Abstract
Charcoal rot incited by Macrophomina phaseolina is one of the major diseases of green gram and black gram in Pakistan reducing yields up to 40%. As there are no long-term control strategies for this seed- and soil-borne pathogen, therefore, in the present study, seven indigenous species of Trichoderma were evaluated for their in vitro and in vivo effectiveness against M. phaseolina with the objective to identify alternatives to pernicious fungicides. All seven species of Trichoderma significantly retarded the growth of M. phaseolina in vitro. Maximum reduction (79.63%) was observed with T. harzianum followed by T. hamatum (76.3%) while T. pseudokoningii caused the minimum decrease (58.14%) in growth of the fungus. Similarly, Trichoderma species had significant effects on number and size of sclerotia. M. phaseolina produced the minimum number of sclerotia in the presence of T. hamatum followed by T. harzianum causing reductions of 69.5 and 66.84% over control, respectively. The maximum reduction in size of sclerotia was caused by T. harzianum. The maximum plant survival of green and black gram was obtained with T. harzianum followed by T. hamatum and T. viride. The maximum individual germination of 86.67% was achieved with T. harzianum at a concentration of 2 × 108 (propagules/ml), while the minimum (33.33%) was recorded with T. pseudokoningii at 2 × 104. Trichoderma concentrations also had significant effects on plant survival, being the maximum at the highest concentration. The plant survival decreased as the concentrations of the antagonists decreased showing a direct relationship between plant survival and concentrations.
Zusammenfassung
Die durch Macrophomina phaseolina hervorgerufene Holzkohlenfäule ist eine der Hauptkrankheiten bei der Mungbohne und der Urdbohne in Pakistan und reduziert die Erträge um bis zu 40 %. Da es keine langfristigen Bekämpfungsstrategien für diesen samen- und bodenbürtigen Erreger gibt, wurden in der vorliegenden Studie sieben einheimische Trichoderma-Arten auf ihre in-vitro- und in-vivo-Wirksamkeit gegen M. phaseolina mit dem Ziel untersucht, Alternativen zu schädlichen Fungiziden zu finden. Alle sieben Trichoderma-Arten verzögerten das Wachstum von M. phaseolina in vitro signifikant. Die maximale Reduktion (79,63 %) wurde bei T. harzianum beobachtet, gefolgt von T. hamatum (76,3 %), während T. pseudokoningii die geringste Abnahme (58,14 %) des Pilzwachstums verursachte. In ähnlicher Weise hatten die Trichoderma-Arten signifikante Auswirkungen auf Anzahl und Größe der Sklerotien. M. phaseolina produzierte die minimale Anzahl von Sklerotien in Anwesenheit von T. hamatum, gefolgt von T. harzianum, was zu einer Verringerung um 69,5 % bzw. 66,84 % gegenüber der Kontrolle führte. Die maximale Verringerung der Größe der Sklerotien wurde durch T. harzianum verursacht. Das maximale Überleben der Mungbohne und der Urdbohne wurde mit T. harzianum erreicht, gefolgt von T. hamatum und T. viride. Die maximale Keimung von 86,67 % wurde mit T. harzianum bei einer Konzentration von 2 × 108 (propagules/ml) erreicht, während das Minimum (33,33 %) mit T. pseudokoningii bei 2 × 104 erfasst wurde. Die verwendeten Trichoderma-Konzentrationen hatten ebenfalls signifikante Auswirkungen auf das Überleben der Pflanzen, wobei das Maximum bei der höchsten Konzentration erreicht wurde. Das Pflanzenüberleben nahm mit der Reduktion der Konzentrationen ab, was eine direkte Beziehung zwischen dem Pflanzenüberleben und den Konzentrationen zeigte.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green gram (Vigna radiata L.) Wilczek and black gram (Vigna mungo L.) Hepper are two important summer pulse crops of Pakistan, cultivated on an area of 245.9 and 32.5 thousand hectares with a total production of 177.7 and 17.3 thousand tons, respectively, (Anonymous 2018) under a wide range of agro-ecological zones. The average yields of these pulses in Pakistan are very low as compared to their potential yields obtained in many other countries which can be imputed to legions of biotic and abiotic constraints. Among biotic factors, diseases are the most destructive (Aslam et al. 2019a, 2019b; Hussain and Mukhtar 2019; Mukhtar and Hussain 2019; Mukhtar and Kayani 2019; Tariq-Khan et al. 2020). The losses due to diseases to pulse crops have been estimated up to 44%, depending upon the crop variety (Bashir and Malik 1988). Green gram and black gram are invaded by about 26 diseases in the world (Charles 1978); charcoal rot incited by Macrophomina phaseolina (Tassi) Goid, is of prime significance in reducing crop yield especially in arid regions of the world (Hoes 1985). The pathogen is distributed in diverse climatic conditions from arid to tropical regions and has a broad host range. There are more than 500 hosts of the fungus including legumes and cereal plants. In Pakistan, it has 67 important economic host plant species including mungbean, mashbean, soybean, sunflower, sorghum, maize, linseed, chickpea and alfalfa (Javaid et al. 2017). M. phaseolina is a soil- and seed-borne pathogenic fungus. It produces cushion shaped black sclerotia and disease severity is correlated with viable sclerotia present in the soil (Iqbal and Mukhtar 2014).
All the growth stages of plants are infected by charcoal rot. The disease manifests in the form of dark lesions on the epicotyls and hypocotyls resulting in seedling death due to obstruction of xylem vessels. The pathogen causes red to brown lesions on roots and stems, produces dark mycelia and black microsclerotia. Severe infections cause defoliation and wilting resulting in 100% yield losses (Bashir and Malik 1988). The fungus is fairly distributed in all the agro-ecological zones of Pakistan. Significant morphological and pathogenic variations have been observed among different isolates of the fungus from these agro-ecological zones suggesting that these variations may be considered in disease management and breeding programs of green gram and black gram against charcoal rot (Iqbal and Mukhtar 2014).
The ever-increasing need for incessant supply of food to the bourgeoning human population of the world demands for the management of phytopathogens responsible for considerable reduction in crop yields (Javed et al. 2019a, 2019b; Khan et al. 2019; Nazir et al. 2019; Mukhtar and Kayani 2020). Currently, the management of charcoal rot is mainly relied on synthetic fungicides and resistant cultivars (Mukhtar et al. 2017; Iqbal and Mukhtar 2020). The commonly used fungicides against M. phaseolina in Pakistan are Propineb, Copper + Mancozeb, Mancozeb, Carbendazim, Captan, Benomyl, Mtalaxyl + Mancozeb, Copper oxychloride, Chlorothalonil. Synthetic fungicides give an instant and effective control of plant pathogens but their use is inimical to humans, livestock and environment. Moreover, the effectiveness of these fungicides as seed treatment is not long term and their soil application is expensive and causes imbalance in soil microbial communities which affect normal activities of beneficial organisms. In addition, continuous use of the same fungicides for the same pathogen results in the development of resistant strains of the pathogens (Iqbal et al. 2014). As no resistant cultivars of green gram and black gram are available against M. phaseolina in the country, and the use of fungicides is costly and hazardous, therefore, deployment of biocontrol agents could be one of the feasible and best alternative strategies to abate yield losses by the fungus. Among fungal biocontrol agents, Trichoderma species have been most widely studied against different phytopathogens. Trichoderma species are naturally occurring soil fungi that colonize roots and stimulate plant growth. Such fungi have been applied to a wide range of plant species for the purpose of growth enhancement, with a positive effect on plant weight, crop yields, and disease control. The large scale production of potential Trichoderma spp. would be economical and ecofriendly. In Pakistan, very little work has been done on the biocontrol potential of indigenous Trichoderma species for the management of charcoal rot of green gram and black gram. For these reasons, in the present study, seven indigenous species of Trichoderma were evaluated for their in vitro and in vivo effectiveness against M. phaseolina with the objective to identify alternatives to pernicious fungicides and to update disease management strategies.
Materials and Methods
Isolation, Purification and Identification of Macrophomina phaseolina
The fungus, Macrophomina phaseolina, used in the study was isolated from stem bark tissues of black gram bearing fungal sclerotia and characteristic charcoal rot symptoms. The samples were cut into small pieces (5–10 mm long), surface sterilized with 1% sodium hypochlorite for 2 min and then rinsed thrice in sterilized distilled water. The pieces were placed on Chloroneb Mercury Rose Bengal Agar (CMRA) medium (Meyer et al. 1973) in petri dishes and incubated in dark at 25 ± 1 °C for 7 d. A small portion of the actively growing colony of M. phaseolina was taken from the periphery of 90 mm diameter petri dish and spread onto petri dishes containing glucose agar medium (glucose, 20 g; agar, 20 g and water, 1000 ml) and incubated in dark at 25 ± 1 °C for 7 d. A small portion of the colony having sclerotia was taken into a drop of sterilized water and agitated with a sterilized needle to separate the sclerotia from the mycelia. Sclerotia were then transferred to 90 mm diameter petri dishes containing CMRA medium. Colonies appearing from single sclerotium were again transferred to CMRA medium in 90 mm petri plates, incubated as mentioned above and identified on the basis of standard key (Barnett and Hunter 1972).
Multiplication of M. phaseolina for Pot Assay
Sorghum seeds were water soaked overnight, air dried under room temperature and placed in conical flasks. The mouth of each flask was plugged with cotton wool, wrapped in aluminum foil and autoclaved at 15 psi (121 °C) for 20 min. After cooling, the seeds in flasks were inoculated with 4 mm mycelial plugs from a 7-day-old culture of M. phaseolina and incubated at 25 ± 1 °C for 15 d. The flasks were shaken at alternate days for uniform colonization of the grains. The inoculum thus produced was used in pot experiments.
Biomass Production of Trichoderma Species
Seven indigenous Trichoderma species viz. Trichoderma harzianum, T. hamatum, T. koningii, T. pseudokoningii, T. viride, T. aureoviride and T. virens used in the experiment were collected from the First Fungal Culture Bank, The University of Punjab, Lahore. The biocontrol agents were grown on potato dextrose agar (PDA). For biomass production of antagonists, Richard’s medium was used. The medium (100 ml) was poured in 250 ml Erlenmeyer flasks, autoclaved at 15 psi for 30 min, inoculated with two scoops of 10 mm diameter taken from a 7-day-old culture of each Trichoderma species on PDA and incubated at 25 ± 1 °C for 15 d. The biomass of each antagonist was centrifuged at 11,000 rpm for 30 s and their slurries were made. The propagules of each fungal slurry were enumerated using a haemocytometer. Based on these counts, the concentrations (2 × 104, 2 × 106 and 2 × 108 propagules/ml) were made by the addition of requisite amount of distilled water to the slurry of fungal antagonist. Two ml of gelatin as an adhesive was added to the fungal suspensions of each concentration of each antagonist. The seeds each of green gram (cv. NM-92) and black gram (cv. Mash-98) were surface sterilized and primed with different concentrations of biocontrol agents. Seeds treated with sterilized distilled water served as controls.
Evaluation of Trichoderma Species for their Efficacy Against M. phaseolina
In Vitro Evaluation of Trichoderma Species
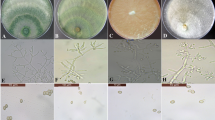
The in vitro antagonistic activities of Trichoderma species were tested against M. phaseolina by seeing their effects on different parameters viz. radial growth, number and size of sclerotia produced by the pathogen. The effects of Trichoderma species on radial growth of M. phaseolina were tested by dual culture technique (Naik et al. 2009). Petri dishes (90 mm dia.) containing 15 ml of autoclaved PDA were inoculated with 5‑mm-diameter mycelial discs each of 7‑day-old culture of the pathogen and antagonists at equi-distance from the periphery and incubated for 6 d at 25 ± 1 °C in an incubator. Petri plates without antagonists served as control. Each treatment was replicated five times. After 6 d, the diameters of the colonies of both the biocontrol agents and the pathogenic fungus were measured in four directions, their averages were calculated and percent inhibition of radial growth of M. phaseolina was calculated (Iqbal et al. 2014). The effect of Trichoderma species on sclerotia production was studied by counting sclerotia per microscopic field (under 10 ×). The size of sclerotia was measured with the help of an ocular micrometer from control and treated plates. To calculate the size, the averages of 50 sclerotia from each treatment were taken.
Greenhouse Assay
To evaluate the efficacy of antagonists in greenhouse, the cornmeal sand inoculum was thoroughly mixed with sterilized potting soil at 2.5 g per kg of potting soil. The infested potting soil was filled in plastic greenhouse flats. Plastic greenhouse flats filled with sterilized un-infested potting soil served as controls. Twenty seeds each of green gram (cv. NM-92) and black gram (cv. Mash-98) treated with different concentrations of Trichoderma species were sown in infested potting soil contained in greenhouse flats in four rows of five seeds. The seeds treated with sterilized distilled water sown in infested flats served as control. Each treatment was replicated five times. The flats were placed in a glasshouse at 25 °C in completely randomized design. Data on percentage germination were recorded after 20 d, and percentage increase or decrease over control was calculated.
Statistical Analysis
The experiment was conducted twice. Percent reduction in mycelial growth, number and size of sclerotia of the pathogenic fungus and increase in seedling emergence were calculated over controls prior to statistical analysis (Mukhtar 2018). All the data were subjected to Analysis of Variance (ANOVA) using GenStat package 2009, (12th edition) version 12.1.0.3278 (www.vsni.co.uk). The differences among means were compared by Fisher’s protected least significant difference test at (P ≤ 0.05). No significant interaction was observed between the data of both the experiments, so the two sets of data were combined for analysis. Standard errors of differences of means were calculated in Microsoft Excel 2010. Taking concentrations of antagonistic fungi as independent variables (X) and plant survival as dependent variable (Y), linear relationships were established and regression equations and correlation coefficients (R2) were calculated in Microsoft Excel 2010. The closer the R2 is to 1.00, the better the fit.
Results
Inhibitory Effects of Trichoderma Species Against M. phaseolina
All the seven species of Trichoderma significantly retarded the growth of M. phaseolina in vitro. Maximum reduction (79.63%) was observed with T. harzianum followed by T. hamatum (76.3%) while T. pseudokoningii caused the minimum decrease (58.14%) in growth of the fungus. The inhibitions in growths caused by other Trichoderma species were found to be intermediate as shown in Fig. 1. Similarly, Trichoderma species had significant effects on number of sclerotia. M. phaseolina produced the minimum number of sclerotia in the presence of T. hamatum followed by T. harzianum causing reductions of 69.5 and 66.84%, respectively, over control. The reductions caused by these two fungi were statistically similar. On the other hand, T. virens caused the minimum reductions in number of sclerotia produced by M. phaseolina followed by T. pseudokoningii giving 43.44 and 41.31% reductions, respectively, over control as shown in Fig. 2. Likewise, the size of sclerotia of M. phaseolina was also significantly affected by Trichoderma species. The maximum reduction in size of sclerotia was caused by T. harzianum while T. pseudokoningii caused the minimum reduction in size. The reductions in size caused by other Trichoderma species were in-between as shown in Fig. 3.
Effects of Trichoderma Species on Plant Survival of Green Gram and Black Gram
Antagonists exhibited significant variations in their efficacy on plant survival of green gram when used as seed treatment. Maximum average plant survival was obtained with T. harzianum followed by T. hamatum and T. viride while T. pseudokoningii gave the poorest germination of all the test biocontrol agents. Maximum individual germination of 86.67% was achieved with T. harzianum at a concentration of 2 × 108 (propagules/ml), while the minimum (33.33%) was recorded with T. pseudokoningii at 2 × 104. Individual germination of green gram with all the species at three concentrations is given in Table 1. Concentrations also had significant effects on plant survival being the maximum at the highest concentration. The plant survival decreased as the concentrations of the antagonists decreased showing a direct relationship between plat survival and concentrations and has been shown by regression equations in Table 1. The Trichoderma species gave almost the similar kind of results in case of black gram and are shown in Table 2.
Discussion
In the present study, seven indigenous Trichoderma species were tested against the devastating fungus M. phaseolina. All the species significantly reduced the growth of M. phaseolina, number and size of sclerotia, and improved the survival and germination of green gram and black gram. Trichoderma species have been considered effective against a plethora of pathogens. Alice et al. (1996) found T. harzianum and T. viride effective against M. phaseolina infecting jasmine. Similarly, Etebarian (2006) found that T. harzianum (M), T. harzianum (T39) and T. virens (DAR 74290) completely retarded growth of M. phaseolina inciting charcoal rot in melon. Moreover, many soil-borne pathogens including Rhizoctonia solani, Pythium ultimum, Fusarium moniliforme and Sclerotium rolfsii have also been controlled by different Trichoderma species (Ghazanfar et al. 2018; Mukhtar 2018). In the present study, Trichoderma species showed variations in effectiveness in reducing fungal growth and number and size of M. phaseolina; T. harzianum and T. hamatum were found to be the most effective. The variations among different Trichoderma species in their ability to control the growth of phytopathogens have also been reported by many scientists (Laha et al. 1992; Naik et al. 2000; Upmanyu et al. 2002; Singh et al. 2008). This emphasizes the need to select the antagonistic fungi which give best results for the management of phytopathogens under specific agro-ecological conditions. The antagonistic activity of Trichoderma species is attributed to parasitism, production of lytic enzymes such as chitinases and glucanases (Chet, 1987; Woo et al. 2002; Compant et al. 2005) which degraded β‑glucans, chitin and polysaccharides, responsible for fungal cell wall rigidness (Gupta et al. 1995; Howell 2003) or by antibiotic production such as glioviridin and gliotoxin (Di Pietro et al. 1993) or by competition or rhizosphere competence. It has also been reported that, in dual culture technique, Trichoderma hyphae hyper-parasitized the pathogen and coagulated its protoplasm leading to shrinkage, granulated and vacuolated protoplasm of the pathogen (Pandey and Upadhyay 2000). Suppression of fungal growth by antagonists might be due to overgrowth forming inhibition zone (Hashem 2004).
Dressing green gram and black gram seeds with three different concentrations of Trichoderma species in greenhouse decreased the incidence of charcoal rot caused by M. phaseolina and enhanced germination. Earlier, a number of researchers reported similar results by different Trichoderma species against different plant pathogens. Adekunle et al. (2001) showed that dressing of cowpea seeds with different Trichoderma spp. gave maximum plant stand. Dawar et al. (2008) observed a decrease in root rot infection and enhanced plant height and weight in T. harzianum coated seeds of okra and sunflower. Hussain et al. (1990) found that seed treatment with T. harzianum, T. virens, Paecilomyces lilacinus or Streptomyces spp. reduced M. phaseolina infection in sunflower and mungbean. Several fungi such as M. phaseolina, F. semitecum, F. moniliforme and F. solani infecting cowpea, horse gram, black and green gram have also been effectively controlled by seed treatment with conidial suspension of T. harzianum (Krishna et al. 2003). Rettinasbabady et al. (2000) found that T. viride treated black gram seeds decreased sclerotial formation of M. phaseolina. Similarly, the same fungus effectively controlled R. bataticola in vitro (Kaswate et al. 2003). Seed treatment and soil application with T. virens reduced root rot incidence in pigeon pea (Lokesha and Benagi 2007). Seed treatment with both T. harzianum and T. viride also reduced root rot incidence and increased growth in mungbean plants and hence regarded as a suitable method for controlling seed- and soil-borne fungi (Ashraf et al. 2006).
The improvement in germination was due to multiplication of antagonists on seed surface which prevented the fungal entry into seeds by instantly colonizing the roots (Chao et al. 1986; Raguchander et al. 1998). The enhanced germination might also be due to triggering of host plant cell by antagonists to synthesize growth hormones and toxic substances in large quantities which inhibited pathogenic fungi (Hendelsman and Eric 1996). Similar results by Pineda (2001) in sesame, Malik and Dawar (2003) in chickpea and mash bean also confirmed the present findings.
The application of Trichoderma species in soil as seed treatment could lead to the establishment and rapid build-up of these biocontrol agents in the soil which would assist in reducing the infectivity of soil-borne inoculum or suppression of the pathogen. This is particularly applicable for soil-borne pathogens such as M. phaseolina where soil drenching with chemicals is not only deleterious to the environment but is also practically not feasible due to high costs. Considering the cost of seed dressing chemicals which is about three times that of the indigenously produced biocontrol agents (like Trichoderma species tested in the present study), the use of these antagonistic fungi as seed treatment will not significantly affect the cost of production. Furthermore, the continuous application of these antagonistic fungi will enhance their rhizosphere population resulting in increase in disease suppressiveness of the soil. Therefore, the present study identified the antagonistic potential of indigenous Trichoderma species which can be used for the effective management of M. phaseolina in different agro-ecological zones of the country and can be incorporated in an integrated pest management strategy.
References
Adekunle AT, Cardwell KF, Florini DA, Ikotun T (2001) Seed treatment with Trichoderma species for control of damping-off of cowpea caused by Macrophomina phaseolina. Biocontrol Sci Technol 11:449–457
Alice D, Ebenezar EG, Siraprakasan K (1996) Biocontrol of Macrophomina phaseolina causing root rot of jasmine. J Ecobiol 8:17–20
Anonymous (2018) Agricultural Statistics of Pakistan, Ministry of Food, Agriculture and Live Stock, Agriculture and Livestock Division, Islamabad, Pakistan
Ashraf M, Badri ZA, Eswaran A (2006) Bioefficacy of fungal antagonists against Macrophomina phaseolina (Tassi) Goid. Causing root rot of mungbean. Environ Ecol 24:1016–1018
Aslam MA, Javed K, Javed H, Mukhtar T, Bashir MS (2019a) Infestation of Helicoverpa armigera Hübner (Noctuidae: Lepidoptera) on soybean cultivars in Pothwar region and relationship with physico-morphic characters. Pak J Agri Sci 56(2):401–405
Aslam MN, Mukhtar T, Jamil M, Nafees M (2019b) Analysis of aubergine germplasm for resistance sources to bacterial wilt incited by Ralstonia solanacearum. Pak J Agri Sci 56:119–122
Barnett HL, Hunter BB (1972) Illustrated genera of imperfect fungi. Burgress, Minneapolis
Bashir MA, Malik BA (1988) Diseases of major pulse crops in Pakistan. Trop Pest Manag 34:309–314
Chao WL, Nelson EB, Harmen GE, Hoch HC (1986) Colonization of the rhizosphere by biological control agents applied to seeds. Phytopathology 76:60–65
Charles YY (1978) Mungbean diseases and control. The first international mungbean symposium AVRDC, Tiawan, p 262
Chet I (1987) Trichoderma-Application, mode of action and potential as a biocontrol agent of soil-borne pathogenic fungi. In: Chet I (ed) Innovative approaches to plant disease control. John Wiley & Sons Inc., Hoboken, pp 137–160
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases. Principles, mechanism of action and future prospects. Appl Environ Microbiol 71:4951–4959
Dawar S, Hayat S, Anis M, Zaki MJ (2008) Effect of seed coating material in the efficacy of microbial antagonists for the control of root rot fungi on okra and sunflower. Pak J Bot 40:1269–1278
Etebarian HR (2006) Evaluation of Trichoderma Isolates for biological control of charcoal stem rot in melon caused by Macrophomina phaseolina. J Agric Sci Technol 8:243–250
Ghazanfar MU, Raza M, Raza W, Qamar MI (2018) Trichoderma as potential biocontrol agent, its exploitation in agriculture: a review. Plant Prot 2:23–41
Gupta R, Sexena RK, Chaturvedi P, Viride JS (1995) Chitinase production by Streptomyces viridificans: Its potential in fungal cell wall lysis. J Appl Bacteriol 78:378–383
Hashem M (2004) Biological control of two phytopathogenic fungal species isolated from the rhizoplane of soybean (Glycine max). Czech Mycol 56:223–238
Hendelsman J, Eric VS (1996) Biocontrol of soil borne plant pathogens. Plant Cell 8:1855–1869
Hoes JA (1985) Macrophomina phaseolina causal agent of charcoal rot of sunflower and other crops. Agric Res Stn, Morden Manitoba, Canada
Howell CR (2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis 87:4–10
Hussain MA, Mukhtar T (2019) Root-knot nematodes infecting okra in major vegetable growing districts of Punjab, Pakistan. Pak J Zool 51(3):1137–1143
Hussain S, Ghaffar A, Aslam M (1990) Biological control of Macrophomina phaseolina charcoal rot of sunflower and mungbean. J Phytopathol 130:157–160
Iqbal U, Mukhtar T (2014) Morphological and pathogenic variability among Macrophomina phaseolina isolates associated with mungbean (Vigna radiata L.) Wilczek from Pakistan. Sci World J. https://doi.org/10.1155/2014/950175. Article ID 950175
Iqbal U, Mukhtar T (2020) Inhibitory effects of some fungicides against Macrophomina phaseolina causing charcoal rot. Pak J Zool 52(2):709–715
Iqbal U, Mukhtar T, Iqbal SM (2014) In vitro and in vivo evaluation of antifungal activities of some antagonistic plants against charcoal rot causing fungus, Macrophomina phaseolina. Pak J Agri Sci 51:691–696
Javaid A, Afzal L, Shoaib A (2017) Biological control of charcoal rot of mungbean by Trichoderma harzianum and shoot dry biomass of Sisymbrium irio. Plant Daninha 35:e17165756
Javed K, Javed H, Mukhtar T, Qiu D (2019a) Efficacy of Beauveria bassiana and Verticillium lecanii for the management of whitefly and aphid. Pak J Agri Sci 56(3):669–674
Javed K, Javed H, Mukhtar T, Qiu D (2019b) Pathogenicity of some entomopathogenic fungal strains to green peach aphid, Myzus persicae Sulzer (Homoptera: Aphididae). Egypt J Biol Pest Control 29:92. https://doi.org/10.1186/s41938-019-0183-z
Kaswate NS, Shinde SS, Rathod RR (2003) Effect of biological agents against different isolates of Rhizoctonia bataticola (Taub.) Butler in vitro. Ann Plant Physiol 17:167–168
Khan MTA, Mukhtar T, Saeed M (2019) Resistance or susceptibility of eight aubergine cultivars to Meloidogyne javanica. Pak J Zool 51(6):2187–2192
Krishna M, Niranjana SR, Shetty HS (2003) Effects of chemical fungicides and biological agent on seed quality improvement in pulses. Seed Res 31:121–124
Laha GS, Singh RP, Verma JP (1992) Biocontrol of Rhizoctonia solani in cotton by fluorescent pseudomonads. Indian Phytopathol 45:412–415
Lokesha NM, Benagi VI (2007) Biological management of pigeonpea dry root rot caused by Macrophomina phaseolina. Karnataka J Agric Sci 20:54–56
Malik G, Dawar S (2003) Biological control of root infecting fungi with Trichoderma harzianum. Pak J Bot 35:971–975
Meyer WA, Sinclair JB, Khare MN (1973) Biology of Macrophomina phaseolina in soil studies with selective media. Phytopathology 63:613–620
Mukhtar T (2018) Management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pak J Zool 50:1589–1592
Mukhtar T, Hussain MA (2019) Pathogenic potential of Javanese root-knot nematode on susceptible and resistant okra cultivars. Pak J Zool 51(5):1891–1897
Mukhtar T, Kayani MZ (2019) Growth and yield responses of fifteen cucumber cultivars to root-knot nematode (Meloidogyne incognita). Acta Sci Pol Hortorum Cultus 18(3):45–52
Mukhtar T, Kayani MZ (2020) Comparison of the damaging effects of Meloidogyne incognita on a resistant and susceptible cultivar of cucumber. Bragantia. https://doi.org/10.1590/1678-4499.20190359
Mukhtar T, Arooj M, Ashfaq M, Gulzar A (2017) Resistance evaluation and host status of selected green gram germplasm against Meloidogyne incognita. Crop Prot 92:198–202
Naik MK, Madhukar HM, Devika Rani GS (2009) Evaluation of biocontrol efficacy of Trichoderma isolates and methods of its applications against wilt of chilli caused by Fusarium solani. J Biol Control 23:31–36
Naik MK, Singh SJ, Sinha P (2000) Mechanism of bio-control of wilt of chilli caused by Fusarium oxysporum f. sp. capsici. Proc. Golden Jubilee Int. Conf., on Integrated Plant Disease Management for Sustainable Agriculture.. Indian Phytopathological Society, New Delhi, p 665
Nazir K, Mukhtar T, Javed H (2019) In vitro effectiveness of silver nanoparticles against root-knot nematode (Meloidogyne incognita). Pak J Zool 51(6):2077–2083
Pandey KK, Upadhyay JP (2000) Microbial population from rhizosphere and non rhizosphere soil of pigeon pea. Screening for antagonist and mode of mycoparasitism. J Mycol Plant Pathol 30:7–10
Di Pietro A, Lorito M, Hayes CK, Broadway RM, Harman E (1993) Endochitinase from Gliocladium virens: isolation, characterisation and synergistic antifungal activity in combination with gliotoxin. Phytopathology 83:308–312
Pineda JB (2001) Evaluation of Trichoderma harzianum application methods to the soil for control of Macrophomina phaseolina in sesame. Fitopatol Venezol 14:31–34
Raguchander T, Rajappan K, Samiyappan R (1998) Influence of biocontrol agents and organic amendments on soybean root rot. Int J Trop Agric 16:247–252
Rettinassababady C, Ramadoss N, Ramanadane T (2000) Effect of culture filtrates of Trichoderma viride isolates on germination of black gram and sclerotia of Macrophomina phaseolina. Seed Res 28:193–195
Singh S, Chand H, Varma PK (2008) Screening of bioagents against root rot of mung bean caused by Rhizoctonia solani. Legum Res 31:75–76
Tariq-Khan M, Mukhtar T, Munir A, Hallmann J, Heuer H (2020) Comprehensive report on the prevalence of root-knot nematodes in the Poonch division of Azad Jammu and Kashmir, Pakistan. J Phytopathol. https://doi.org/10.1111/jph.12895
Upmanyu S, Gupta SK, Shyam KR (2002) Innovative approaches for the management of root rot and web blight (Rhizoctonia solani) of french bean. J Mycol Plant Pathol 32:317–331
Woo S, Fogliano V, Scala F, Lorito M (2002) Synergism between fungal enzymes and bacterial antibiotics may enhance biocontrol. Antonie Van Heuwwenhock 81:3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
U. Iqbal and T. Mukhtar declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Iqbal, U., Mukhtar, T. Evaluation of Biocontrol Potential of Seven Indigenous Trichoderma Species against Charcoal Rot Causing Fungus, Macrophomina phaseolina. Gesunde Pflanzen 72, 195–202 (2020). https://doi.org/10.1007/s10343-020-00501-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-020-00501-x