Abstract
Two pot experiments were directed under open field conditions where green bean (Phaseolus vulgaris L.) plants cv. Valentino were irrigated with four levels of salinity (1000, 2000, 3000 and 4000 ppm) combined with two anti-salinity agents (Arbuscular Mycorrhizal fungi [AMF] Glomus irradicans 10% w/w, Bacillus megaterium [10 ml/pot] and non-inoculated plants as control) to counteract the negative effect of salt stress, improve the growth, yield, enzymes activity and chemical composition of green bean plants during 2017and 2018 growing seasons. All salinity amelioration treatments (AMF and Bacillus megaterium) significantly improved vegetative growth, shoots biomass (total fresh and dry weight per plant), chlorophyll and antioxidant enzymatic activity at all verified salinity levels compared with non-inoculated plants (control) which showed severe growth retardation especially under the higher salt concentration (4000 ppm). The lowest values of membrane permeability and maximum leaf relative water content were significantly obtained with AMF and B. megaterium. Plants irrigated with lower concentrated saline water (1000 ppm) significantly accumulated lower Na and Cl and higher K than plants irrigated with higher concentrated salinity irrigation water (4000 ppm). The anti-salinity application increased green bean pod yield under all salinity stress levels particularly with AMF followed by B. megaterium compared with non-inoculated plants.
Zusammenfassung
In zwei Topfexperimenten unter Freilandbedingungen wurden grüne Bohnen (Phaseolus vulgaris L.) cv. Valentino mit salzhaltigem Wasser bewässert (vier Salinitätsstufen: 1000, 2000, 3000 und 4000 ppm), kombiniert mit zwei unterschiedlichen Behandlungsmethoden (arbuskuläre Mykorrhizapilze [AMF] Glomus besticansans 10 % w/w, Bacillus megaterium [10 ml/Topf], nicht beimpfte Pflanzen als Kontrolle). Ziel war es, der negativen Auswirkung von Salzstress entgegenzuwirken sowie das Wachstum, den Ertrag, die Enzymaktivität und die chemische Zusammensetzung von grünen Bohnenpflanzen in den Vegetationsperioden 2017 und 2018 zu verbessern. Beide Behandlungen verbesserten signifikant bei allen Salzgehalten das vegetative Wachstum, die Biomasse der Triebe (Gesamt-Frisch- und -Trockengewicht pro Pflanze), die enzymatische Chlorophyll- und antioxidative Aktivität im Vergleich zu nicht beimpften Pflanzen. Die Kontrollpflanzen zeigten eine starke Wachstumsverzögerung, insbesondere bei der höheren Salzkonzentration (4000 ppm). Die geringste Membranpermeabilität und der maximale relative Wassergehalt der Blätter wurden durch die Behandlung mit AMF und B. megaterium erreicht. Pflanzen, die mit niedriger konzentriertem Salzwasser (1000 ppm) bewässert wurden, akkumulierten signifikant weniger Na und Cl und mehr K als Pflanzen, die mit höher konzentriertem Salzwasser (4000 ppm) bewässert wurden. Die Behandlungen erhöhten die Ausbeute der grünen Bohnenschoten, insbesondere durch AMF, gefolgt von B. megaterium, verglichen mit nicht beimpften Pflanzen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At recent days Egypt is severely suffering from a lack of fresh irrigation water particularly after the building of the Grand Ethiopian Renaissance Dam (Hegazi et al. 2015). Such situation pushed the farmers to utilize semi-saline underground water or reuse of poor-quality drainage water (Hegazi et al. 2017). The effects of salinity on plants include ion toxicity, osmotic stress, impaired growth, mineral deficiencies, photosynthetic imbalance, and combinations of these effects (Galmés et al. 2011). A lot of studies worked to reduce salinity negative effects on the growth and production of various vegetable crops (Ashraf and Foolad 2007; Gupta and Huang 2014; Hegazi et al. 2015, 2017). Green bean is a low-cost and nutritious food from the fabaceae family (Farooq et al. 2017) and also it is a major protein source for Egyptian families (Abdel-Mawgoud 2006). Arbuscular mycorrhizal fungi (AMF) show a positive effect on abiotic stress (Augé 2001). The alleviation of salt stress in plants by AMF is mediated by growth hormones (Barker and Tagu 2000). Various studies revealed that AMF improve plant growth and yield under salt stress conditions (Al-Karaki et al. 2001). Plant growth-promoting rhizobacteria (PGPR) improve plant growth and yield (Noel et al. 1996). It can also protect plants from the deleterious effects of environmental stresses, including flooding (Grichko and Glick 2001), drought (Mayak et al. 2004a), salt (Mayak et al. 2004b). The B. megaterium strain grows in both the rhizosphere and the roots. This bacterial strain promotes clover growth under drought conditions. Also, this B. megaterium strain increases its own levels of proline and indole acetic acid (an auxin) when grown in vitro under osmotic stress conditions without affecting its own growth (Marulanda et al. 2009). Thus, it is approved that a specific B. megaterium strain is capable to amend the plant response to numerous abiotic stresses in different plant species. For this reasons, this study is an attempt to discover an applied technique to alleviate the harmful effect of salinity on growth, yield and the quality of green bean plants using B. megaterium (PGPR) and AMF.
Materials and Methods
Location and Growth Conditions
Two pot experiments were proposed out at the Experimental Station Farm, Faculty of Agriculture, Ain Shams University, Cairo, Egypt (30° 4′ 37.28N, 31° 17′ 6.06″ E), during the two seasons (2017 and 2018). Green bean plants (Phaseolus vulgaris L.) cv. Valentino (a bush bean of the fine type group) seeds were seeded on the 1st of September in both seasons in black polyethylene bags weighted 15 kg of washed sand (soil physical properties were 89.4% sand, 6.9% silt, and 3.7% clay with a pH of 7.8, EC of 1.68 dS/m). The experimental location has a prevalent arid weather, with cool winters and a warm dry summer.
Plant Material and Treatments
The present trial incorporated four NaCl treatments (1000, 2000, 3000 and 4000 ppm) with two anti-salinity applications (B. megaterium, AMF and a control without treatment). Normal farming practices were followed for green bean production, and disease and pest control were followed referring to the recommendation of the Egyptian Ministry of Agriculture. Seeds were seeded in four pits per pot. Ten days after seed germination, seedlings were thinned to five plants per pot/replicate, and after 21 days, NaCl treatments were started. Each experimental plot involved 15 pots arranged in 3 rows, with 5 pots in each row. Two litres of Hoagland’s nutrient solution (full strength) was applied weekly to each pot. The experiment was organized in a completely randomized design with three replicates.
Inoculation with Bacillus megaterium and AMF
The AMF treatments used in this study were a non-mycorrhizal inoculum as a control and a mycorrhizal inoculum (a mixture of stock cultures of Glomus spp. isolate) created by adding 100 g of a mycorrhizal inoculum per bag at sowing time. Inocula consisted of spores, extra-radical mycelium, and mycorrhizal roots, which were achieved from the Department of Agricultural Microbiology, College of Agriculture, Ain Shams University. The active strain of B. megaterium (108 CFU/ml) was added at 10 ml/pot. The soil inoculation with B. megaterium and AMF was repeated three times at 15-day intervals.
Sampling and Data Recording
Vegetative Growth Parameters
A random sample of three plants/replicate was collected 45 days after sowing to evaluate vegetative growth elements, namely plant length, shoot fresh and dry weight, and root fresh and dry weight. The plants were weighed to record the plant fresh weight and were placed in an oven at 70 ºC until a constant weight was achieved, which was recorded as the plant dry weight.
Determination of Photosynthetic Pigments
At 45 days after sowing, a portable chlorophyll meter (SPAD-502; Konica Minolta Sensing, Inc., Japan) was used to determine the leaf greenness of the plants. For each plant, measurements were taken at four locations on each leaf (two on each side of the midrib on all fully expanded leaves) and then averaged (Khan et al. 2003).
Determination of Membrane Permeability (MP)
To measure membrane permeability (MP), 10 leaf discs (10 mm in diameter) from the young, fully expanded leaves from two plants per replicate were placed in 50-ml glass vials and washed with distilled water to remove electrolytes released during leaf disc excision. Electrolyte leakage was calculated as a percentage of EC1/EC2 (Shi et al. 2006).
Determination of Leaf Relative Water Content (LRWC)
Leaf samples were taken from two plants per replicate (the sixth leaf from the top) to determine fresh weight (FW), dry weight (DW) and turgid weight (TW). FW, TW, and DW values were used to calculate LRWC using the following equation (Kaya et al. 2003): LRWC (%) = [(FW − DW)/(TW − DW)] × 100.
Biochemical Composition
Determination of Catalase Activity (CAT)
CAT activity was measured following a published method (Montavon et al. 2007). Fresh tissues were homogenized in 50 mmol sodium phosphate buffer (pH 7.0, 1/10, w/v) including 150 mmol NaCl and 0.5 mmol EDTA. One unit of CAT activity was expressed as the amount of enzyme needed to reduce 1 µmol of H2O2 per min CAT activity and was expressed as unit min−1 mg−1 protein.
Determination of Superoxide Dismutase Activity (SOD)
The activity of superoxide dismutase (EC1.15.1.1) was analysed using the method of Beauchamp and Fridovich (1971) by measuring the ability of the enzyme to inhibit the photochemical reduction of nitro blue tetrazolium (NBT). A reaction mixture (3 ml) containing 40 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 µM NBT, 2 µM riboflavin, 0.1 mM EDTA and 100 µl of crude enzyme extract was shaken and placed 30 cm below a 15 W fluorescent lamp as a light source. The absorbance was recorded at 650 nm. One unit of SOD activity is the amount of protein required to inhibit 50% of the initial reduction of NBT under light. The activity of SOD was expressed as unit min−1 mg−1 protein.
Determination of Peroxidase Activity (POD)
The activity of peroxidase (EC1.11.1.7) was assayed by the method of Hammmerschmidt et al. (1982). The reaction mixture (2.9 ml) consisted of 0.25% (v/v) guaiacol in 10 mM sodium phosphate buffer (pH 6 containing 10 mM H2O2). One hundred microlitres of crude enzyme extract was added to initiate the reaction, which was measured spectrophotometrically (CT200 spectrophotometer) at 470 nm per min. One international unit (IU) of enzyme activity was expressed as ∆OD = 0.01, and POX activity was expressed as unit min−1 mg−1 protein.
Determination of Na, K, Cl and P Concentrations
The extraction and determination of Na and K concentrations were conducted according to (Xu et al. 2006). Na and K concentrations were measured using an atomic absorption spectrophotometer (Varian spectra AA 220, Varian, Palo Alto, CA, USA). Chloride was measured using an ion chromatography analyser (Model 926, Sherwood Scientific Ltd., Cambridge, UK). Total phosphorus was determined using a spectrophotometer according to the method of Jackson (1973).
Pod Yield Parameters
Green pods were collected 55–60 days after sowing, and the total number of pods per plant, pod length and pod yield (kg/m2) were calculated according to AOAC (1990).
Experimental Design and Statistical Analysis
Data for all experiments were investigated by analysis of variance (ANOVA) using the general linear model procedure of CoState. The significance of differences between means was tested using an “F” test, and the LSD (p = 0.05) was calculated (Snedecor and Cochran 1967).
Results and Discussion
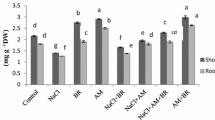
Vegetative Growth
The results in Table 1 show that plants grown in 4000 ppm were significantly harmfully affected, as validated by the vegetative growth traits of green bean plants (e.g., plant length, shoot fresh and dry weight, and root fresh and dry weight). The best growth significantly occurred with 1000 ppm. Salinity amelioration treatments (Mycorrhiza and B. megaterium) compared to uninoculated plants (control), upgraded all mentioned plant vegetative growth characteristics affected by salt stress. B. megaterium was significantly best in mitigating the salt stress effects at low level (1000 ppm) but it was moderately neutralising the destructive effects of high-salt stress (4000 ppm) for all vegetative growth parameters, followed by Mycorrhiza application. Similar trend was detected in the two seasons. The obtained results confirmed previous findings of Ferri et al. (2000) who observed that salt stress reduced the growth of common bean (P. vulgaris L). However, AMF was more effective in improving growth of Chickpea and Zucchini plants (Garg and Bhandari 2016; and Colla et al. 2008). AMF gave a progressive general impact on plant biomass under salt stress conditions in sweet pepper plants (Tian et al. 2004). Other studies conveyed similar growth promotion in various leguminous vegetable crops with AM inoculation. These crops included green beans (Neeraj and Singh 2005; Salim and Abou El-Yazied 2015) and pea (Estaún et al. 1987). This effect could be related to a sufficient supply of nutrients (principally phosphorus), with the support of AMF in the host plant (Marschner 1986; Al-Karaki 2000; and Ghoname et al.2012). B. megaterium retained a better root growth, which is considered a salt tolerance trait (Farooq et al. 2017). The B. megaterium strain supposed to produce indole-3-acetic acid (an auxin) (Marulanda et al. 2009), which might lead to better root growth and development under saline conditions (Wang et al. 2009). A lot of studies show that the PGPR led to increase root fresh weight and dry weight and shoot fresh weight and dry weight in okra (Habib et al. 2016) and strawberry (Karlidag et al. 2013). PGPR isolates can lessen salt stress effect in plants and enhance shoot/root length, dry matter production in numerous plants (Egamberdieva and Lugtenberg 2014). The progressive effects of PGPR treatments on growth of plants can be attributed to the release of phytohormones, such as indole-3-acetic acid and cytokinins as well as N2 fixation, phosphate-solubilizing, and production of antimicrobial materials., amino acids, and enzymes, (Gunes et al. 2015).
Photosynthetic Pigments
Data in Fig. 1 show that chlorophyll concentrations were significantly better with lower salinity level (1000 ppm) and were considerably lower with the high salinity level (4000 ppm) which gave the lowest chlorophyll concentration. Anti-salinity applications positively minimized the harmful effect of salt stress and generated a stimulatory effect on all plant photosynthetic pigment concentrations when compared to uninoculated plants. Generally at low salinity level (1000 ppm), B. megaterium enhanced chlorophyll content followed by AMF, with no significant differences between them. Higher chlorophyll content in the leaves of mycorrhiza-inoculated plants under salt stress conditions was conveyed by numerous investigators (Giri and Mukerji 2004; Shekoofeh and Sepideh 2011; and Elhindi et al. 2017). The chlorophyll decline under salt stress can be accredited to the antagonistic effects of Na+ ions on Mg2+ absorption. AMF improved the absorption of Mg2+ ions, which in turn can intensify chlorophyll synthesis in salt-stressed plants (Giri and Mukerji 2004). The improved photosynthetic pigmentation due to mycorrhizal colonization in plants might be attributed to the inhibition of Na+ transport, which leads to better function of the photosynthetic mechanism (Borde et al. 2010; García-Garrido and Ocampo 2002). Similar improvement in chlorophyll content were stated after mycorrhizal inoculation in cowpea (Arumugam et al. 2010), fava bean (Ismaiel et al. 2014), snap bean (Salim and Abou El-Yazied 2015), pea (Shinde and Thakur 2015), and lentil (Yaseen et al. 2016). In this respect, Yildirim et al. (2008) reported that PGPR improved the chlorophyll contents of radish leaves under salt stress. PGPR inoculation alleviated the damaging effects of salinity which improved the chlorophyll synthesis, thus enhancing salt tolerance in strawberry plants (Karlidag et al. 2013).
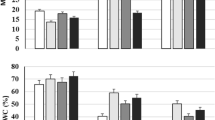
Leaf Relative Water Content (LRWC) and Membrane Permeability (MP)
Results shown in Fig. 2 pointed that high salinity stress level (4000 ppm) deleteriously affected leaf relative water content (LRWC) and membrane permeability (MP), with significant differences among salinity levels. Salinity amelioration treatments positively improved LRWC and significantly reduced the damaging effect of salinity on MP compared to uninoculated plants, with significant differences between the inoculated plants and uninoculated plants (control). The achieved results confirmed former reports of (Kaya et al. 2009), which revealed that pigeon pea (Cajanus cajan) demonstrated higher relative MP when treated with AMF. Moreover, the electrical conductivity of mycorrhizal plants was higher in pigeon pea plant roots (Garg and Manchanda 2008). As well, Colla et al. (2008) conveyed water status enhancement of Zucchini plants colonized by Glomus intraradices when exposed to salinity stress. PGRB treatments initiated an increment in LRWC under salt stress (Yildirim et al. 2008). Additional investigation of Yildirim et al. (2008) indicated that PGRB-inoculated plants had better electrolyte leakage than their relevant uninoculated controls.
Antioxidant Enzyme Activity
Antioxidant enzyme activity (CAT, POD and SOD), as demonstrated in Fig. 2, revealed a significant progressive effect with salinity amelioration application. The best results were obtained by B. megaterium. The most negative effect of high saline condition (4000 ppm) was recorded in the non-inoculated plants (control) On the other hand, salinity amelioration treatments significantly increased enzyme activity at the 1000 ppm salinity levels. Previous data for other investigations showed that both salt and drought stress could induce oxidative stress, as indicated by the increased level of lipid peroxidation (Hossain et al. 2015). The activities of SOD and POD were increased by salt and drought stress, but activity of CAT was declined (Pan et al. 2006). To resist the stressful environment, plants provides numerous antioxidant enzymes to preserve them from the damaging effects of ROS. So, antioxidative enzymes have a key character as a resistance mechanism in several plant species (Koyro et al. 2012). Improved antioxidant enzymes involved with AMF plants have been verified by many researchers. Mycorrhizal plants had a higher accumulation of antioxidative enzymes and thus improve general plant growth under stress (Miller et al. 2010; Scheibe and Beck 2011). Similar results were conveyed by Han and Lee (2005) for lettuce. Heidari and Golpayegani (2012) indicated that inoculation with Bacillus lentus and Azospirillum rasilens significantly increased Ascorbate peroxidase and Glutathione peroxidase activity of basil leaves grown under drought stress.
Mineral Content
The data in Fig. 3 show that highest Na+ and Cl− content was obtained with the highest salinity stress level (4000 ppm) while, the maximum K+ and P levels were achieved with the low salinity stress level (1000 ppm). The salinity amelioration treatments led to a significant increases in K+ and P, level with a premier result obtained with B. megaterium treatment. There were a significant differences between salinity amelioration treatments and the control (uninoculated) on Na+ and Cl− content. The achieved results confirmed those of a previous study (Juniper and Abbott 1993), who revealed that salinity initiates an imbalances in the K+/Na+ ratio, and harmfully affecting plant growth. As mycorrhizal plants retain higher Na+/K+ (higher K+ uptake in shoots), such plants by a dilution effect has the ability to alleviate salt stress. Under saline conditions, higher levels of sodium (Na+) not only interrupt the uptake of other nutrients but also lead to specific ion toxicity (Ashraf 1994). A high K+/Na+ ratio is essential for salinity tolerance and preservation of plant osmotic potential (Hamdia et al. 2004). The promoting effect of mycorrhizal inoculation on snap bean growth is possibly due to the effects of AMF on enhancing soil structure (Miller and Jastrow 2000). Also, salt stressed zucchini plants when colonized by Glomus intraradices had a better nutrient content (Colla et al. 2008). Growth improvement of plants inoculated with AMF has been partially correlated to that mycorrhiza enriched nutrient accomplishment, particularly P nutrition (Sharifi et al. 2007). Furthermore, PGPR can improve the uptake of mineral element by motivating root formation and growth (Yildirim et al. 2008).
Pod Yield Parameters
The collected data in Table 2 show that yield (number of pods per plant, total pods yield per plant and average pods weight) was positively superior under low salinity level (1000 ppm). Meanwhile, low salinity level gave the maximum yield compared to the highest salinity stress level (4000 ppm). However, anti-salinity applications positively enhanced the pod yield of green bean plants under saline stress conditions. Hence, the highest pod yields of bean plants were significantly observed with B. megaterium, followed by AMF, compared with control (uninoculated) plants at all salinity stress levels. Similar findings of Khan et al. (2016) showed that salinity stress delayed flowering and lessened flower numbers and pod set in green bean plants. Youssef et al. (2017) showed that AMF inoculation significantly increased pod number and green pods yield of snap bean. Also, Colla et al. (2008) conveyed an improvement in fruit quality of Glomus intraradices colonized zucchini plants grown under salinity stress. Al-Karaki (2000) indicated a higher fresh fruit yield, fruit weight and fruit number in a mycorrhizal inoculated.
Conclusion
This investigation affirmed the significance of both AMF and Bacillus megaterium application on ameliorating the negative effect of salinity on growth and pod yield of green beans. Chlorophyll and antioxidant enzymatic activity at all verified salinity levels were markedly enhanced by AMF and Bacillus megaterium treatments compared with non-inoculated plants (control) which showed severe growth retardation especially under the higher salt concentration.
References
Abdel-Mawgoud AMR (2006) Growth, yield and quality of green bean (Phaseolus vulgaris) in response to irrigation and compost applications. J Appl Sci Res 2(7):443–450
Al-Karaki GN (2000) Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza, vol 10, pp 51–54
Al-Karaki GN, Hammad R, Rusan M (2001) Response of two tomato cultivars differing in salt tolerance toinoculation with mycorrhizal fungi under salt stress. Mycorrhiza 11:43–47. https://doi.org/10.1007/s005720100098
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington DC
Arumugam R, Rajasekaran S, Nagarajan SM (2010) Response of Arbuscularmycorrhizal fungi and Rhizobium inoculation on growth and chlorophyll content of Vignaunguiculata (L) Walp Var. Pusa 151. J Appl Sci Environ Manage. https://doi.org/10.4314/jasem.v14i4.63282
Ashraf M (1994) Organic substances responsible for salt tolerance in Eruca sativa. Biol Plant 36:255–259
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Augé RM (2001) Water relations, drought and vesicular-arbuscularmycorrhizal symbiosis. Mycorrhiza 11:3–42. https://doi.org/10.1007/s005720100097
Barker SJ, Tagu D (2000) The roles of auxins and cytokinins in mycorrhizal symbioses. J Plant Growth Regul 19:144–154
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Borde M, Dudhane M, Jite PK (2010) AM fungi influences the photosynthetic activity, growth and antioxidant enzymes in Allium sativum L. under salinity condition. Not Sci Biol 2(4):64–71
Colla G, Rouphael Y, Cardarelli M, Tullio M, Rivera CM, Rea E (2008) Alleviation of salt stress by arbuscularmycorrhizal in zucchini plants grown at low and high phosphorus concentration. Biol Fertil Soils 44:501–509
Egamberdieva D, Lugtenberg B (2014) Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses, vol 1. Springer, New York, pp 73–96
Elhindi KM, El-Din AS, Elgorban AM (2017) The impact of arbuscularmycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimumbasilicum L.). Saudi J Biol Sci 24(1):170–179. https://doi.org/10.1016/j.sjbs.2016.02.010
Estaún V, Calvet C, Hayman DS (1987) Influence of plant genotype on mycorrhizal infection: response of three pea cultivars. Plant Soil 103(2):296–298. https://doi.org/10.1007/bf02370406
Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N et al (2017) Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem 118:199–217. https://doi.org/10.1016/j.plaphy.2017.06.020
Ferri A, Lluch C, Ocaña A (2000) Effect of salt stress on carbon metabolism and bacteriod respiration in root nodules of common bean (PhaseolusvulgariesL.). Plant Biol 2:396–402. https://doi.org/10.1055/s-2000-5956
Galmés J, Ribas-Carbó M, Medrano H, Flexas J (2011) Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J Exp Bot 62:653–665. https://doi.org/10.1093/jxb/erq303
García‐Garrido JM, Ocampo JA (2002) Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J Exp Bot 53(373):1377–1386. https://doi.org/10.1093/jexbot/53.373.1377
Garg N, Bhandari P (2016) Silicon nutrition and mycorrhizal inoculations improve growth nutrient status, K +/Na+ ratio and yield of Cicer arietinum L. Genotypes under salinity stress. Plant Growth Regul 78:371–387. https://doi.org/10.1007/s10725-015-0099-x
Garg N, Manchanda G (2008) Effect of arbuscularmycorrhizal inoculation of saltinduced nodule senescence in CajanuscajanL. (pigeonpea). J Plant Growth Regul 27:115–124. https://doi.org/10.1007/s00344-007-9038-z
Ghoname AA, El-Bassiouny AM, Abdel-Mawgoud AMR, El-Tohamy WA, Gruda N (2012) Growth, yield and blossom-end rot incidence in Bell pepper as affected by phosphorus level and amino acid applications. Gesunde Pflanzen 64:29–37
Giri B, Mukerji KG (2004) Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbaniagrandiflora under field condition: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 14:307–312
Grichko VP, Glick BR (2001) Amelioration offlooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem 39:11–17
Gunes A, Karagoz K, Turan M, Kotan R, Yildirim E, Cakmakci R, Sahın F (2015) Fertilizer efficiency of some plant growth promoting rhizobacteria for plant growth. Res J Soil Biol 7(2):28–45. https://doi.org/10.3923/rjsb.2015.28.45
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecularcharacterization. Int J Genom. https://doi.org/10.1155/2014/701596
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Research International. https://doi.org/10.1155/2016/6284547
Hamdia MA, Shaddad MAK, Doaa MM (2004) Mechanisms of salt tolerance and interactive effect of Azospirillum brasilense inoculation on maize cultivars grown under salt stress. Plant Growth Regul 44:165–174
Hammerschmidt R, Nuckles EM, Kuc J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichumlagenarium. Physiol Plant Pathol 20:73–82
Han HS, Lee KD (2005) Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res J Agric Biol Sci 1(3):210–215
Hegazi AM, El-Shraiy AM, Ghoname AA (2015) Alleviation of salt stress adverse effect and enhancing phenolic anti-oxidant content of eggplant by seaweed extract. Gesunde Pflanzen 67(1):21–31
Hegazi AM, El-Shraiy AM, Ghoname AA (2017) Mitigation of salt stress negative effects on sweet pepper using ArbuscularMycorrhizal fungi (AMF), bacillus megaterium and Brassinosteroids (BRs). Gesunde Pflanzen 69(2):91–102
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agricul Sci 11(1):57–61. https://doi.org/10.1016/j.jssas.2011.09.001
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M et al (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Ismaiel AA, Hegazy HS, Azb MA (2014) Physiological response of Viciafaba L. to inoculation with Rhizobium and arbuscularmycorrhizal fungi: comparative study for irrigation with Nile water and wastewater. Aust J Crop Sci 8(5):781–790
Jackson ML (1973) Soil chemical analysis. Prentice-Hall, Englewood Cliffs
Juniper S, Abbott L (1993) Vesicular-Arbuscularmycorrhizas and soil salinity. Mycorrhiza 4:45–58
Karlidag H, Yildirim E, Turan M, Pehluvan M, Donmez F (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria× ananassa). HortScience 48(5):563–567
Kaya C, Higgs D, Ince F, Amador BM, Cakir A, Sakar E (2003) Ameliorative effects of potassium phosphate on salt-stressed pepper and cucumber. J Plant Nutr 26:807–820
Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA (2009) The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci Hortic 121:1–6. https://doi.org/10.1016/j.scienta.2009.01.001
Khan HA, Siddique KHM, Colmer TD (2016) Vegetative and reproductive growth of salt-stressed chickpea are carbon-limited: sucrose infusion at the reproductive stage improves salt tolerance. J Exp Bot. https://doi.org/10.1093/jxb/erw177
Khan W, Prithiviraj B, Smith DL (2003) Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol 160(5):485–492
Koyro HW, Ahmad P, Geissler N (2012) Abiotic stress responses in plants: an overview. In: Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 1–28
Marschner H. (1986) Mineral nutrition in higher plants. Wd Ltd. The Greystone Press, Antrim, Northern Ireland
Marulanda A, Barea JM, Azcón R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial eVectiveness. J Plant Growth Regul 28:115–124
Mayak S, Tirosh T, Glick BR (2004a) Plant growth-promoting bacteria that confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Mayak S, Tirosh T, Glick BR (2004b) Plant growth-promoting bacteria that confer resistance to water stress in tomato and pepper. Plant Sci 166:525–530
Miller RM, Jastrow JD (2000) Mycorrhizal fungi influence soil structure. In: Kapulnik Y, Douds DD Jr. (eds) Arbuscularmycorrhizas physiology and function. Kluwer, Dordrecht, pp 3–18
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stress. Plant Cell Environ 33:453–467
Montavon P, Kukic KR, Bortlik K (2007) A simple method to measure effective catalase activities: optimization, validation, and application in green coffee. Anal Biochem 360:207–215
Neeraj K, Singh K (2005) Impact of VA-mycorrhiza, Rhizobium and phosphorus on growth and yield of Phaseolus vulgaris L. J Phytol Res 18(1):59–63
Noel TC, Sheng C, Yost CK, Pharis RP, Hynes MF (1996) Rhizobiumleguminosarumas a plant growth-promoting rhizobacterium: direct growth promotion of canola and lettuce. Can J Microbiol 42:279–283
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49(2–3):157–165
Salim BBM, Abou El-Yazied A (2015) Effect of mycorrhiza on growth, biochemical constituents and yield of snap bean plants under water deficit conditions. J Hortic Sci Ornam Plants 7(3):131–140
Scheibe R, Beck E (2011) Drought, desiccation, and oxidative stress. In: Lüttge U, Bech E, Bartels D (eds) Plant desiccation tolerance, vol 215. Springer, Berlin, pp 209–231
Sharifi M, Ghorbanli M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with pre-treated mycorrhizal fungi. J Plant Physiol 164:1144–1151. https://doi.org/10.1016/j.jplph.2006.06.016
Shekoofeh E, Sepideh H (2011) Effect of mycorrhizal fungi on some physiological characteristics of salt stressed Ocimumbasilicum L. Iraninan. J Plant Physiol 1(4):215–222
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135
Shinde BP, Thakur J (2015) Influence of arbuscularmycorrhizal fungi on chlorophyll, proteins, proline and total carbohydrates content of the pea plant under water stress condition. Int J Curr Microbiol App Sci 4(1):809–821
Snedecor GW, Cochran WG (1967) Statistical methods, 6th edn. Iowa state University, Ames
Tian CY, Feng G, Li XL, Zhang FS (2004) Different effects of arbuscularmycorrhizal fungal isolates from saline or non-saline on salinity tolerance of plants. Appl Soil Ecol 26:143–148
Wang Y, Li K, Li X (2009) Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J Plant Physiol 166:1637–1645
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell, 125:1347–1360
Yaseen T, Ali KAWSAR, Munsif F, Rab A, Ahmad M, Israr M, Baraich K (2016) Influence of arbuscularmycorrhizal fungi, Rhizobium inoculation and rock phosphate on growth and quality of lentil. Pak J Bot 48(5):2101–2107
Yildirim E, Turan M, Donmez MF (2008) Mitigation of salt stress in radish (Raphanus sativus L.) by plant growth promoting rhizobacteria. Roumanian Biotechnol Lett 13:3933–3943
Youssef SM, Riad GS, Elhady SAA (2017) Effect of phosphorus sources and Arbuscular Mycorrhizal inoculation on growth and productivity of snap bean (Phaseolus vulgaris L.). Gesunde Pflanzen 69(3):139–148
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.A. Abdel Motaleb, S.A. Abd Elhady and A.A. Ghoname declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Abdel Motaleb, N.A., Abd Elhady, S.A. & Ghoname, A.A. AMF and Bacillus megaterium Neutralize the Harmful Effects of Salt Stress On Bean Plants. Gesunde Pflanzen 72, 29–39 (2020). https://doi.org/10.1007/s10343-019-00480-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-019-00480-8
Keywords
- Salt stress
- Arbuscular mycorrhizal fungi
- Bacillus megaterium
- Antioxidant enzymes
- Membrane permeability