Abstract
Recent studies have highlighted that higher species richness can increase the resistance and/or the resilience to disturbances and stresses. The present study quantifies the overall tree species richness effect on growth and intrinsic water-use efficiency (iWUE) in three target tree species (i.e. Fraxinus excelsior, Juglans spp. and Prunus avium) after drought in six deciduous plantations in the Friuli-Venezia Giulia plain, North-eastern Italy. Planting densities, management, climatic and soil characteristics were the same at all the plantations. Stands differed only for their total surface area and for their total tree species richness (3, 4, 6, 7, 8 and 9). We double-sampled 15 dominant trees for each of the three target species, we measured tree-ring width, and we removed age-related trends using a detrending function. We selected 2006 as the driest year at the sites and 2014 as the reference year using the De Martonne Index. For both years, we measured δ13C signature in tree rings to calculate iWUE. Tree species richness had a positive effect on the response to drought both in terms of normalized ring width and iWUE, but only at a lower number of consociated species (< 5), when facilitation and/or complementarity mechanisms prevailed. Instead, negative responses were typical at higher levels (≥ 5), when competition was the dominant process within the stand. Moreover, species richness had no effect on tree growth in 2014, maybe because either competition or complementarity processes did not occur, or these processes could cancel out each other, when environmental conditions were not limiting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last decades, anthropogenic impacts are threatening natural ecosystems worldwide and are exacerbating extreme climate events, which are increasing both in intensity and in frequency (IPCC 2014; Trenberth et al. 2014; Williams et al. 2013). Consequently, forests are becoming more vulnerable to these events (i.e. windstorms, drought events, heatwaves or wildfires), which are causing increasingly loss of biodiversity and loss in several ecosystem functions (EFs) and services (ESs) (Allen et al. 2010; Scholze et al. 2006). In fact, stresses and disturbances may induce changes of ecosystem structure and species composition, leading to multiple and less predictable successional pathways and influence the overall ecosystem functioning (Beckage et al. 2008; Christmas et al. 2016; Jump and Peñuelas 2005; Kremer et al. 2012; Parmesan 2006). Thus, it has become important to understand the extreme events’ effects on forest ecosystems in order to identify and adopt the best management and mitigation policies and practices in the short/medium term (Mori et al. 2017).
Over the last three decades, findings in community ecology have led to the widespread agreement that species diversity plays a critical role for EFs and ESs (Cardinale et al. 2012; Hector et al. 2011; Morin et al. 2011; Jactel et al. 2018; Ammer 2019). Some earlier studies have also highlighted that higher species richness can increase the resistance and/or the resilience to disturbances and stresses, especially to pests, pathogens and other diseases (Jactel and Brockerhoff 2007; Zhu et al. 2000). However, studies about the role of species diversity in enhancing forest resistance (i.e. the ability to withstand harsh events) and/or resilience (i.e. the ability to regain the pre-disturbance growth rates) (Merlin et al. 2015) to climatic extremes have been published only recently (Baeten et al. 2013; Bruelheide et al. 2014; Verheyen et al. 2015; Grossiord 2019). Even if the effects of tree species diversity on the response to drought and heatwaves are still debated and the results are often contrasting (Yin and Bauerle 2017; Grossiord 2019), there is a general agreement that tree species diversity could eventually contribute in reducing the ecosystem’s vulnerability to these stresses through resources partitioning, facilitation and selection effects (Grossiord 2019). During drought events, plant’s physiological activity and growth can be seriously compromised: hydraulic failure and/or depletion of carbon (C) pools (i.e. C starvation), interacting with pests and other biotic attacks, are considered the main factors leading in reduced growth or higher tree mortality (McDowell 2011). In some cases, different tree species, with different functional traits (e.g. including genetic diversity and physiological characteristics), both above and below ground, have been seen to positively influence forest resistance to drought with a better resource acquisition and/or use (Lebourgeois et al. 2013; Pretzsch et al. 2013). In fact, species diversity has been reported to increase the temporal stability of forest growth, thanks to the different responses of the single tree species to fluctuating environmental conditions (Aussenac et al. 2017) as well as thanks to the existing interspecific interactions (Aussenac et al. 2019; Steckel et al. 2020). In particular, root stratification (Schwendenmann et al. 2015), different stomatal egulation strategies (West et al. 2012) as well as active hydraulic redistribution (Zou et al. 2005) and nocturnal water release (Prieto et al. 2012) have been considered the major mechanisms behind the increased resistance/resilience to drought in tree mixtures. However, several other studies have shown that species sharing the same ecological niches strongly compete for the same resources, thus leading in a reduced resistance or resilience to drought (Grossiord et al. 2014a, b; Ammer 2019; Grossiord 2019).
In most of this studies, dendrochronological analyses have been used to assess the impact of well- documented dry years on forests and targeted tree species (Lloret et al. 2011; Merlin et al. 2015; Vitali et al. 2017; Steckel et al. 2020). Such data have been sometimes combined with stable C isotope analyses to compare dry and wet years and to better underline the effects of drought on tree physiological processes (Grossiord et al. 2014a; Guerrieri et al. 2019; Schwarz and Bauhus 2019). In fact, every tree ring is the result of plant-intrinsic factors (i.e. photosynthetic rate, water potential regulation, hormonal regulation) and plant-external factors (i.e. climate, pathogens, stress factors), which can modulate the quantity (i.e. width) and the composition (i.e. isotopic signature) of annual wood production (Battipaglia et al. 2009; De Micco et al. 2010; Deslauriers and Morin 2005; Lupi et al. 2010). Hot and dry vegetative seasons can lead plants to important hydraulic deficits coupled to drops in photosynthetic rate. As a result, annual tree rings will be thinner than those formed during favourable seasons (Gao et al. 2018). On the other hand, the analysis of stable C isotopes in tree rings gives additional information about the occurrence of drought events and on the amount of carbon assimilated as biomass per unit of water used by trees (i.e. intrinsic water-use efficiency; iWUE) (Farquhar et al. 1982). During drought events, plants can close stomata to avoid unnecessary water loss. Consequently, stomatal conductance to CO2 decreases and rubisco fixes a higher-than-usual proportion of 13C compared to 12C. The results are rings enriched in heavier isotope, which translates in less negative isotopic signatures (Francey and Farquhar 1982). Few studies (Li 1999; Walker et al. 2015) have specifically investigated, so far, the role of tree species richness on the tree growth’s response to drought by combining both dendrochronological indexes and C isotope in forests or in controlled experiments.
In Friuli-Venezia Giulia plain (North-eastern Italy), several deciduous plantations were established at the end of last century to sustain regional wood production, but also to increase C storage and/or biodiversity. Such plantations might be composed by target tree species only (i.e. commercial ones) or by target species consociated with accessory tree species. The same climate and soil conditions make these plantations a useful tree species richness gradient experiment in homogenous environmental conditions to assess the role of tree diversity in mitigating extreme event impacts on forest ecosystems. The aim of our study was to understand the role of the overall tree species richness in determining growth and iWUE patterns in the target tree species (i.e. Fraxinus excelsior, Juglans spp. and Prunus avium) after drought, by coupling dendrochronology and C isotope analysis. We hypothesized that the higher is the number of consociated species (i.e. tree species’ diversity), the lower is the impact of drought on tree growth and iWUE.
Materials and methods
Study area
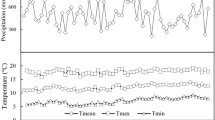
Six plantations were identified in Friuli-Venezia Giulia plain (Italy; 46°5′50″N, 13°1′7″E, 121 a.s.l.). Climatic conditions and soil characteristics were the same across all sites: mean annual temperature was 13.3 °C and mean annual precipitation was 1500 mm from 1995 to 2017 data recorded at Fagagna meteorological station (46°06′51″N; 13°04′50″E; www.osmer.fvg.it); soil was alluvial mesic Udifluvent (sand 58%, loam 28%, clay 14%). All plantations were established in 1999 and stand planting density was between 1905 and 2020 plant ha−1. No fertilization, irrigation (with the exception of emergency irrigation during the first 4–5 years) and pruning have been performed at all sites. Plantations differed only for their total surface area and for tree species richness (3, 4, 6, 7, 8 and 9). A detailed description of each selected stand (i.e. species composition and dendrometric characteristics) is reported in Online resource 1 and 2 and in Palandrani and Alberti (2020).
Sampling
The study focused on the three commercial tree species (target species), which were present at all sites (i.e. Fraxinus excelsior, Juglans spp. and Prunus avium). In each plantation, 15 dominant trees for each target species (45 trees per site) were selected in 2018, avoiding individuals at the border of the stand. Two perpendicular wood cores at 30 cm height above the ground for each selected individual were taken using an increment borer, stored in cardboard and naturally dried. Once in the laboratory, samples were glued on woody supports and sanded with paper with 60, 120, 240, 320 and 600 grit to make tree rings more visible.
Dendrochronological analysis
Tree-ring width was estimated for each core using Dendrotab 2003 (© Walesch Electronic GmbH). Chronologies were visually and statistically cross-dated using TSAP (© TSAPWin Scientific, version 4.81, 2002–2018, Frank Rinn/RINNTECH) and COFECHA (Grissino-Mayer 2001; Holmes 1983) softwares, respectively. Cross-dated chronologies were then normalized using the Hugershoff correction (Fang et al. 2010; Warren 1980; Warren and MacWilliam 1981) with the function detrend in dplR package in RStudio (© Rstudio, version 1.2.1335, ©2009-2019 Rstudio, inc.) in order to remove noises associated with age trends, which may be significant especially in young plants and during the first years of growth.
Climate index
The driest and the reference year (i.e. a year representing average climate conditions at the experimental sites, neither too warm and dry nor too cold and wet) since tree planting were identified by calculating the De Martonne Index (DMI; Maliva and Missimer 2012; Vitali et al. 2017):
where P is the total rainfall (mm) and T is the mean temperature (°C) at the experiment sites, calculated yearly on the vegetative season (from April to October) from 1995 to 2017 data from Fagagna meteorological station, the nearest station to our selected sites (www.osmer.fvg.it). We considered the years below the 10th percentile of DMI distribution as the driest and the years between the 45th and the 55th percentile as the references (i.e. years representing mean climate conditions at the experimental sites). Using such an approach, 2006 and 2014 were identified as the driest and the reference year, respectively. Even though 2003 was identified as the driest year ever, we decided to not consider it as the selected plantations were 4-year old, canopies were not completely closed and emergency irrigations were still performed according to local records.
Isotopes analysis and iWUE
Three of the collected wood cores for each species in each plot were randomly selected. Rings corresponding to 2006 and 2014 were accurately cut and stored in different plastic tubes. To avoid contamination, samples were washed with pure ethanol and dried at 40 °C overnight. Then, samples were milled and weighted in tin capsules and δ13C was assessed using a CHNS Elemental Analyser (Vario Microcube, © Elementar) coupled to a stable isotope ratio mass spectrometer (IRMS; Isoprime 100, © Elementar). The analytical precision for 13C analysis was less than 0.06‰.
We calculated iWUE (μmol CO2 mol H2O−1), defined as the ratio between photosynthesis and leaf transpiration, using δ13C values of the single tree ring according to the model of Farquhar et al. (1982):
knowing that C isotopic discrimination is equal to (Farquhar et al. 1982):
where Ca (ppm) is mean annual atmospheric CO2 concentration, a is the fractionation during CO2 diffusion (a = 4.4‰), b is the fractionation during carboxylation (b = 27‰), δ13Ca is the isotopic mean annual isotopic composition of atmosphere, and δ13Cp is the isotopic composition of tree-ring samples. Ca and δ13Ca were downloaded from Mauna Loa Observatory database (https://www.esrl.noaa.gov/gmd/).
Data analysis
We considered as control (ctrl) the plantation without any consociate species (plantation n. 1; Online resource 1), while we expressed tree species diversity in the other plantations as the total number of consociated species. Then, we compared normalized tree-ring width and iWUE of the target species between the selected years (2006 and 2014), between species richness and years × species richness using a two-way ANOVA. All data were eventually transformed before doing the statistical analysis to meet the requirements for parametric statistical tests using powerTransform and bcPower functions in car package. Post hoc Tukey test was done when a significant difference was detected. All statistical analysis were applied using RStudio software (Rstudio, version 1.2.1335, ©2009-2019 Rstudio, inc.).
Results
The annual growth rates of the three target tree species were similar, on average, across all the considered plantations both considering raw and normalized tree-ring width (Fig. 1a, b): all target species showed peaks in tree-ring width in 2002 and in 2004 followed by a constant decrease during the last 8–9 years. Similarly, they showed a significant drop in the growth rate in 2003, 2006 and 2015, while synchronous increases were recorded in 2007, 2014 and 2016. When normalized ring width is considered across all plantations, Fraxinus excelsior, Juglans sp.p. and Prunus avium grew 36%, 47%, 30% less in the driest (2006) than in the reference year (2014), respectively (p < 0.001; Fig. 2). Such a difference in ring width between those two years is also confirmed when each plantation (i.e. with different number of associated species) is considered individually (p < 0.05; Fig. 3). However, while in the driest year (2006) increasing the number of consociated species had a positive influence on the ring width of the target species (+ 14% when compared to control; p = 0.001), in the reference year (2014) such a difference was not significant (− 8%; p > 0.05; Fig. 3). Moreover, in 2006 the major difference (+ 23%, p < 0.001) was detected in the plantation with only one consociated species, while lower differences were found at higher levels (+ 3% and p = 0.30 when consociated species = 6). In 2014 (reference year), significant differences were only found between plantations with five and six consociated species (p = 0.002).
Normalized tree-ring width with the increase in associated tree species in the different plantations. “ctrl” represents the control plantation (i.e. the three commercial species, only). Black triangles indicate mean values for each boxplot. Different lowercase letters in “Ass. Sp.” box indicate significant differences among consociated species levels in 2006 (p < 0.05), while different capital letters in the same box indicate significant differences among plantations in 2014 (p < 0.05)
As far as C isotopic signature is concerned, δ13C values ranged from − 26.45 ± 0.65‰ and − 26.54 ± 0.84‰ in 2006 and 2014, respectively, reflecting typical ranges for C3 plants. We measured a significant lower mean iWUE across all plantations (− 6%; p < 0.001; Fig. 4) in the driest year (2006) than in the reference year (2014). Increasing the number of consociated species (Fig. 5) caused a significant difference in iWUE in the driest year, only (p = 0.02). In particular, major and significant differences with control were detected when five consociated tree species were present (+ 11%; p = 0.02), while minor and non-significant differences were found when six consociated species were present (+ 4%; p = 0.84).
Intrinsic water-use efficiency (iWUE μmol CO2 mol H2O−1) with increasing the number of associated species. “ctrl” represents the control plantation (i.e. the three commercial species, only). Black triangles indicate mean values for each boxplot. No significant differences have been found among 2006 (dry year) and 2014 (reference year) inside the same plantation along the entire richness gradient (p > 0.05). Different lowercase letters in “Ass. Sp.” box indicate significant differences among treatments in 2006 (p < 0.05), while different capital letters in the same box indicate significant differences among plantations in 2014 (p < 0.05)
Discussion
Tree species respond differently to drought events depending on their intensity, frequency and timing (i.e. spring or summer droughts) (Merlin et al. 2015), on tree age, site characteristics and forest management (Sohn et al. 2016).
In our study, we observed a strong age-related trend in tree-ring width for each of the considered commercial species (Fig. 1a): major widths were recorded between 2001 and 2005, followed by a subsequent and constant decline. Such a growth pattern reflects the typical growth behaviour in young trees (Fang et al. 2010), thus confirming our choice to use a normalizing function to eliminate these age-related trends (Fig. 1b). Each of the target tree species showed a significant drop in tree-ring width values during 2003, which was recorded as one of the driest and hottest years in Europe since the beginning of XX century (De Bono et al. 2004; Rebetez et al. 2006; Schär and Jendritzky 2004). Other growth declines were also observed in 2006 and in 2015, which have been reported as exceptionally hot and dry years at regional scale (www.osmer.fvg.it), as also confirmed by our DMI index analysis (Online resource 3). On the contrary, increases in the ring width values were measured in 2004 and 2014, years with vegetative seasons characterized by mild temperatures and relatively abundant precipitations (www.osmer.fvg.it).
Looking at normalized tree-ring widths, the three target species responded differently during the selected dry (2006) or the reference year (2014), with a 37% decrease in the former compared to the latter (Fig. 2). Such a difference was consistently maintained throughout the tree species richness gradient, even though with different intensities (from 48% in the reference to 23% in the plantation with one consociated tree species; Fig. 3). Moreover, comparing the control plantations to all the other stands, the difference in the normalized ring width values was more evident in the driest year than in the reference year (Fig. 3). Plantations with less than five consociated tree species may experience enhanced tree growth in the target commercial tree species through niche complementarity and/or facilitation processes (Pacala and Tilman 1994; Petchey 2003; Schoener 1974), while higher numbers of consociated species (≥ 5) may decrease growth during the harshest seasons because of competition for resources (Lang et al. 2010; Wagner and Radosevich 1998). Such a behaviour has been already observed in the literature, even though the majority of the studies have been performed on forest species as fir, pine, beech and spruce. Metz et al. (2016) found that European beech performed better (higher tree growth) when associated with pine compared to pure beech stands as tree ecophysiology, canopy structure and sunlight tolerance were different between these two species. Nevertheless, at higher tree species richness levels, a stronger competition for water was recorded and a reduced tree-ring growth in beech was measured (Metz et al. 2016). Moreover, Pretzsch and Dieler (2012) highlighted facilitative effects of oak on beech when conditions are less favourable, for example, during drought periods. Del Rio et al. (2014) report that when environmental conditions are favourable, a strong competition between beech and other species, and consequently, a decrease in tree growth is detected while when condition are less favourable (i.e. drought year) the opposite occurs (the so-called “stress-gradient hypothesis”, as defined by Bertness and Callaway 1994). These results were also confirmed by Grossiord et al. (2014a) and, in a wider study across Europe, by Jucker et al. (2016). Thus, competitive and facilitative mechanisms can interact simultaneously inside the same community, depending on the local climatic conditions, producing a wide spectrum of positive or negative effects on tree growth (Callaway 1998; Callaway and Walker 1997). Moreover, species richness does not influence tree growth when environmental conditions are not limiting, as observed in 2014 at our experimental sites, maybe because neither competition nor complementarity processes occur, or because the two processes cancel out each other (Pretzsch et al. 2013).
We also measured a significant lower iWUE in 2006 than in 2014 (Fig. 4). During the 2006 drought, significant differences in iWUE were observed between different levels of species richness, with the highest values in plantations with five consociated species, and the lowest values in plantations with four and six consociated species (Fig. 5). However, these differences were not observed during the year 2014. This confirms again that tree species richness has a positive effect on tree growth only when environmental conditions are harsher. Similar patterns in iWUE with species diversity have been also observed in boreal forests by Grossiord et al. (2014a) and by Gebauer et al. (2012).
Conclusions
Our results partially confirmed our working hypothesis that the higher is the number of consociated species (i.e. tree species’ diversity), the less the target commercial tree species are impacted by a drought event. In fact, a positive species richness effect on tree growth and iWUE was found only when less than five consociated species are present and only when environmental conditions are harsher (2006). For higher tree species richness levels, competition for resources prevails, thus leading to a decrease in radial tree growth and iWUE. Similarly, our data support the ‘stress gradient hypothesis’: the absence of both complementarity and competition or an equilibrium between these two mechanisms when environmental conditions are not limiting (2014) bring to no effect of tree species richness on tree growth.
References
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell D, Vennetier M, Kitzberger T, Rigling A, Bershears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim JH, Allard G, Running SW, Semerci A, Cobb N (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259(4):660–684. https://doi.org/10.1016/j.foreco.2009.09.001
Ammer C (2019) Diversity and forest productivity in a changing climate. New Phytol 221:50–66. https://doi.org/10.1111/nph.15263
Aussenac R, Bergeron Y, Ghotsa Mekontchou C, Gravel D, Pilch K, Drobyshev I (2017) Intraspecific variability in growth response to environmental fluctuations modulates the stabilizing effect of species diversity on forest growth. J Ecol 105:1010–1020. https://doi.org/10.1111/1365-2745.12728
Aussenac R, Bergeron Y, Gravel D, Drobyshev I (2019) Interactions among trees: a key element in the stabilising effect of species diversity on forest growth. Funct Ecol 33:360–367. https://doi.org/10.1111/1365-2435.13257
Baeten L, Verheyen K, Wirth C, Bruelheide H, Bussotti F, Finér L, Jaroszewicz B, Selvi F, Valladares F, Allan E, Ampoorter E, Auge H, Avǎcǎriei D, Barbaro L, Bǎrnoaiea I, Bastias CC, Bauhus J, Beinhoff C, Benavides R, Benneter A, Berger S, Berthold F, Boberg J, Bonal D, Brüggemann W, Carnol M, Castagneyrol B, Charbonnier Y, Chećko E, Coomes D, Coppi A, Dalmaris E, Dǎnilǎ G, Dawud SM, de Vries W, Wandeler HD, Deconchat M, Domisch T, Duduman G, Fischer M, Fotelli M, Gessler A, Gimeno TE, Granier A, Grossiord C, Guyot V, Hantsch L, Hättenschwiler S, Hector A, Hermy M, Holland V, Jactel H, Joly FX, Juckers T, Kolb S, Koricheva J, Lexer MJ, Liebergesell M, Milligan H, Müller S, Muys B, Nguyen D, Nichiforel L, Pollastrini M, Proulx R, Rabasa S, Radoglou K, Ratcliffe S, Raulund-Rasmussen K, Seiferling I, Stenlid J, Vesterdal L, von Wilpert K, Zavala MA, Zielinski D, Scherer-Lorenzen M (2013) A novel comparative research platform designed to determine the functional significance of tree species diversity in European forests. Perspect Plant Ecol 15:281–291. https://doi.org/10.1016/j.ppees.2013.07.002
Battipaglia G, Saurer M, Cherubini P, Siegwolf RTW, Cotrufo MF (2009) Tree rings indicate different drought resistance of a native (Abies alba Mill.) and a non-native (Picea abies (L.) Karst.) species co-occurring at a dry site in Southern Italy. Fort Ecol Manag 257:820–828. https://doi.org/10.1016/j.foreco.2008.10.015
Beckage B, Osborne B, Gavin DG, Pucko C, Siccama T, Perkins T (2008) A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. P Natl Acad Sci USA 105(11):4197–4202. https://doi.org/10.1073/pnas.0708921105
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9(5):191–193. https://doi.org/10.1016/0169-5347(94)90088-4
Bruelheide H, Nadrowski K, Assmann T, Bauhus J, Both S, Buscot F, Chen XY, Ding B, Durka W, Erfmeier A, Gutknecht JLM, Guo D, Guo LD, Härdtle W, He JS, Klein AM, Kühn P, Liang Y, Liu X, Michalski S, Niklaus PA, Pei K, Scherer-Lorenzen M, Scholten T, Schuldt A, Seidler G, Trogisch S, von Oheimb G, Welk E, Wirth C, Wubet T, Yang X, Yu M, Zhang S, Zhou H, Fischer M, Ma K, Schmid B (2014) Designing forest biodiversity experiments: general considerations illustrated by a new large experiment in subtropical China. Methods Ecol Evol 5:74–89. https://doi.org/10.1111/2041-210X.12126
Callaway RM (1998) Competition and facilitation on elevation gradients in subalpine forests of the northern rocky mountains, USA. Oikos 82(3):561–573. https://www.jstor.org/stable/3546376
Callaway RM, Walker LR (1997) Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78(7):1958–1965. https://doi.org/10.1890/0012-9658(1997)078%5b1958:CAFASA%5d2.0.CO;2
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486:59–67. https://doi.org/10.1038/nature11148
Christmas MJ, Breed MF, Lowe AJ (2016) Constraints to and conservation implications for climate change adaptation in plants. Conserv Genet 17(2):305–320. https://doi.org/10.1007/s10592-015-0782-5
De Bono A, Peduzzi P, Kluser S, Giuliani G (2004) Impacts of summer 2003 heat wave in Europe. United Nations Environment Programme. Environment Alert Bulletin 2. https://archive-ouverte.unige.ch/unige:32255
De Micco V, Ruel K, Joseleau JP, Aronne G (2010) Building and degradation of secondary cell walls: are there common patterns of lamellar assembly of cellulose microfibrils and cell wall delamination? Planta 232(3):621–627. https://doi.org/10.1007/s00425-010-1202-1
Del Rio M, Schütze G, Pretzsch H (2014) Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol 16(1):166–176. https://doi.org/10.1111/plb.12029
Deslauriers A, Morin H (2005) Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 19(4):402–408. https://doi.org/10.1007/s00468-004-0398-8
Fang K, Gou X, Peters K, Li J, Zhang F (2010) Removing biological trends from tree-ring series: testing modified Hugershoff curves. Tree-Ring Res 66(1):51–59. https://doi.org/10.3959/2008-18.1
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9(2):121–137. https://doi.org/10.1071/PP9820121
Francey RJ, Farquhar GD (1982) An explanation for the 12C/13C variations in tree rings. Nature 297:28–31
Gao S, Liu R, Zhou T, Fang W, Yi C, Lu R, Zhao X, Luo H (2018) Dynamic responses of tree-ring growth to multiple dimensions of drought. Glob Change Biol 24(11):5380–5390. https://doi.org/10.1111/gcb.14367
Gebauer T, Horna V, Leuschner C (2012) Canopy transpiration of pure and mixed forest stands with variable abundance of European beech. J Hydrol 442–443:2–14. https://doi.org/10.1016/j.jhydrol.2012.03.009
Grossiord C (2019) Having the right neighbors: how tree species diversity modulates drought impacts on forests. New Phytol. https://doi.org/10.1111/nph.15667
Grossiord C, Gessler A, Granier A, Pollastrini M, Bussotti F, Bonal D (2014a) Interspecific competition influences the response of oak transpiration to increasing drought stress in a mixed Mediterranean forest. Forest Ecol Manag 318:54–61. https://doi.org/10.1016/j.foreco.2014.01.004
Grossiord C, Granier A, Gessler A, Jucker T, Bonal D (2014b) Does drought influence the relationship between biodiversity and ecosystem functioning in boreal forests? Ecosystems 17(3):394–404. https://doi.org/10.1007/s10021-013-9729-1
Guerrieri R, Belmecheri S, Ollinger SV, Asbjornsen H, Jennings K, Xiao J, Stocker BD, Martin M, Hollinger DY, Bracho-Garrillo R, Clark K, Dore S, Kolb T, Munger JW, Novick K, Richardson AD (2019) Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1905912116
Hector A, Philipson C, Saner P, Chamagne J, Dzulkifli D, O’Brien M, Snaddon JL, Ulok P, Weilenmann M, Reynolds G, Godfray HCJ (2011) The Sabah biodiversity experiment: a long-term test of the role of tree diversity in restoring tropical forest structure and functioning. Philos Trans R Soc B 366:3303–3315. https://doi.org/10.1098/rstb.2011.0094
IPCC, 2014: Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Core writing team Pachauri RK, Meyer LA (eds) IPCC, Geneva, Switzerland, 151 pp
Jactel H, Brockerhoff EG (2007) Tree diversity reduces herbivory by forest insects. Ecol Lett 10(9):835–848. https://doi.org/10.1111/j.1461-0248.2007.01073.x
Jactel H, Gritti ES, Drössler L, Forrester DI, Mason WL, Morin X, Pretzsch H, Castagneyrol B (2018) Positive biodiversity–productivity relationships in forests: climate matters. Biol Lett 14:20170747. https://doi.org/10.1098/rsbl.2017.0747
Jucker T, Avăcăriței D, Bărnoaiea I, Duduman G, Bouriaud O, Coomes DA (2016) Climate modulates the effects of tree diversity on forest productivity. J Ecol 104(2):388–398. https://doi.org/10.1111/1365-2745.12522
Jump AS, Peñuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8(9):1010–1020. https://doi.org/10.1111/j.1461-0248.2005.00796.x
Kremer A, Ronce O, Robledo-Arnuncio JJ, Guillaume F, Bohrer G, Nathan R, Bridle JR, Gomulkiewicz R, Klein EK, Ritland K, Kuparinen A, Gerber S, Schueler S (2012) Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecol Lett 15(4):378–392. https://doi.org/10.1111/j.1461-0248.2012.01746.x
Lang AC, Härdtle W, Bruelheide H, Geißler C, Nadrowski K, Schuldt A, Yue M, von Oheimb G (2010) Tree morphology responds to neighbourhood competition and slope in species-rich forests of subtropical China. For Ecol Manag 260:1708–1715. https://doi.org/10.1016/j.foreco.2010.08.015
Lebourgeois F, Gomez N, Pinto P, Mérian P (2013) Mixed stands reduce Abies alba tree-ring sensitivity to summer drought in the Vosges mountains, western Europe. For Ecol Manag 303(1):61–71. https://doi.org/10.1016/j.foreco.2013.04.003
Li C (1999) Carbon isotope composition, water-use efficiency and biomass productivity of Eucalyptus microtheca populations under different water supplies. Plant Soil 214(1–2):165–171. https://doi.org/10.1023/A:1004708815973
Lloret F, Keeling EG, Sala A (2011) Components of tree resilience: effects of successive low-growth episodes on old ponderosa pine forests. Oikos 120(12):1909–1920. https://doi.org/10.1111/j.1600-0706.2011.19372.x
Lupi C, Hubert M, Deslauriers A, Rossi S (2010) Xylem phenology and wood production: resolving the chicken-or-egg dilemma. Plant Cell Environ 33(10):1721–1730. https://doi.org/10.1111/j.1365-3040.2010.02176.x
Maliva R, Missimer T (2012) Arid lands water evaluation and management. Springer, Berlin, New York. https://doi.org/10.1007/978-3-642-29104-3
McDowell NG (2011) Mechanisms linking drought, hydraulics, carbon metabolism and vegetation mortality. Plant Physiol 155:1051–1059. https://doi.org/10.1104/pp.110.170704
Merlin M, Perot T, Perret S, Korbulewsky N, Vallet P (2015) Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scot pine. For Ecol Manag 339:22–33. https://doi.org/10.1016/j.foreco.2014.11.032
Metz J, Annighöfer P, Schall P, Zimmermann J, Kahl T, Schulze ED, Ammer C (2016) Site-adapted admixed tree species reduce drought susceptibility of mature European beech. Glob Change Biol 22(2):903–920. https://doi.org/10.1111/gcb.13113
Mori AS, Lertzman KP, Gustafsson L (2017) Biodiversity and ecosystem services in forest ecosystems: a research agenda for applied forest ecology. J Appl Ecol 54(1):12–27. https://doi.org/10.1111/1365-2664.12669
Morin X, Fahse L, Scherer-Lorenzen M, Bugmann H (2011) Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol Lett 14(12):1211–1219. https://doi.org/10.1111/j.1461-0248.2011.01691.x
Pacala SW, Tilman D (1994) Limiting similarity in mechanistic and spatial models of plant competition in heterogeneous environments. Am Nat 143:222–257. https://doi.org/10.1086/285602
Palandrani C, Alberti G (2020) Tree derived soil carbon is enhanced by tree species richness and functional diversity. Plant Soil 446:457–469. https://doi.org/10.1007/s11104-019-04381-7
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669. https://doi.org/10.1146/annurev.ecolsys.37.091305.110100
Petchey OL (2003) Integrating methods that investigate how complementarity influences ecosystem functioning. Oikos 10:323–330. https://doi.org/10.1034/j.1600-0706.2003.11828.x
Pretzsch H, Dieler J (2012) Evidence of variant intra- and inter-specific scaling of tree crown structure and relevance for allometric theory. Oecologia 169(3):637–649. https://doi.org/10.1007/s00442-011-2240-5
Pretzsch H, Schütze G, Uhl E (2013) Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation. Plant Biol 15(3):483–495. https://doi.org/10.1111/j.1438-8677.2012.00670.x
Prieto I, Armas C, Pugnaire FI (2012) Water release through plant roots: new insights into its consequences at the plant and ecosystem level. New Phytol 193:830–841. https://doi.org/10.1111/j.1469-8137.2011.04039.x
Rebetez M, Mayer H, Dupont O, Schindler D, Gartner K, Kropp JP, Menzel A (2006) Heat and drought 2003 in Europe: a climate synthesis. Ann For Sci 63:569–577. https://doi.org/10.1051/forest:2006043
Schär C, Jendritzky G (2004) Climate change: hot news from summer 2003. Nature 432:559–560. https://doi.org/10.1038/432559a
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39. https://doi.org/10.1126/science.185.4145.27
Scholze M, Knorr W, Arnell NW, Prentice IC (2006) A climate-change risk analysis for world ecosystems. Proc Natl Acad Sci USA 103(35):13116–13120. https://doi.org/10.1073/pnas.0601816103
Schwarz JA, Bauhus J (2019) Benefits of mixtures on growth performance of silver fir (Abies alba) and European beech (Fagus sylvatica) increase with tree size without reducing drought tolerance. Front For Glob Change. https://doi.org/10.3389/ffgc.2019.00079
Schwendenmann L, Pendall E, Sanchez-Bragado R, Kunert N, Hölscher D (2015) Tree water uptake in a tropical plantation varying in tree diversity: interspecific differences, seasonal shifts and complementarity. Ecohydrology 8:1–12. https://doi.org/10.1002/eco.1479
Sohn JA, Saha S, Bauhus J (2016) Potential of forest thinning to mitigate drought stress: a meta-analysis. For Ecol Manag 380:261–273. https://doi.org/10.1016/j.foreco.2016.07.046
Steckel M, del Río M, Heym M, Aldea J, Bielak K, Brazaitis G, Černý J, Coll L, Collet C, Ehbrecht M, Jansons A, Nothdurft A, Pach M, Pardos M, Ponette Q, Reventlow DOJ, Sitko R, Svoboda M, Vallet P, Wolff B, Pretzsch H (2020) Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak [Quercus robur L., Quercus petraea (Matt.) Liebl.]—Site water supply and fertility modify the mixing effect. For Ecol Manag 461:117908. https://doi.org/10.1016/j.foreco.2020.117908
Trenberth KE, Dai A, van der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J (2014) Global warming and changes in drought. Nat Clim Change 4:17–22. https://doi.org/10.1038/nclimate2067
Verheyen K, Vanhellemont M, Auge H, Baeten L, Baraloto C, Barsoum N, Bilodeau-Gauthier S, Bruelheide H, Castagneyrol B, Godbold D, Haase J, Hector A, Jactel H, Koricheva J, Loreau M, Mereu S, Messier C, Muys B, Nolet P, Paquette A, Parker J, Perring M, Ponette Q, Potvin C, Reich P, Smith A, Weih M, Scherer-Lorenzen M (2015) Contributions of a global network of tree diversity experiments to sustainable forest plantations. Ambio 45(1):29–41. https://doi.org/10.1007/s13280-015-0685-1
Vitali V, Büntgen U, Bauhus J (2017) Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob Change Biol 23(12):5108–5119. https://doi.org/10.1111/gcb.13774
Wagner RG, Radosevich SR (1998) Neighbourhood approach for quantifying interspecific competition in coastal Oregon forests. Ecol Appl 8:779–794. https://doi.org/10.1890/1051-0761(1998)008%5b0779:NAFQIC%5d2.0.CO;2
Walker XJ, Mack MC, Johnstone JF (2015) Stable carbon isotope analysis reveals widespread drought stress in boreal black spruce forest. Glob Change Biol 21(8):3102–3113. https://doi.org/10.1111/gcb.12893
Warren WG (1980) On removing the growth trend from dendrochronological data. Tree-Ring Bull 40:35–44
Warren WG, MacWilliam SL (1981) Test of a new method for removing the growth trend from dendrochronological data. Tree-Ring Bull 41:55–66
West AG, Dawson TE, February EC, Midgley GF, Bond WJ, Aston TL (2012) Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol 195:396–407. https://doi.org/10.1111/j.1469-8137.2012.04170.x
Williams AP, Allen CD, Macalady AK, Griffin D, Woodhouse CA, Meko DM, Swetnam TW, Rauscher SA, Seager R, Grissino-Mayer HD, Dean JS, Cook ER, Gangodagamage C, Cai M, McDowell NG (2013) Temperature as a potent driver of regional forest drought stress. Nat Clim Change 3:292–297. https://doi.org/10.1038/nclimate1693
Yin J, Bauerle TL (2017) A global analysis of plant recovery performance from water stress. Oikos 126(10):1377–1388. https://doi.org/10.1111/oik.04534
Zhu Y, Chen H, Fan J, Wang Y, Li Y, Chen J, Fan JX, Yang S, Hu L, Leung H, Mew TW, Teng PS, Wang Z, Mundt CC (2000) Genetic diversity and disease control in rice. Nature 406(6797):718–722. https://doi.org/10.1038/35021046
Zou CB, Barnes PW, Archer S, McMurtry CR (2005) Soil moisture redistribution as a mechanism of facilitation in savanna tree–shrub clusters. Oecologia 145:32–40. https://doi.org/10.1007/s00442-005-0110-8
Acknowledgements
We thank Diego Chiabà for helping during field sampling and Federico Toffoletti for helping during field sampling, sample preparation and data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Rüdiger Grote.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palandrani, C., Battipaglia, G. & Alberti, G. Influence of tree species richness on tree growth and intrinsic water-use efficiency after drought in tree plantations in north-eastern Italy. Eur J Forest Res 139, 869–877 (2020). https://doi.org/10.1007/s10342-020-01291-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-020-01291-7