint to testing models of herbivore diversification stems from insufficient accounts of variations in observed trends. In an attempt to overcome this limitation, several studies have employed methods that express feeding style as a continuous variable across multiple taxa, rather than simply classifying animals into discrete groups of browsers, grazers, and mixed-feeders (Janis 1995; Clauss et al. 2003; Sponheimer et al. 2003; Perez-Barberia et al. 2004). Such approaches represent advances on earlier methods because they account for dietary variations across the browser–grazer continuum. However, these studies do not control for dietary variations within species, which even among predominantly browsing and grazing taxa are often substantial across space and time (data reviewed in Owen-Smith 1997 and du Toit 2003). Moreover, in vivo digestibilities of at least some African ungulate species were shown to change significantly through the seasonal cycle (see Meissner et al. 1996 for impala Aepyceros melampus; Bodenstein et al. 2000 for Burchell’s zebra Equus burchellii and blue wildebeest Connochaetes taurinus). Our data provide some insights into changes in food quality over space and time, which may offer support to refine models of adaptation.
In the current study, fiber digestibility of tree foliage was highest in the dry season, and possibly not different to that of grass during this period. In the wet season, grass clearly had higher DMD than tree foliage, and grass %N also increased substantially from the dry to the wet season. These patterns may explain why mixed-feeders, and some grazer species, increase browse consumption during the dry season and consume more grass in the wet season (e.g., Skinner and Smithers 1990; Owen-Smith 1997).
In terms of regional changes, surprisingly few variations in plant nutrient content across landscapes were observed, despite the variety of habitat types represented in this study, including areas that received above and below average rainfall. Spatial differences were recorded, however, between sites within certain landscapes. Fiber digestibility of tree foliage appeared to be highest at our riparian site, lowest at the waterhole site, and grass %N was highest at the dense, closed canopy site near Punda Maria in the far northern Kruger Park. Although these sites represent isolated cases, patterns recorded might explain feeding variations recorded for impala from Kruger Park; stable carbon isotope evidence from feces revealed that impala exhibit maximum browse (C3 vegetation) intake in riparian areas, minimum browse intake around artificial watering points, and maximum grass (C4 plant) consumption at Punda Maria, even though the latter landscapes are characterized by heavily wooded, even forested, habitats (Codron et al. 2006).
While evidence presented here for spatiotemporal variations is incomplete, and food choice is likely to occur at many different scales not covered by our study, the trends do suggest that it may be premature to rule out plant food quality as a key driver of herbivore diversification. Indeed, a number of field studies showed that phenological changes in plants seem to account for at least some of the variation in food choice through the seasonal cycle (Owen-Smith and Cooper 1989; Meissner et al. 1996; Bodenstein et al. 2000; Watson and Owen-Smith 2002). Owen-Smith (1997) provided the only real attempt to integrate feeding variations within taxa into the context of ecological differentiation between browsers and grazers, but highlighted the lack of data for most species. Future studies employing multifactorial approaches to ungulate ecological complexity (e.g., Perez-Barberia et al. 2004) would benefit from improved controls of intraspecific variations in diet, diet quality, and digestive efficiency.
References
Bodenstein V, Meissner HH, van Hoven W (2000) Food selection by Burchell’s zebra and blue wildebeest in the Timbavati area of the Northern Province Lowveld. S Afr J Wildl Res 30:63–72
Bodmer RE (1990) Ungulate frugivores and the browser grazer continuum. Oikos 57:319–325
Chesselet P, Wolfson MM, Ellis RP (1992) A comparative histochemical study of plant polyphenols in southern African grasses. J Grassl Soc South Afr 9:119–125
Clauss M, Lechner-Doll M (2001) Differences in selective reticulo-ruminal particle retention as a key factor in ruminant diversification. Oecologia 129:321–327
Clauss M, Lechner-Doll M, Streich W (2002) Faecal particle size distribution in captive and wild ruminants: an approach to the browser/grazer dichotomy from the other end. Oecologia 131:343–349
Clauss M, Lechner-Doll M, Streich W (2003) Ruminant diversification as an adaptation to the physicomechanical characteristics of forage. A reevaluation of an old debate and a new hypothesis. Oikos 102:253–262
Clauss M, Hummel J, Streich W (2006) The dissociation of the fluid and particle phase in the forestomach as a physiological characteristic of large grazing ruminants: an evaluation of available, comparable ruminant passage data. Eur J Wildl Res 52:88–98
Codron D, Codron J, Lee-Thorp JA, Sponheimer M, de Ruiter D, Brink JS (2006) High-resolution dietary variation in impala Aepyceros melampus reorded by carbon isotope composition of feces. Acta Zool Sin (in press)
Cooper SM, Owen-Smith N (1985) Condensed tannins deter feeding by browsing ruminants in a South African savanna. Oecologia 67:142–146
Demment MW, Van Soest PJ (1985) A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am Nat 125:641–672
Ditchkoff SS (2000) A decade since ‘diversification of ruminants’: has our knowledge improved? Oecologia 125:82–84
du Toit JT (2003) Large herbivores and savanna heterogeneity. In: du Toit JT, Rogers KH, Biggs HC (eds) The Kruger experience. Island Press, Washington DC, pp 292–309
Ellery WN, Scholes RJ, Scholes MC (1995) The distribution of sweetveld and sourveld in South Africa’s grassland biome in relation to environmental factors. Afr J Range Forage Sci 12:38–45
Gagnon M, Chew AE (2000) Dietary preferences in extant African bovidae. J Mammal 81:490–511
Georgiadis NJ, McNaughton SJ (1988) Interactions between grazers and a cyanogenic grass, Cynodon plectostachyus. Oikos 51:343–350
Gordon IJ, Illius AW (1994) The functional significance of the browser–grazer dichotomy in African ruminants. Oecologia 98:167–175
Hay L, van Hoven W (1988) Tannins and digestibility in the steenbok (Raphicerus campestris). Comp Biochem Physiol 91:509–511
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78:443–457
Hofmann RR, Stewart DRM (1972) Grazer or browser: a classification based on the stomach structure and feeding habits of East African ruminants. Mammalia 36:226–240
Hummel J, Südekum KH, Streich WJ, Clauss M (2006) Forage fermentation patterns and their implications for herbivore ingesta retention times. Funct Ecol (in press)
Illius AW (1997) Physiological adaptation in savanna ungulates. Proc Nutr Soc 56:1041–1048
Janis CM (1995) Correlations between craniodental morphology and feeding behavior in ungulates: reciprocal illumination between living and fossil taxa. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, New York, pp 76–98
Jarman PJ (1974) The social organization of antelope in relation to their ecology. Behaviour 48:215–266
Jung HG, Deetz DA (1993) Cell wall lignification and degradability. In: Jung HG, Buxton DR, Hatfield RD, Ralph J (eds) Forage cell wall structure and digestibility. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison WI, p 315
Jung HG, Mertens DR, Payne AJ (1997) Nutrition, feeding, and calves. Correlation of acid detergent lignin and Klason lignin with digestibility of forage dry matter and neutral detergent fiber. J Dairy Sci 80:1622–1628
McNaughton SJ, Georgiadis NJ (1986) Ecology of African grazing and browsing mammals. Ann Rev Ecolog Syst 17:39–65
Meissner HH, Pieterse E, Potgieter JHJ (1996) Seasonal food selection by male impala Aepyceros melampus in two habitats. S Afr J Wildl Res 26:56–63
Meissner HH, Zacharias PJK, O’Reagain PJ (1999) Forage quality (feed value). In: Tainton NM (ed) Veld management in South Africa. University of Natal Press, Pietermaritzburg, South Africa, pp 139–168
Milton K, Dintzis FR (1981) Nitrogen-to-protein conversion factors for tropical plant samples. Biotropica 13:177–181
Minson DJ (1990) Forage in ruminant nutrition. Academic, San Diego, California
Molloy L, Hart JA (2002) Duiker food selection: palatability trials using natural foods in the Ituri Forest, Democratic Republic of Congo. Zoo Biol 21:149–159
Moore JE, Coleman SW (2001) Forage intake, digestibility, NDF and ADF: how well are they related? In: Terrill TM (ed) Proceedings of the American Forage and Grassland Council Conference, Springdale, AR AFGC, Georgetown, TX, 20 May 2001, pp 238–242
Mould ED, Robbins CT (1982) Digestive capabilities in elk compared to white-tailed deer. J Wildl Manage 46:22–29
Owen-Smith RN (1982) Factors influencing the consumption of plant products by large herbivores. In: Huntley BJ, Walker BH (eds) Ecology of tropical savannas. Springer, Berlin Heidelberg New York, pp 359–404
Owen-Smith RN (1988) Megaherbivores—the influence of very large body size on ecology. Cambridge University Press, Cambridge
Owen-Smith N (1997) Distinctive features of the nutritional ecology of browsing versus grazing ruminants. Z. Säugetierkd-Int J Mamm Biol 62:176–191
Owen-Smith N, Cooper SM (1989) Nutritional ecology of a browsing ruminant, the kudu (Tragelaphus strepsiceros), through the seasonal cycle. J Zool Lond 219:29–43
Perez-Barberia FJ, Elston DA, Gordon IJ, Illius AW (2004) The evolution of phylogenetic differences in the efficiency of digestion in ruminants. Proc R Soc Lond 271B:1081–1090
Robbins CT (1993) Wildlife feeding and nutrition, 2nd edn. Academic, New York
Robbins CT, Hanley TA, Hagerman AE, Hjejord O, Baker DL, Schwartz CC, Mautz WW (1987) Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology 68:98–107
Robbins CT, Spalinger DE, van Hoven W (1995) Adaptation of ruminants to browse and grass diets: are anatomical-based browser–grazer interpretations valid? Oecologia 103:208–213
Shipley LA, Felicetti L (2002) Fiber digestibility and nitrogen requirements of blue duikers (Cephalophus monticola). Zoo Biol 21:123–134
Skinner JD, Smithers RHN (1990) The mammals of the southern African subregion, 2nd edn. University of Pretoria Press, Pretoria
Sponheimer M, Lee-Thorp JA, de Ruiter D, Smith JM, Van der Merwe NJ, Reed K, Grant CC, Ayliffe LK, Robinson TF, Heidelberger C, Marcus W (2003) Diets of Southern African bovidae: stable isotope evidence. J Mammal 84:471–479
Van Soest PJ (1994) Nutritional ecology of the ruminant, 2nd edn. Comstock, Ithaca, New York
Van Soest PJ, Wine RH (1967) Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J Assoc Anal Chem 50:50–55
Van Wieren SE (1996) Browsers and grazers: foraging strategies in ruminants. In: Van Wieren SE (ed) Digestive strategies in ruminants and nonruminants. Ph.D. thesis, University of Wageningen, The Netherlands, pp 119–146
van Wyk B, van Wyk P, van Wyk B-E (2000) Photographic guide to trees of southern Africa. Briza Publications, Pretoria
Venter FJ, Scholes RJ, Eckhardt HC (2003) The abiotic template and its associated vegetation pattern. In: du Toit JT, Rogers KH, Biggs HC (eds) The Kruger experience. Island Press, Washington DC, pp 83–129
Watson LH, Owen-Smith N (2002) Phenological influences on the utilization of woody plants by eland in semi-arid shrubland. Afr J Ecol 40:65–75
Wenninger PS, Shipley LA (2000) Harvesting, rumination, digestion, and passage of fruit and leaf diets by a small ruminant, the blue duiker. Oecologia 123:466–474
Acknowledgements
We thank Darryl de Ruiter, Todd Robinson, James Brink, and Norman Owen-Smith for advice and comments on the study; Charles T. Robbins for commenting on an earlier draft of this manuscript; and Marcus Clauss and an anonymous reviewer for thorough efforts that greatly improved the clarity of the paper. We also thank Rina Grant, Angela Gaylard, Abri de Buys, and Wilson Dinda for facilitating and assisting with research in Kruger National Park; the Limpopo Tourism and Parks Board for access to Hans Merensky Nature Reserve; Carl Morrow and Jo Crane for facilitating fiber analyses; John Lanham and Ian Newton for assistance with mass spectrometry; and Nick Zambatis (Kruger Park) and the South African Weather Bureau for climate data. Funding was provided by the Palaeontological Scientific Trust (RSA), the National Research Foundation (RSA), the National Science Foundation (USA), and the University of Cape Town (RSA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Codron, D., Lee-Thorp, J.A., Sponheimer, M. et al. Nutritional content of savanna plant foods: implications for browser/grazer models of ungulate diversification. Eur J Wildl Res 53, 100–111 (2007). https://doi.org/10.1007/s10344-006-0071-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-006-0071-1