Abstract
The potential impacts of species colonization on the structure and functioning of ecosystems are poorly understood. We propose a novel approach for understanding the consequences of habitat colonization, highlighting the influence of colonists on the availability of limiting resources to resident species. We studied how colonization of dry oak woodlands by pines (Pinus halepensis) is affecting water stress of resident oaks (Quercus calliprinos). We monitored predawn leaf water potential (PLWP) of oaks monthly for 2 years. Using maximum likelihood and multi-model inference, we evaluated how the PLWP of oaks was affected by pine colonists. The influence of colonizing pines on PLWP of resident oaks varied in time and space from negative to positive depending on season, oak size, pine size, and proximity to pines presence. The water stress of oaks increased along the dry season (− 1.5 to − 4.5 MPa), with small oaks becoming more severely stressed than large ones (up to 60% difference). During the dry season, neighboring pine colonists increased the water stress of oaks (up to − 0.4 MPa difference), but during the wet season, they reduced the water stress mainly for large oaks. Our findings indicate that pine colonization differentially affects water limitation for resident oaks with implications for future development and regeneration. The influence of pine colonists shifted from positive to negative along an increasing water stress gradient, contrary to predictions by the stress gradient hypothesis. Our work demonstrates how colonization by non-resident species can influence key ecosystem processes through the redistribution of limiting resources. Identifying these processes is fundamental for understanding the consequences of colonization, mitigating these influences, and predicting future change in the structure and function of ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colonization of plant communities by non-resident species (i.e., species that were not part of the community; hereafter ``colonization'') is expected to become more prevalent with increasing human influences. Climate change, land-use change, species introduction, and other human-driven processes are leading to both the spread of plant species into new habitats (Mooney and Hobbs 2000; Root et al. 2003; Hoegh-Guldberg et al. 2008) and increased susceptibility of plant communities to colonization (Thuiller et al. 2008; Reu et al. 2014). However, the potential impacts of colonization on the organization of plant communities and the functioning of ecosystems are poorly understood and hard to assess (Crystal-Ornelas and Lockwood 2020; Sagoff 2020).
Colonization can cause significant changes in the structure of the colonized plant community (e.g., weed growth in the forest understory, shrub encroachment in a prairie, tree colonization in a grassland; Wiser et al. 1998; Asner et al. 2008; Eldridge et al. 2011) and as a consequence influence various ecosystem processes (e.g., primary productivity and nutrient cycling; Chapin et al. 1997; Hibbard et al. 2001). These modifications can, in turn, change the local conditions for resident species and lead to further modifications in the structure and function of the ecosystem (Foster and Dickson 2004). The effects of colonization on the resident vegetation largely depend upon the way by which the colonizer influences the availability of limiting resources (nutrients, light, or water, Funk and Vitousek 2007). Thus, understanding how colonization influences patterns of resource availability in space and time is key for studying and predicting consequences of colonization (Prevosto et al. 2006).
Colonizing plants can compete for resources with residents or indirectly increase the availability of limiting resources to resident plants. According to the stress gradient hypothesis (sensu Bertness and Callaway 1994; Callaway et al. 2002), plant–plant interactions will shift from competitive in resource-rich (low stress) environments to facilitative in resource-limited (high stress) environments. For example, in arid, semiarid, and Mediterranean ecosystems, where water is a limiting resource, facilitation (plant A alleviates water stress for plant B) is more prevalent in drier sites compared to competition (plants A and B mutually increase water stress) (e.g., Pugnaire and Luque 2001, Malkinson and Tielborger 2010). The latter is more prevalent in mesic sites.
East Mediterranean woodlands, representing the xeric end of the Mediterranean Basin, are mostly composed of sclerophyllous tree and shrub species, dominated by the common oak, Quercus calliprinos Web. The vegetation in these woodlands is adapted to high intra- and inter-annual variation in water availability including severe droughts (Grunzweig et al. 2008). Throughout the Mediterranean Basin, many areas that were historically dominated by oak woodlands are now covered by pine plantations (mostly P. halepensis), established more than half a century ago as part of vast afforestation projects following centuries of over-exploitation of the local vegetation (Maestre et al. 2003). The pioneer nature of P. halepensis, which made this species a successful plantation tree in degraded dry habitats (Ne’eman and Trabaud 2000), has also facilitated its spontaneous expansion into neighboring oak woodlands several decades later (Osem et al. 2011; Sheffer et al. 2014a) and to the creation of mixed pine–oak communities (Sheffer 2012).
Quercus calliprinos is a sclerophyllous evergreen tree species common to the eastern Mediterranean Basin. This species varies significantly in size and architecture ranging from multiple-stem low shrubs to single-stem tall trees (> 10 m) according to genetics, age, environmental conditions, and browsing pressure. In dry woodlands Q. calliprinos rarely exceed 6 m height, much shorter than mature P. halepensis trees growing under similar conditions. Several drought resistance mechanisms, including sclerophyllous leaves, a deep root system, and physiological tolerance to low water potentials, allow the survival of Q. calliprinos during hot, dry Mediterranean summers (Schiller et al. 2003; Grunzweig et al. 2008; Klein et al. 2013b). Pinus halepensis grows mainly in the southwestern Mediterranean, but it is also native to Israel. This species was extensively used for afforestation in Israel during the previous century (Osem et al. 2008). It is a light-demanding and drought-resistant species known for its isohydric water conserving strategy (stomatal control of transpiration; Schiller 2000; Klein et al. 2011, 2013a).
The establishment of pine trees in oak woodlands forms a new tall canopy stratum, overtopping the oak canopies (Fig. 1a; Sheffer et al. 2014b). The formation of this new canopy layer can affect various processes including light penetration to lower canopy strata, water and nutrient availability, microclimate, litter composition, rates of litter decomposition, as well as above- and belowground plant–plant interactions (e.g., Conn and Dighton 2000; Gonzalez-Moreno et al. 2011; Van Wilgen et al. 2008; Sheffer et al. 2015). Considering all these, the net effect of pine colonization on the performance of resident oaks can be either negative (e.g., competition) or positive (e.g., facilitation), with the nature of this pine–oak interaction varying in space and time depending on local abiotic conditions (e.g., the stress gradient hypothesis; Bertness and Callaway 1994), species characteristics (e.g., stress resistance and strategy of resource utilization; Maestre et al. 2009), and vegetation structure (e.g., height, density, and spatial configuration of interacting species; Bullock 2009; Rodriguez-Garcia et al. 2011).
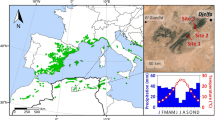
Vegetation and landscape structure of our study site. a Tall P. halepensis colonist growing in-between smaller shrubs and trees, dominated by Q. calliprinos; b location of our site (starred) in the map of Israel; c map of the region of our oak woodland study, indicating the distribution of planted P. halepensis stands in the landscape as potential seed sources for colonization by pines. The region of our study site is indicated by the black rectangle
The effect of pine colonization on water availability to resident vegetation is particularly complex as it involves a series of processes including rain interception by the tall pine canopy, reduced evaporation by pine shading (Raz Yaseef et al. 2012), and vegetative uptake from both shallow and deep soil layers (Raz Yaseef et al. 2012, Sarris et al. 2013). Indeed, previous studies looking at the effects of overstory pine cover on understory water regime in water-limited P. halepensis forests are inconsistent, varying among climatic regions, seasons, forest structures, and soil characteristics (Maestre et al. 2003; Bellot et al. 2004; Prevosto et al. 2011). As water is the most fundamental resource in these ecosystems, it implies that the consequences of pine colonization may be determined primarily by pine-induced changes in the availability and the distribution of water. The ongoing process of pine colonization in dry oak woodlands allowed us to evaluate the influence of pine colonists on water stress of resident oaks across a range of pine sizes, oak sizes, and pine–oak distances.
To better understand the potential ecological implications of colonization by pines, we examined the effect of colonizing pine trees on the water stress of resident oaks. Specifically, we asked (1) how neighboring pine colonists affect the water stress of resident oaks and how this effect varies as a function of (2) time (i.e., seasonal and inter-annual variation); (3) the size of the resident oak; (4) the size of the colonizing pine; and (5) the spatial proximity between the oak and the pine. Our hypothesis was that drought-adapted P. halepensis colonists (Schiller 2000) would compete with the resident vegetation for the limiting water resource and increase the water stress for local oaks. However, we expected that the competitive effect incurred by pines would change to a facilitative effect with increasing water limitation, as proposed by the stress gradient hypothesis (sensu Callaway et al. 2002). The proposed shift in the effect of pine colonists on the water status of resident oaks during spring vs. summer should be strengthened by the isohydric water use strategy of pines which close their stomata and diminish their water consumption during the summer period (Klein et al. 2011, Helman et al. 2017). We took an observational approach in which we monitored the variation in water stress of resident oaks for 2 years, and analyzed this variation with respect to neighboring pine colonists. Using maximum likelihood analyses and comparison of different models to explain these effects allowed us to reduce the dimensionality of this complex interaction and evaluate it in the context of the natural heterogeneity of our field site.
Methods
Study system
The study was conducted in the area of Britannia Park (34° 55′ 49″ E 31° 40′ 54″ N), in the Judean lowland region, Israel (Fig. 1b). The climate is typical East Mediterranean with long, hot, and rainless summers and mild, rainy winters. Mean annual precipitation is 480 mm, mainly concentrated between December and March. The local soil is a mix of brown forest Rendzina with bright Rendzina (Haploxeroll or Xerothent, USDA) formed on rolling hills of early Eocene chalk sediments, covered by a petrocalcic horizon (or calcrete, locally termed Nari; Dan et al. 2007). The study site is a highly diverse Mediterranean woodland with Q. calliprinos as the dominant species accompanied by a variety of other trees (e.g., Pistacia palestina, and Ceratonia siliqua), shrubs (e.g., Pistacia lentiscus and Calicotome villosa), and dwarf shrubs (e.g., Sarcopoterium spinosum), intermingled with patches of ephemeral herbaceous vegetation. Total woody vegetation cover at the study site ranges between 40 and 60% with Q. calliprinos trees constituting about 60–80% of that cover. The site is at the driest edge of the distribution of Q. calliprinos (Feinbrun-Dothan and Danin 1991), where water limitation is acute (Klein et al. 2013a).
Experimental setup
For the study, we designated a typical natural woodland area of about 100 ha. The woodland is dominated by Quercus calliprinos trees with the density of these trees ranging from 400 to 800 tree ha−1 (5–3.5 m distance between stems, respectively), their heights from few centimeters up to six meters, and their number of stems from one to six. Oak densities and heights are randomly distributed in space. The studied woodland is subjected to an ongoing colonization by P. halepensis trees (Sheffer et al. 2014a). Pine colonization is assumed to be the result of a continuous seed flow originating from seed sources in adjacent pine plantations (Fig. 1b). Pinus halepensis colonists (9–40 years of age) are sparsely distributed (~ 25 trees ha−1), with heights ranging 1–20 m. We selected 48 oak trees that stratified the range of examined factors including the size of oak—1.1 to 5.5 m height (average = 2.89 SE = 0.17 m) and 0.50–6.2 m crown diameter, the size of neighboring pine tree—6.1 to 14.7 m height (average = 9.20 SE = 0.34) and 10–38 cm stem diameter at breast height (average = 20 SE = 1.1 cm), and pine–oak distance—0.5 to 8 m (average = 4.64 SE = 0.28 m). Selected oaks were at least 10 m apart from each other and had only one pine colonist in their immediate neighborhood (8 m radius). Vegetation around the oaks is typically dominated by shrubs (30–50% cover) and open patches which are covered by ephemeral vegetation during mid-winter to early spring (February–April). Vegetation below the oaks canopy is very limited due to their closed evergreen canopy and thick litter layer.
Water potential measurements
We used predawn water potential of oak shoots as a proxy of water stress of oak trees and applied a series of measuring events of predawn water potential to monitor changes in the oak water stress. Leaf water potential is evaluated by measuring the amount of pressure applied on the leaf blade (often measured on twigs or shoots) required to force out water through the petiole. When measured before dawn (predawn), it is a well-accepted robust measure of water availability at the root–soil interface for plants in situ (Turner 1988; Reich and Hinckley 1989; Saha et al. 2008). We focused on measuring water potential only because in the conditions of these dry Mediterranean woodlands soil moisture varies considerably in space, depth, and time, and the terrain is highly rocky and stony, making it virtually impossible to measure soil moisture at relevant depths representing the trees’ root zones which typically reach soil and rock crevices few meters deep. However, predawn water potential was shown to be well correlated with relative extractable water in dry forest soils (Breda et al. 1995). Furthermore, in previous studies conducted in similar East Mediterranean environments we found predawn water potential to be closely correlated with both daily mean stomatal conductance and photosynthetic activity and therefore highly representative of tree water status in Q. calliprinos (Väänänen et al. 2020) as well as in another East Mediterranean oak species—Q. ithaburesis (Cooper et al. 2014).
We conducted monthly campaigns during two full hydrological years (2010/11–2011/12) to measure predawn water potential of 48 Q. calliprinos individuals. At each sampling campaign, we collected from each oak tree three shoots carrying healthy-looking fully developed leaves located at ~ 1 m height aboveground. Sample collection began two hours prior to dawn and ended just before dawn. Collected shoots were sealed in plastic bags and kept in a dark cooler. We measured the predawn water potential of each shoot using a gas chamber (PMS Instrument Company, Oregon USA), immediately after sample collection.
Data analysis
We used maximum likelihood estimation (Burnham and Anderson 2002) to analyze how the predawn water potential of the oak is affected by: (1) oak height; (2) size of the nearest neighbor pine colonist; and (3) distance to the nearest neighboring pine. Pine stem basal area and height were both tested and compared (repeating all model types once with pine height and once with basal area but not with both, Appendix A) to test two alternative hypotheses. The first hypothesis is that large pines compete with resident oaks for water belowground. The alternative hypothesis is that pines are not only competing with local oak trees for water, but tall pines can also shade local oaks and by that either increase competition for light, or reduce evaporative pressure in the dry hot summer. Stem basal area is a good measure of the potential water uptake by the pine (Lopez-Serrano et al. 2000), while height represents the shading effect of the pine and less closely its water demand. Although both measures are usually correlated (Appendix B), the nature of this height–diameter allometry varies as a function of vegetation structure and habitat conditions (Lines et al. 2012).
The analysis was conducted for each measurement date separately, to include only independent samples and to understand the temporal variability of the pine–oak effects by comparing the results (model fit and maximum likelihood estimates of the parameters) across measurement dates. We used a basic additive model of the form:
where the predawn water potential of the ith oak (i = 1…48) at the jth measurement date (j = 1…13), Pi j, is a function of the basal predawn water potential of oaks at the jth date P0 j (estimated intercept) and the additive effects of the independent variables: height of the focal oak HQ, size (height or basal area) of the neighboring pine SP, and oak–pine distance D, respectively, for the ith oak; αj, βj, and γj are estimated linear slope parameters that quantify the magnitude of the effects of each of the independent variables at the jth measurement date. We compared several types of functional relationships for model effects, to evaluate different ecological hypotheses (Appendix A), including (1) linear models, in which each effect is independent of the others; for example, the influence of pine is constant regardless of the size of the oak or its proximity (null model); (2) multiplicative models, in which the effects of different independent variables are multiplied by one another (instead of summed), and therefore test for the product of interactions among these effects; (3) neighborhood models, in which the effect of the nearest neighboring pine is divided by the distance to this pine (Canham and Uriarte 2006), which tests for a spatial sensitivity in the effect of the pine; and (4) neighborhood threshold models, in which the effect of the neighboring pine is different for small vs. large oak trees, and the height threshold is another parameter in the model for which we look for the maximum likelihood value. The “threshold model” therefore allowed us to test for a possible shift from facilitation to competition as a function of oak size. For example, for a linear threshold model there will be two β parameters: βS for small oaks with HQi< HQ-threshold and βL for large oaks with HQi ≥ HQ-threshold, where HQ-threshold is another estimated parameter. To understand the relative importance of each of the independent variables, we tested models with different grouping of the effects and different partial combinations of them. The complete set of models is detailed in Appendix A.
Parameter estimation and model evaluation
We used the mean predawn water potential of each oak tree, calculated as the mean of the water potential measurements of three shoots. We solved for the maximum likelihood parameter values for each model in our set of models and for each measurement date separately, using a search algorithm based on the “simulated annealing” method in the “likelihood 1.3” package in R (Murphy 2012). For each scientific model, we assume an additional error model for which we included the residuals (ε) in the model as normally distributed. We used asymptotic two-unit support intervals to assess the strength of evidence for individual maximum likelihood parameter estimates (Edwards 1992). All analyses were done using the R programming environment version 2.8.0 (R Development Core Team 2008).
Model comparison and multi-model inference
We compared alternative models on the basis of Akaike information criterion corrected for a small sample size (AICc). However, we chose the multi-model inference approach to encompass the multidimensional complexity in our data because we found that: (a) the most parsimonious model differed between measurement dates, and (b) in many measurement dates there was no single best model, that is, several models had similar AICc values (ΔAICc < 2). Therefore, we inferred the pine–oak effects from the full set of models analyzed for each measurement date based on Akaike weights ωi as indicators of the probability that each model is the best among the whole set of our tested models (Burnham and Anderson 2002) and as a measure of the relative strength of evidence for competing hypotheses. We then used the sum of Akaike weights Σωi of all the models that included a specific effect (e.g., the effect of oak height), representing the combined percentage weight for those models, as a measure of the strength of evidence for the significance of that effect. We calculated the size of each effect using the model-averaged parameter estimates (appendix C) as a measure of the direction and magnitude of each of these effects, which reduce the bias in parameter estimates compared to using only the estimates from the best model (Burnham and Anderson 2002).
Results
Temporal pattern
Water stress of Q. calliprinos in the studied dry woodland increased from early spring (April) toward the end of the summer (September), as indicated by increasingly negative values of the predawn water potential, and recovered during the wet winter (November–March, Fig. 2). Furthermore, our results show that the predawn water potential of the oaks tracked the potential changes in soil water availability following precipitation events during the 2 years of our experiment. That is, the amounts and timing of precipitation events corresponded to the values of oak predawn water potentials (Fig. 2). Low precipitation in the first winter (2010/11, 65% of the annual average) resulted in extremely negative water potentials at the end of the following summer (October 2011, < –5 MPa average; i.e., severe water stress). In comparison, higher precipitation in the second winter (2011/12 110% of the average) resulted in a milder decrease in the water potential along the following summer (September 2012, > –4 MPa average, Fig. 2).
Inter-annual and seasonal patterns of predawn water potential of resident oaks. Monthly precipitation in the 2 years of our experiment (gray bars, right-side axis) and the predawn water potential of oaks as estimated by: the multi-model-averaged estimated basal water potential (intercept parameter P0) (black triangles); the estimated basal water potential intercept in the best model, with two upper and lower support intervals (black dot with error bars); and the mean of all water potential measurements (n = 127–288) at each measurement date (gray open circles)
Water stress and oak size
Our findings show that the predawn water potential of oaks, at a given time of the year, was positively related to oak size, that is, small trees experienced significantly more severe water stress (more negative water potential) compared to large ones (Fig. 3a, b). The effect size of oak height (i.e., average maximum likelihood estimate for the slope of the effect of oak height): (1) was a positive number, which means that the predawn water potential will be less negative (reduced water stress) the taller the oak is and (2) increased with increasing water stress (Fig. 3c, appendix C), which means that the difference in predawn water potential between short and tall oaks increases with increasing drought conditions. The same positive effect of oak height was also captured in the inverse multiplicative models we tested (models 15–18 in Appendix A), where the predicted predawn water potential (a negative number) was divided by the height of the oak, and thus became less negative with increasing oak height.
Oak predawn water potential as a function of oak height and influence of the size of neighboring pine. Measurements of the predawn water potential as a function of oak height along 2 years: 2011 (a) and 2012 (b), some of the months were excluded from the figure to allow a clearer view. Effect sizes, calculated as multi-model-averaged slope parameters, for c the effect of the height of the oak [MPa m−1], d the basal area [MPa m−2], and e the height of a neighboring pine [MPa m−1] on the predawn water potential of oaks, as a function of the multi-model-averaged basal predawn water potential, at each sampling date. The rainy season is marked by gray-shaded area, and white area marks the rainless dry season
Effect of pine colonists
Our multi-model inference indicates that the effect of pine colonists on the predawn water potential of resident oaks was important throughout the 2 years of our study, especially in the transition between the rainy and rainless seasons (Table 1). The effect of pine colonists depended either on their height or on their basal area. Although the influence of pine height or basal area had a very similar level of support in parts of the year, the pine effect tended to clearly be driven by either pine height or pine basal area but not both during the transition from the rainy to the rainless season, when the effect of pine was especially high.
The magnitude and direction of pine colonists’ effect on the water potential of oaks varied substantially along the seasonal water stress gradient (Fig. 3d, e). When the predawn water potential of the oaks was low (< –2 MPa; dry season), the effect size of neighboring pine colonists was negative (Fig. 3d, e), meaning: (1) that neighboring pines reduced the predawn water potential (i.e., increased the water stress) of resident oaks and (2) that this negative effect increased with increasing size of the pine. The negative effect of pine colonists became more pronounced with increasing drought. However, when the predawn water potentials of oaks were high (i.e., close to zero; wet season), the effect size of neighboring pine colonists (estimated slope) was close to zero. Furthermore, at the wettest time of the year the effect size of a neighboring pine was positive, indicating that pine colonists reduced, significantly, the water stress experienced by resident oaks. Interestingly, significant negative effects were usually more related to pine basal area, and significant positive effects were more related to pine height.
We made a further attempt to evaluate under what conditions pine colonists reduce rather than increase the water stress experienced by resident oaks. Based on the strong support for models with an interaction between the effects of oak and pine heights (Σωi > 0.6 for multiplicative neighborhood models during the spring, Table 1), we tested threshold interaction models in which the effects of pine colonists were allowed to change depending on the size of resident oaks.
Indeed, we found that the effect of pine colonists on oak water potential shifted from neutral or negative to positive depending on the size of the affected oak and pine height (Fig. 3d, e). It should be noted, however, that strong support for such models was found only in spring, at the time of transition from the rainy to the rainless season (May 2011 and May–June 2012). Our models indicate that large oaks (e.g., larger than HQ-threshold = 1.65 and 2.2 m in May and June 2012, respectively; Appendix D) experienced a decrease in water stress (positive effect by pine colonists) while small oaks were not affected by pines (slope ≈ 0, May 2011 and 2012) or experienced increased stress (negative pine effect, June 2012) (Appendix E). The size of the positive effect (slope parameter) imposed by pine colonists on large oaks was more than twofold larger in magnitude than their negative effect on small oaks (Appendices D,E).
Effect of pine–oak proximity
We found that the effects of pine colonists were also strongly influenced by their proximity to focal oak trees. Most of the support for this spatial effect was found in the neighborhood models, in which the effect of the size of the pine was divided by the distance to the pine (Canham and Uriarte 2006; Table 1, Appendices A, D), therefore indicating that the influence of pine decreased with increasing distance from the focal oak.
Discussion
Temporal–seasonal pattern
The predawn water potentials (PLWP) of resident oak trees declined gradually along the dry season indicating increasing drought stress which became severe (PDWP = − 4 to − 5 MPa) during late summer (Väänänen et al. 2020). The water stress decreased with increasing oak size, and the difference in the extent of water stress between small and large oaks became more pronounced as drought conditions became more severe. This observed size-related variation in the water stress level of oaks likely indicates variation in the size of their root systems, whereby larger oaks have deeper root systems allowing them to exploit deep water resources that are unavailable to small oaks. The assumption that large oaks have a deeper root system than small ones is supported by previous work showing high correlation between canopy and root size in various forest systems (see, for example, Tatarinov et al. 2008; Christina et al. 2011; Stahl et al. 2013) and on evidence relating variation in predawn leaf water potential of neighboring individuals to their rooting depths (Otieno et al. 2006; Stahl et al. 2013). Our results suggest that forest vulnerability to future increase in drought conditions may depend on the size–structure of the community and that small individuals are likely to be more severely affected than large ones. However, our models explained only ~ 30% of the variability in the predawn water potential of these oak trees. Other parts of the variation could be related to additional process such as regulation within the tree (e.g., stomatal conductance) or belowground distribution of the water and of the roots of the oak trees and all their neighboring shrubs and trees (Breda et al. 1995).
Pine colonists–resident oaks interaction
Our results revealed a complex interaction in which the effect of pine colonists on the water status of resident oaks depended on the size of both pine colonists and resident oaks, their proximity, and the season.
The influence of pine colonists on the water stress of resident oaks was spatially limited (up to 5 m from the pine, see appendix E). This coincides with an overlap in the rooting zones, assuming that the lateral size of the rooting zone of these trees is similar to that of their canopy (a conservative estimate; rooting zones tend to be larger than canopies in resource-limited environments; Schenk and Jackson 2002). Within this spatial range, the pattern of pine–oak interaction varied between seasons and oak sizes. In the spring, pines had a negative influence on the water status of small oaks but a positive influence on large oaks. We propose that this differential influence reflects a delicate balance between belowground and aboveground pine–oak interactions.
Belowground, pine colonists compete for water with the resident oaks. This competitive effect (manifested mainly through pine stem basal area, Sun et al. 2019) is likely to increase with increasing overlap between the root zones of the two species (i.e., closer proximity and/or denser colonization). We hypothesize that the observed stronger competitive effect of pines on small compared to large oaks is the outcome of greater overlap in the root zones of pines and small oaks, as both are assumed to have shallower root systems than large oaks. This assumption is based on evidence demonstrating the capacity of P. halepensis to develop an extensive lateral root system in dry shallow soil rocky terrains (Ganatsas and Spanos 2005) and of Q. calliprinos to penetrate rock cracks and develop a deep root system (Schiller et al. 2010). Furthermore, this is in agreement with del-Castillo et al. (2016) who proposed, based on stable isotope composition, that in a mixed oak (Quercus ilex)-pine (Pinus halepensis) Mediterranean forest, the use of shallow water in oaks was limited by pines, which forced them to shift to deep soil water use, whereas pines had more restricted access to deep water in the presence of oaks. On the other hand, Voltas et al. (2015) have shown in a typical Mediterranean pine forest of eastern Spain that P. halepensis shifts into deeper water pools during the summer.
Aboveground, pine colonists usually are taller than the tallest oaks (Sheffer et al. 2014b) and therefore intercept sunlight, affecting the exposure of resident oaks to direct beam radiation (shading effect). It has been previously shown in drylands that shading, to a certain extent, can ameliorate the water stress of understory vegetation and facilitate its growth (Gomes-Aparicio et al. 2009; Semchenko et al. 2012). When sparsely distributed, pine colonists may exert a moderate shading effect, manifested mainly through the effect of pine height, thereby reducing the evaporative demand for the oaks without causing substantial light limitation for their photosynthetic activity (Holmgren et al. 2012). Our findings regarding a positive effect of pine colonists on the water status of large oaks, but not of small oaks, might be related to vertical variation in shading experienced by large vs. small oaks. The influence of shading from pines is proportionally smaller for small oaks, which are already shaded by large oaks growing around them, than for the mostly unshaded large oaks, which can be shaded only by tall pine colonists. The proposed positive influence of pine colonists on resident oaks, through shading, may be regarded as a facilitative effect. Nevertheless, it may be the result of other processes that should not be considered as facilitation per se, such as reduced competition by understory shrubs and herbs, growing around the oaks, due to the shading of pines.
We found two conditions under which pine colonists had a positive influence on the water status of resident oaks: in early spring (beginning of the rainless season; March–May), on all oaks, and in late spring (May–June), on large oaks only. In both cases, water stress was moderate. However, further along the rainless season, as conditions became increasingly drier, the influence of pines on the oak water status shifted and became increasingly negative, with both small and large oaks experiencing increasingly stronger negative effects by the neighboring pine colonists. We propose that the interaction between large oaks and large colonizing pines shifted from facilitation to competition along the seasonal water stress gradient (Holmgren and Scheffer 2010). This finding is at odds with the stress gradient hypothesis, which suggested that the net balance between competition and facilitation shifts toward higher importance of facilitation with increasing stress (Callaway et al. 2002, Malkinson and Tielborger 2010), with the caveat that the stress gradient hypothesis has been proposed mostly in the context of a spatial stress gradients and not a temporal one. Whereas the stress gradient hypothesis predicts a positive linear relationship between abiotic stress level and the relative importance of facilitation, Maestre and Cortina (2004) as well as Michalet (2006) proposed that under certain circumstances nonlinear relationships, such as unimodal, might be expected and that the functional form of this relationship might not only depend on the stress level, but rather on multiple additional factors including the life history of the interacting species (Maestre et al. 2009).
Furthermore, the pattern we found contradicts our expectations that the relatively isohydric water use strategy of P. halepensis (e.g., relative to Quercus calliprinos, Klein et al. 2013b) will result in stronger belowground competition during the spring season, when P. halepensis is at peak activity and not in the summer when it avoids water uptake almost completely (Klein et al. 2011; 2013a, b; Helman et al. 2017).
The positive influence of pine colonists during the early rainless season can be highly important for the development of the resident oaks, as this is the most active season for Mediterranean woody vegetation (Klein et al. 2013b; Väänänen et al. 2020). Nevertheless, the negative influence of pines during the summer also can be meaningful, as this is the most stressful season in terms of water limitation. Altogether, our results show that pine colonization in the oak woodland influences the availability of resources to the resident vegetation and alludes to a possible shift in the distribution of water among plants in the woodland (e.g., large vs. small oaks). This may incur substantial consequences for the future structure of the colonized woodland.
Consequences of colonization
Based on our findings, we predict that the influence of pine colonization on the water status of resident oaks will become more pronounced as a result of further growth of established pine colonists, further colonization, and increasing density of pine colonists (i.e., decreasing the average distance between oaks and pines). This is in agreement with Moreno-Gutierrez et al. (2015) who demonstrated, by conducting a retrospective analysis of growth rings and stable C isotopes, how P. halepensis afforestation reduced water and nutrient availability for resident understory vegetation in a semiarid Mediterranean ecosystem.
The variation in pine–oak interactions for small vs. large oaks probably reflects a more complex influence of pine colonization, which changes the distribution of above- and belowground resources among the woodland components. According to our findings, pine colonization can severely increase the local water stress for small resident oaks while at the same time having a relatively minor negative influence or even a positive influence (reducing the water stress) for large oaks. While consequences on oak stand dynamics were not addressed explicitly in this study, we propose that the size-dependent differential effect of colonizing pines on the water status of resident oaks may result in decreased oak recruitment, reduced growth of small oaks, and consequently increased allocation of woodland resources toward the existing large oaks (and the colonizing pines). Overall, such processes can lead to the development of a taller pine–oak forest. This idea agrees with Pretzsch and Schutze (2014), who demonstrated how conversion from pure to mixed tree species composition with different resource requirements affects the size–structure dynamics of forest stands.
The differential effect of pine colonists on the water stress of small vs. large oaks may suggest that large oak trees and colonizing pine trees are somewhat complementary in their water capture (taking water from different soil layers). Thus, a mixed pine–oak forest growing under the same rainfall regime may support more biomass compared to pure oak or pine dominated woodlands, which possibly results in an improved water use efficiency for this dry climate. Such efficiency in capturing the most critical resource can explain the recent emergence of oak–pine ecosystems across the Mediterranean biome (Sheffer 2012). Increasing biomass densities in these ecosystems could increase long-term carbon sequestration in semiarid regions (Knapp et al. 2008; Poulter et al. 2014), but also increase the risk of wildfire (Wittenberg and Malkinson 2009).
Our results should be understood in the context of the extreme water limitation in a xeric Mediterranean site. The influence of pine colonization can vary along increasing water availability levels, as the severity of the drought stress gradually decreases, while the importance of competition for light increases.
Our work demonstrates the complexity of the interactions between a local plant community, non-resident colonizing species, and limiting resources. New colonists can affect not only the total availability of resources but also their distribution among resident vegetation components. The redistribution of resources can further influence vegetation dynamics and lead to altered patterns of vegetation structure, resource use, and ecosystem functioning. These findings highlight the importance of looking at vegetation processes (e.g., succession, colonization, and invasion) through interactions over limiting resources. Such an approach can provide meaningful insights and improve the predictive capacity regarding vegetation dynamics under changing environmental conditions and increasing human impacts.
References
Asner GP, Hughes RF, Vitousek PM, Knapp DE, Kennedy-Bowdoin T, Boardman J, Martin RE, Eastwood M, Green RO (2008) Invasive plants transform the three-dimensional structure of rain forests. Proc Natl Acad Sci 105:4519–4523
Bellot J, Maestre FT, Chirino E, Hernandez N, de Urbina JO (2004) Afforestation with Pinus halepensis reduces native shrub performance in a Mediterranean semiarid area. Acta Oecologia 25:7–15
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Breda N, Granier A, Barataud F, Moyne C (1995) Soil-water dynamics in an oak stand. I. Soil moisture, water potentials and water uptake by roots. Plant Soil 172:17–27
Bullock JM (2009) A long-term study of the roles of competition and facilitation in the establishment of an invasive pine following heathland fires. J Ecol 97:646–656
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York, p 488
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Canham CD, Uriarte M (2006) Analysis of neighborhood dynamics of forest ecosystems using likelihood methods and modeling. Ecol Appl 16:62–73
Chapin FS, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D (1997) Biotic control over the functioning of ecosystems. Science 277:500
Christina M, Laclau JP, Goncalves JLM, Jourdan C, Nouvellon Y, Bouillet JP (2011) Almost symmetrical vertical growth rates above and below ground in one of the world’s most productive forests. Ecosphere. https://doi.org/10.1890/ES10-00158.1
Conn C, Dighton J (2000) Letter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 4:489–496
Cooper A, Shapira O, Zaidan S, Moshe Y, Zangy E, Osem Y (2014) Oak restoration in water limited pine plantations: interactive effects of overstory light interception and water availability on understory oak performance. Eur J For Res 133:661–670
Crystal-Ornelas R, Lockwood JL (2020) The ‘known unknowns’ of invasive species impact measurement. Biol Invasions. https://doi.org/10.1007/s10530-020-0
Dan J, Fine P, Lavee H (2007) The soils of the land of Israel. Tel Aviv University, Tel Aviv, Eretz
Del-Castillo J, Comas C, Voltas J, Ferrio JP (2016) Dynamics of competition over water in a mixed oak–pine Mediterranean forest: spatio temporal and physiological components. For Ecol Manage 382:214–224
Edwards AWF (1992) Likelihood-expanded edition. Johns Hopkins University Press, Baltimore, p 275
Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol Lett 14:709–722
Feinbrun-Dothan N, Danin A (1991) Analytical flora of Eretz Israel. Cana Publishing House, Jerusalem
Foster BL, Dickson TL (2004) Grass land diversity and productivity: the interplay of resource availability and propagule pools. Ecology 85:1541–1547
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low resource systems. Nature 446:1079–1081
Ganatsas P, Spanos I (2005) Root system asymmetry of Mediterranean pines. Plant Soil 278:75–83
Gomes-Aparicio L, Zavala MA, Bonet FJ, Zamora R (2009) Are pine plantations valid tools for restoring Mediterranean forests? An assessment along abiotic and biotic gradients. Ecol Appl 19:2124–2141
Gonzalez-Moreno P, Quero JL, Poorter L, Bonet FJ, Zamora R (2011) Is spatial structure the key to promote plant diversity in Mediterranean forest plantations? Basic Appl Ecol 12:251–259
Grunzweig JM, Carmel Y, Riov J, Sever N, McCreary DD, Flather CH (2008) Growth, resource storage, and adaptation to drought in California and eastern Mediterranean oak seedlings. Canad J For 38:331–342
Helman D, Osem Y, Yakir D, Lensky IM (2017) Relationship between climate, topography, water use and productivity in two key Mediterranean forest types with different water-use strategies. Agric For Meteorol 232:319–330
Hibbard KA, Archer S, Schimel DS, Valentine DW (2001) Biogeochemical changes accompanying woody plant encroachment in a subtropical savanna. Ecology 82:1999–2011
Hoegh-Guldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP, Thomas CD (2008) Assisted colonization and rapid climate change. Science 321:345–346
Holmgren M, Scheffer M (2010) Strong facilitation in mild environments: the stress gradient hypothesis revisited. J Ecol 98:1269–1275. https://doi.org/10.1111/j.1365-2745.2010.01709.x
Holmgren M, Gomes-Aparicio L, Quero JL, Valladares F (2012) Non-linear effects of drought under shade: reconciling physiological and ecological models in plant communities. Oecologia 169:293–305
Klein T, Cohen S, Yakir D (2011) Hydraulic adjustments underlying drought resistance of Pinus halepensis. Tree Physiol 31:637–648
Klein T, Di Matteo G, Rotenberg E, Cohen S, Yakir D (2013a) Differential ecophysiological response of a major Mediterranean pine species across a climatic gradient. Tree Physiol 33:26–36
Klein T, Shpringer I, Fikler B, Elbaz G, Cohen S, Yakir D (2013b) Relationships between stomatal regulation, water-use, and water-use efficiency of two coexisting key Mediterranean tree species. For Ecol Manage 302:34–42
Knapp AK, Briggs JM, Collins SL, Archer SR, Bret-Harte MS, Ewers BE, Peters DP, Young DR, Shaver GR, Pendalle E, Cleary MB (2008) Shrub encroachment in North American grasslands: shifts in growth form dominance rapidly alters control of ecosystem carbon inputs. Glob Change Biol 14:615–623
Lines ER, Zavala MA, Purves DW, Coomes DA (2012) Predictable changes in aboveground allometry of trees along gradients of temperature, aridity and competition. Glob Ecol Biogeogr 21:1017–1028
Lopez-Serrano FR, Landete-Castillejos T, Martinez-Millan J, del Cerro-Barja A (2000) LAI estimation of natural pine forest using a non-standard sampling technique. Agric For Meteorol 101:95–111
Maestre FT, Cortina J (2004) Do positive interactions increase with abiotic stress? A test from a semi-arid steppe. Proc R Soc 271:331–333
Maestre FT, Cortina J, Bautista S, Bellot J (2003) Does Pinus halepensis facilitate the establishment of shrubs in Mediterranean semi-arid afforestations? For Ecol Manage 176:147–160
Maestre FT, Callaway RM, Valladares F, Lortie CJ (2009) Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol 97:199–205
Malkinson D, Tielborger K (2010) What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos 119:1546–1552
Michalet R (2006) Is facilitation in arid environments the result of direct or complex interactions. New Phytol 169:3–6
Mooney HA, Hobbs RJ (eds) (2000) Invasive species in a changing world. Island Press, Washington DC
Moreno-Gutierrez C, Battipaglia G, Cherubini P, Huertas AD, Querejeta JI (2015) Pines afforestation decreases the long-term performance of understory shrubs in a semi-aris Mediterranean ecosystem: a stable isotope approach. Funct Ecol 29:15–25
Murphy L (2008) Likelihood: methods for maximum likelihood estimation. R package version 1.4. http://www.sortie-nd.org/lme/lme_R_code_tutorials.html
Murphy L (2012) Likelihood: methods for maximum likelihood estimation. R package version 1.3. http://CRAN.R-project.org/package=likelihood
Ne’eman G, Trabaud L (eds) (2000) Ecology, biogeography and management of Pinus halepensis and P. brutia forest ecosystems in the Mediterranean basin. Backhuys, Leiden
Osem Y, Ginsberg P, Tauber I, Atzmon N, Perevolotsky A (2008) Sustainable management of Mediterranean planted coniferous forests: an Israeli definition. J Forest 106:38–46
Osem Y, Lavi A, Rosenfeld A (2011) Colonization of Pinus halepensis in Mediterranean habitats: consequences of afforestation, grazing and fire. Biol Invasions 13:485–498
Otieno DO, Kurz-Besson C, Liu J, Schmidt MWT, Vale-Lobo do R, David V, Siegwolf R, Pereira JS, JD Tenhunen (2006) Seasonal variations in soil and plant water status in a Quercus suber L. Stand: roots as determinants of tree productivity and survival in the Mediterranean-type ecosystem. Plant Soil 283:119–135
Poulter B, Frank D, Ciais P, Myneni RB, Andela N, Bi J, Broquet G, Canadell JG, Chevallier F, Liu YY, Running SW, Sitch S, van der Werf GR (2014) Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 509:600–603
Pretzsch H, Schutze G (2014) Size-structure dynamics of mixed versus pure forest stands. For Syst 23:560–572
Prevosto B, Dambrine E, Coquillard P, Robert A (2006) Broom (Cytisus scoparius) colonization after grazing abandonment in the French Massif Central: inpact on vegetation composition and resource availability. Acta Oecologia 30:258–268
Prevosto B, Monnier Y, Ripert C, Fernandez C (2011) Can we use shelterwoods in Mediterranean pine forests to promote oak seedling development? For Ecol Manage 262:1426–1433
Pugnaire FI, Luque MT (2001) Changes in plant interactions along a gradient of environmental stress. Oikos 93:42–49
R Development Core Team (2008) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Raz Yaseef N, Yakir D, Schiller G, Cohen S (2012) Dynamics of evapotranspiration partitioning in a semi-arid forest as affected by temporal rainfall patterns. Agric For Meteorol 157:77–85
Reich PB, Hinckley TM (1989) Influence of pre-dawn water potential and soil-to-leaf hydraulic conductance on maximum daily leaf diffusive conductance in two oak species. Funct Ecol 3:719–726. https://doi.org/10.2307/2389504
Reu B, Zaehle S, Bohn K, Pavlick R, Schmidtlein S, Williams JW, Kleidon A (2014) Future no-analogue vegetation produced by no-analogue combinations of temperature and insolation. Glob Ecol Biogeogr 23:156–167. https://doi.org/10.1111/geb.12110
Rodriguez-Garcia E, Ordonez C, Bravo F (2011) Effects of shrub and canopy cover on the relative growth rate of Pinus pinaster Ait. seedlings of different sizes. Ann For Sci 68:337–346
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. https://doi.org/10.1038/nature01333
Sagoff M (2020) Fact and value in invasion biology. Conserv Biol. https://doi.org/10.1111/cobi.13440
Saha S, Straziser TM, Menges ES, Ellsworth P, Sternberg L (2008) Linking the patterns in soil moisture to leaf water potential, stomatal conductance, growth, and mortality of dominant shrubs in the Florida scrub ecosystem. Plant Soil 313:113–127
Sarris D, Siegwolf R, Körner C (2013) Inter- and intra-annual stable carbon and oxygen isotope signals in response to drought in Mediterranean pines. Agric For Meteorol 168:59–68
Schenk HJ, Jackson RB (2002) Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J Ecol 90:480–494. https://doi.org/10.1046/j.1365-2745.2002.00682.x
Schiller G (2000) Inter- and Intra-specific genetic diversity of Pinus halepensis Mill, and P. Brutia Ten. In: Ne’eman G, Trabaud L (eds) Ecology, biogeography and management of Pinus halepensis and P. brutia forest ecosystems in the Mediterranean basin. Backhuys, Leiden, pp 13–36
Schiller G, Unger ED, Moshe Y, Cohen S, Cohen Y (2003) Estimating water use by sclerophyllous species under east Mediterranean climate: II. The transpiration of Quercus calliprinos Webb. in response to silvicultural treatments. For Ecol Manage 179:483–495
Schiller G, Unger ED, Cohen S, Her N (2010) Water use by Tabor and Kermes oaks growing in their respective habitats in the Lower Galilee region of Israel. For Ecol Manage 259:1018–1024
Semchenko M, Lepik M, Götzenberger L, Zobel K (2012) Positive effect of shade on plant growth: amelioration of stress or active regulation of growth rate? J Ecol 100:459–466. https://doi.org/10.1111/j.1365-2745.2011.01936.x
Sheffer E (2012) A review of the development of Mediterranean pine–oak ecosystems after land abandonment and afforestation: are they novel ecosystems? Ann For Sci 69:429–443
Sheffer E, Canham CD, Kigel J, Perevolotsky A (2014a) An integrative analysis of the dynamics of landscape-and local-scale colonization of Mediterranean woodlands by Pinus halepensis. PLoS ONE 9:e90178
Sheffer E, Canham CD, Kigel J, Perevolotsky A (2014b) Predicting the formation of a new upper canopy strata after colonization of native shrublands by pines. For Sci 60:841–850
Sheffer E, Canham CD, Kigel J, Perevolotsky A (2015) Countervailing effects on pine and oak leaf litter decomposition in human-altered Mediterranean ecosystems. Oecologia 177:1039–1051
Stahl C, Herault B, Rossi V, Burban B, Brechet C, Bonal D (2013) Depth of soil water uptake by tropical rainforest trees during dry periods: does tree dimension matter? Oecologia 173:1191–1201
Sun J, Wang M, Lyu M, Niklas KJ, Zhong Q, Li M, Cheng D (2019) Stem diameter (and not length) limits twig leaf biomass. Front Plant Sci 10:1–10
Tatarinov F, Urban J, Cermak J (2008) Application of “clump technique” for root system studies of Quercus robur and Fraxinus excelsior. For Ecol Manage 255:495–505
Thuiller W, Albert C, Araújo MB, Berry PM, Cabeza M, Guisan A, Hickler T, Midgley GF, Paterson J, Schurr FM, Sykes MT, Zimmermann NE (2008) Predicting global change impacts on plant species’ distributions: future challenges. Perspect Plant Ecol Evol Syst 9:137–152
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308
Väänänen PJ, Osem Y, Cohen S, Grüenzweig JM (2020) Differential drought resistance strategies of co-existing woodland species enduring the long rainless Eastern Mediterranean summer. Tree Physiol 40:305–320
van Wilgen BW, Reyers B, Le Maitre DC, Richardson DM, Schonegevel L (2008) A biom-scale assessment of the impact of invasive alien plants onecosystem services in South Africa. J Environ Manag 89:336–349
Voltas J, Lucabaugh D, Chambel MR, Ferrio JP (2015) Intraspecific variation in the use of water sources by the circum-Medirerranean conifer Pinus halepensis. N Pytologist 208:1031–1041
Wiser SK, Allen RB, Clinton PW, Platt KH (1998) Community structure and forest invasion by an exotic herb over 23 years. Ecology 79:2071–2081
Wittenberg L, Malkinson D (2009) Spatio-temporal perspectives of forest fires regimes in a maturing Mediterranean mixed pine landscape. Eur J For Res 128:297–304
Acknowledgements
We thank Ezra Ben-Moshe, Paivy Yuval, and many more for their help in the field work, Charles D. Canham for helpful advice on data analysis, and the Hedin lab members for advice on the manuscript. This work was funded by the Israeli Science Foundation Grant #514/10.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Miren del Rio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sheffer, E., Cooper, A., Perevolotsky, A. et al. Consequences of pine colonization in dry oak woodlands: effects on water stress. Eur J Forest Res 139, 817–828 (2020). https://doi.org/10.1007/s10342-020-01287-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-020-01287-3