Abstract
Alpine ecosystems face multiple environmental impacts caused by extraordinary rapid warming. One of the impacts caused by rising temperature is the prolongation of the growing season. To determine temperature-driven variation in leaf and xylem phenology, we studied Norway spruce and European beech along two elevational transects in the Northern Limestone Alps, southern Germany. Phenology was observed, and microcore samples were taken regularly from May to October 2011. Microcore thin sections were analyzed for expanding, wall thickening and mature cells in the forming tree ring. Leaf phenology and xylem phenology were compared in respect of onset dates, elevational responses and aspects. Both tree species showed similar responses to elevation in early spring leaf and xylem phenological phases. Delayed start dates and advanced end dates led to shortened growing seasons with elevation for both leaf and xylem development. Elevational responses of phenological phases were similar to each other throughout the growing season in spruce, but only at the beginning of the season for beech. Xylem growth periods from cell expansion until the end of lignification were strongly reduced with elevation for beech, especially the period in which new cells were formed. This was mainly due to an earlier end of xylem cell formation in higher elevations. For spruce, the length of the xylem cell growth period decreased less with elevation. Colder conditions in high elevations seemed to lead to a longer period of cell maturation in spruce. We conclude that with warmer temperature conditions, the tree-ring growth period could be lengthened more in beech compared to spruce, as long as it is not limited by drought stress or unfulfilled chilling requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mountain ecosystems are considered to be especially vulnerable to a changing climate (e.g., IPCC 2013). Among the most apparent consequences are shifts in plant phenological phases, which have been demonstrated in many studies for the mid- and high latitudes of the northern hemisphere (e.g., Menzel and Fabian 1999; Studer et al. 2005; Menzel et al. 2006; Rosenzweig et al. 2008). Changes in the extent of the growing season further affect the temporal patterns of secondary growth of perennial organisms, explaining in part also changes in forest productivity (McCarty 2001; Tegel et al. 2014). Increasing temperature is expected to increase tree growth in temperature-limited environments (Jyske et al. 2014). This is of special interest for mountain forests, since trees at high elevations or high latitudes reveal stronger growth responses to climatic change due to their pronounced sensitivity to temperature (e.g., Briffa et al. 2002; Frank and Esper 2005). With warming having been proven to be above-average in alpine regions (Beniston 2003; Nogués-Bravo et al. 2007), pronounced changes in the duration of wood formation (Pinus leucodermis, Deslauriers et al. 2008), height growth (Picea mariana, Gamache and Payette 2005) and reproductive success (Pinus sylvestris, Kullman 2007) can be expected for higher altitudes. In particular, species-specific responses to changes in regional patterns of temperature and precipitation deserve deeper investigation.
Understanding the temperature-mediated adjustment in leaf phenology and its connection to growth dynamics may allow novel insights into climate change impacts on mountain forest ecosystems. The impact of global warming on tree phenology and the vegetation period was first reported by Menzel and Fabian (1999). Spring onset dates were advanced and autumn onset dates were delayed by higher temperature which resulted in a prolongation of the foliated period (e.g., Estrella and Menzel 2006; Vitasse et al. 2009; Schuster et al. 2013). Higher temperature also advanced the beginning of xylogenesis (Deslauriers et al. 2008; Rossi et al. 2013). The well-known pattern of decreasing tree-ring increment with increasing elevation (e.g., Kozlowski et al. 1991; Dobbertin and Giuggiola 2006) is considered to be closely linked to the length of the growing season (Moser et al. 2010; Rossi et al. 2013). Intra-annual phenology of phloem and xylem formation is a current issue in tree-ring research (Semeniuc et al. 2014; Swidrak et al. 2014; Gričar et al. 2015), since for many tree species it is still unknown how the development in both xylem and phloem growth is triggered by environmental conditions. Temporal dynamics in wood formation is highly variable among species, sites (e.g., elevation) and years; the latter one is related to a highly plastic response of radial growth to climate conditions (Martinez del Castillo et al. 2016). If and how tree species could profit in forest productivity and carbon uptake by a lengthened vegetation period caused by climate warming are among the recent open questions (Rossi et al. 2013, 2014). Since few studies include both xylem and leaf phenology, the demand for further research in this area is reflected in recent studies (Moser et al. 2010; Michelot et al. 2012; Prislan et al. 2013; Čufar et al. 2015). In particular, how these interactions differ with elevation has not been satisfactorily investigated (Moser et al. 2010).
To address this knowledge gap, we studied leaf phenology and intra-annual tree-ring development for two species on eight sampling sites on two elevational transects from 800 to 1400 m a.s.l. in the Bavarian Alps. Lacking associated long-term studies, we used this space-for-time approach (Körner 2003) to approximate responses in tree growth to global warming scenarios by responses to different elevational temperature regimes. We focused on the deciduous European beech (Fagus sylvatica L.) and the evergreen Norway spruce (Picea abies (L.) Karst.) since, together with silver fir (Abies alba Mill.) and sycamore maple (Acer pseudoplatanus L.), they form the predominant vegetation from the lower montane to the subalpine elevational belt of the Northern Alps (Ewald 1997). These species are characterized by a wide geographic range, longevity, and sensitivity to climate (Piovesan et al. 2005; Gryc et al. 2012), which makes them suitable for both dendroecological and phenological studies.
The aim of our study was to distinguish the timing of cell formation including cell expansion, wall thickening and mature xylem cells. We expected delayed start dates and advanced end dates of xylogenesis and leaf phenology with elevation and clear differences between species and aspects. Therefore, three important research questions could be examined:
-
1.
How does the timing of phenology and xylogenesis vary with elevation?
-
2.
How do leaf phenology and tree-ring growth differ between species and aspects?
-
3.
Are there hints to a linkage between leaf phenology and wood formation?
For these purposes, we included four different elevational levels, two aspects (north and south), two tree species (beech and spruce), phenological observations and microcore sampling in the study.
Materials and methods
Study site
Our study site was located in the Bavarian Wetterstein Mountain range (Northern Limestone Alps) comprising two elevational transects (one northern and one southern aspect; Fig. 1a). The highest mountain in Germany, the Zugspitze (2962 m a.s.l., 47°25′N, 10°59′E), is situated above the Loisach valley and the city of Garmisch-Partenkirchen (708 m a.s.l., 47°30′N, 11°5′E). Mean annual precipitation and mean annual temperature are 1363 mm and 6.5 °C at Garmisch and 2003 mm and −4.8 °C at the Zugspitze (German Meteorological Service data 1961–90). Mixed forests cover the sub-montane to subalpine slopes from the valley floor up to tree line at 1700–1800 m a.s.l.
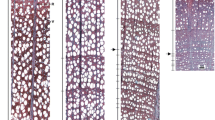
a Sampling sites on the two transects. b Example for model fits of the annual course of cell type increments: Gompertz growth model for mature cells and total xylem (blue, black) and an adjusted Hadwiger model for expanding and wall-thickening cells (light blue, violet). c Exemplary thin sections of beech (11. July 2011) and spruce (25. July 2011) at 800 m a.s.l
European beech (Fagus sylvatica L.) is the most abundant deciduous tree and Norway spruce (Picea abies (L.) Karst.) the most common evergreen tree species, accompanied inter alia by Acer pseudoplatanus, Abies alba and Pinus sylvestris. At higher elevations, Pinus mugo, Larix decidua and Pinus cembra are present. European beech and Norway spruce are dominant tree species over a wide vertical range at both the northern and southern slopes which were chosen for microcore sampling. Their upper elevational limits are at about 1500 m a.s.l. (north and south aspect) for beech and at about 1700 (south aspect) to 1800 m a.s.l. (north aspect) for spruce. On the two transects Garmisch-Partenkirchen—Kramer pass (south aspect) and Garmisch-Partenkirchen—Kreuzeck (north aspect), sample sites were located at 800, 1000, 1200 and 1400 m a.s.l. (Figure 1a). At each site, a temperature/humidity logger (onset HOBO® PRO V2 in a radiation shield fixed at the top of a 2 m pole) measured temperature and relative humidity at 30 min intervals. At each transect, a climate station provided full meteorological information (Felsenkanzel at 1250 m a.s.l. on the south aspect transect and Kreuzeck at 1600 m a.s.l. on the north aspect transect).
Logger temperature measurements along the elevational transect showed strong temperature oscillation throughout the growing season in 2011 (Fig. 2). Daily mean temperature showed the expected decrease with elevation with variable lapse rates from day to day (Fig. 2). Monthly mean lapse rates were similar on both transects, but slightly less pronounced at the southern transect: −0.47, −0.49, −0.49 and −0.39 °C 100 m−1 at the south facing slope, and −0.45, −0.47, −0.47 and −0.35 °C 100 m−1 at the north facing slope (May to August). The data of the meteorological stations revealed higher precipitation from May to August 2011 (269.2, 232.2, 310.4, 310.0 mm) after a dry spring (March 84.8 mm, April 73.2 mm; Felsenkanzel station). Mean temperatures for the months May to August were 10.8, 12.4, 12.0 and 16.1 °C at the Felsenkanzel station (1250 m a.s.l.) and 8.5, 10.3, 9.8 and 14.5 °C at the Kreuzeck station (1600 m a.s.l.).
Phenological observations and microcore sampling
Trees for microcore sampling were selected at each site out of already marked trees of a running phenological observation study. Since not all trees were suitable for microcore sampling, this study only included a small number (three) of trees per site and species. Selected trees were dominant and had stem diameters at breast height ranging from 30 to 60 cm, which equates to an age of about 60–110 years for beech and 50–90 years for spruce. Tree heights ranged from 20 to 30 m for beech and from 18 to 35 m for spruce.
Spring phenology was observed weekly on all trees from April to June 2011 following a modified BBCH-Code after Meier (1997). The most advanced development stage found on each tree was noted (see Schuster et al. 2013). In autumn, the percentage of green, colored and fallen leaves was observed for beech. We selected three spring and three autumn phases to be related to tree-ring growth:
-
BBCH 07: bud burst (first green visible)
-
BBCH 11: beginning of leaf unfolding (beech)/beginning of needle expanding and shoot growth (spruce)
-
BBCH 17: all leaves fully unfolded (beech)/needles and shoots reached final length (spruce)
-
BBCH 93: beginning of leaf coloration (5 % colored)
-
BBCH 95: leaf coloration (50 % colored or fallen, 50 % green leaves)
-
BBCH 97: end of leaf fall (95 % of leaves fallen)
For these phenological phases, specific onset dates were calculated for each site and species based on the observational data (see Schuster et al. 2013).
Microcore samples (1.5 cm length, 2 mm diameter) were taken from the beginning of May to the beginning of October 2011 using the Trephor microcore tool (Rossi et al. 2006a). The sampling interval initially was 1 week and later was reduced to 2 weeks. Sampling days of the year (DOY) were: 122 (May 2), 129, 136, 143, 150, 157, 171, 181, 192, 206, 220, 234, 255, 269, 279, 290 and 297 (October 24). A total of 510 microcore samples were collected from 30 trees (14 beech, 16 spruce) at 17 sampling dates. Sampling took place orthogonal to the slope direction at breast height (app. 1.3 m). The microcores (one per sampling date) were taken in rows from left to right with 3–4 cm horizontal and 6–8 cm vertical separation. When necessary, the bark was carefully removed with a small chisel before sampling. Samples were immediately stored in Eppendorf tubes with a preserving storing solution (Ethanol, Glycol, distilled water, 1:1:1).
Preparation of the samples
In the laboratory, microcore samples were washed and dehydrated in increasing alcohol series (70, 80, 90 and 96 %, 2 h each). After pre-infiltration with Technovit 7100 (7–12 h), sample infiltration followed (infiltration solution I and II, 7–12 h each). Finally, samples were embedded in Technovit 7100 and dried for about 24 h. With a Leica RM 2050 rotation microtome, 10–15-µm-thin sections were cut orthogonal to the fiber direction, in order to obtain transversal cuts of the newest tree rings. Sections were stained (5 min Safranin-O-Astrablau, 1 min acetic acid, 1 min washed in distilled water, 10 min Astrablau, 1 min washed in distilled water) and fixed (alcohol series of 96 %, 100 %, Xylol 1 and Xylol 2, 5–10 min each) before covering by a Leica CV Mount. Completed slices were digitized by photography using the KAPPA Image Base software and camera with 63× magnification. When more than one photograph was required to display the entire tree ring, pictures were compiled using Adobe Photoshop. Measurement was completed in Adobe Photoshop using the analyzing tools.
Measurements and analyzes
In the microcore sections, we distinguished areas with expanding cells (blue-stained, thin-walled, large radial dimension compared to cambium), wall-thickening cells (thick-walled, not lignified) and mature cells (red-stained, lignified), forming the total xylem increment at the sample date. Their respective widths were determined as the mean of three measurements in Adobe Photoshop. Expanding cells, wall-thickening cells, mature cells and total xylem were noted as “cell types” (Fig. 1b, c). In beech, we focused on the development of fibers, as we measured the areas within the tree ring occupied with the different cell types.
Since time series of xylem and mature cell measurements showed a sigmoidal shape over the whole vegetation period and a final maximum plateau (asymptote, Fig. 1b), a Gompertz growth curve was fitted (e.g., Gryc et al. 2012; Swidrak et al. 2014; Jyske et al. 2014). For expanding cells and wall-thickening cells, which display a clear annual maximum, an adjusted three-parametric Hadwiger model was fitted as a bell-shaped unimodal growth model (Hadwiger 1940, Fig. 1b). The applied formula for the Hadwiger model was:
RW = ((a * b * (c/DOY)3/2)/c) * e –b^2 * (c/DOY + DOY/c−2)
with parameters a, b, and c, DOY representing the day of the year and RW representing the predicted tree-ring increment of the respecting cell types. In total, 120 models were fitted (4 cell types, 30 trees). The goodness of fits was above 0.80 (R2) for all Gompertz models and for Hadwiger models they ranged between 0.59 and 0.92 (R2). In two cases for the wall-thickening cells, R2 dropped to 0.42 and 0.49 (Table 1). Since the models for the individual tree-ring measurements fitted very well in most cases, they allowed the calculation of onset dates with high reliability.
For each tree, we calculated start and end onset dates of the different cell types (expanding cells, wall thickening, mature cells, total xylem) on the basis of the fitted curve parameters. The start dates were defined at the date when one cell row had been formed, approximated by 0.02 mm for beech and 0.05 mm for spruce. The end dates were calculated when 95 % of the growth limit was reached. Based on the start and end onset dates, the total growing periods for all cell types were determined. Further, we determined the maximum increment (maximum of the bell-shaped Hadwiger model or plateau for the Gompertz curve) with the respective onset date for all cell types. Additionally, we calculated the maximum growth rate and the respective onset date for the total xylem formation at the inflection point of the Gompertz growth curve. The response of the derived parameters of xylem formation and leaf phenology (onset dates, period, maximum increment…) to elevation was determined for both aspects separately as the slope from a linear regression (parameter on elevation). Onset dates and slopes of leaf and xylem phenology were compared to reveal differences between aspects and assess possible linkages between them. The R version 3.0.0 was used for statistical analyzes and figures (R Development Core Team 2013).
Results
Onset dates of leaf and xylem phenological phases in 2011
To test for differences between the south and the north aspect transect, mean onset dates (day of year, DOY) were compared for both species. Mean onset dates included all sites of a transect, and therefore the deviations were due to the elevational differences between the lowest and the highest sites. Bud burst (BBCH 7) in 2011 started at the beginning of April for beech and end of April for spruce and was delayed with elevation (Fig. 3). No clear differences between aspects were found in spring phenological onset dates for beech. For spruce, onset dates were slightly earlier at the south aspect transect. Mean bud burst dates were DOY 101 ± 8 (south aspect) and DOY 100 ± 10 (north aspect) for beech and DOY 120 ± 12 and DOY 128 ± 9, respectively, for spruce. BBCH 11 occurred at DOY 133 ± 5 (south aspect) and DOY 131 ± 6 (north aspect) for beech and at DOY 150 ± 8 (south aspect) and DOY 156 ± 8 (north aspect) for spruce. All leaves and needles were unfolded and had their final dark color (BBCH 17) in mid-June for beech (DOY 165 ± 4 at south aspect, DOY 162 ± 3 at north aspect) and end of June for spruce (DOY 181 ± 5 at south aspect, DOY 184 ± 8 at north aspect).
Elevational responses in leaf and xylem phenological phases for common beech and Norway spruce at the south (points, straight line) and the north (triangles, dashed lines) aspect transect. Results of linear regressions are presented in Table 1
Onset dates of beech leaf senescence were similar between aspects. Leaf coloring (BBCH 93) of beech started mid-September: DOY 259 ± 9 at the southern, DOY 262 ± 4 at the north aspect transect. BBCH 95 occurred at DOY 277 ± 11 (south aspect) and at DOY 278 ± 6 (north aspect). The end of leaf senescence (BBCH 97) occurred in the beginning of November: DOY 307 ± 12 at south aspect, DOY 311 ± 9 at north aspect. The total foliated period (BBCH 7 to BBCH 97) was on average 208 ± 17 days (south aspect) and 209 ± 18 days (north aspect). Thus, there was no clear difference in leaf phenology between the south and north facing aspects.
Xylem formation of spruce at 800 m a.s.l. started in the beginning of April (north aspect) to late-April (south aspect) and was delayed with elevation to mid-May at 1400 m a.s.l. (both aspects, Fig. 3). The mean start date for xylem formation at all transect sites was DOY 134 ± 6 (south aspect) and DOY 129 ± 11 (north aspect) for beech. For spruce, mean start dates were DOY 127 ± 14 (south aspect) and DOY 118 ± 23 (north aspect). Thus, xylem formation started earlier for spruce and earlier at the northern aspect for both species. On average, wall thickening started 10 days, and maturation 35 days after the first expanding cells for beech (7 and 26 days at south aspect, 12 and 42 days at north aspect). For spruce, respective values were 15 and 40 days (12 and 35 days south aspect and 18 and 45 days north aspect). The total period of xylem formation was on average 116 days for beech (106 days south aspect, 125 days north aspect) and 118 days for spruce (112 days south aspect, 124 days north aspect). The period of xylem formation ended between end-August and end-September for both species (all cells lignified, Fig. 3). On average, all cells were mature at DOY 237 ± 9 (south aspect) and DOY 264 ± 31 (north aspect) for beech and at DOY 236 ± 10 (south aspect) and DOY 249 ± 20 (north aspect) for spruce. Thus, xylem formation started earlier at the north aspect transect but ended earlier at the south aspect transect for both species. The growth period for all cell types was shorter (thus the course of cell differentiation was faster) at the southern aspect. For the period between the beginning of cell wall thickening and the beginning of lignification, the timing of xylogenesis was similar between aspects. Differences between the lowest and highest site in xylogenesis were always larger at the north facing aspect.
Maximum growth rate and maximum increment of cell types
The mean maximum growth rate of xylem formation (all transect sites) was 0.05 ± 0.004 mm day−1 (south aspect) and 0.05 ± 0.02 mm day−1 (north aspect) for beech and 0.04 ± 0.01 mm day−1 (both aspects) for spruce. Hence, maximum growth rate was similar at both transects. The date of the maximum growth rate was similar between aspects for spruce (DOY 162 ± 10 at south aspect, DOY 164 ± 10 at north aspect) but earlier at the southern aspect for beech (DOY 162 ± 10 at south aspect, DOY 175 ± 18 at north aspect).
Mean maximum increment of the tree ring (total xylem) was 1.7 ± 1.0 mm (south aspect) and 2.0 ± 1.9 mm (north aspect) for beech and 2.0 ± 1.9 mm (south aspect) and 3.9 ± 1.9 mm (north aspect) for spruce. Gompertz fits on total xylem measurements for both species and all sites can be found in the supplementary material, and final tree-ring increments are displayed in Fig. 3. Tree-ring increments therefore were larger at the north facing aspect for both species, especially for spruce. The maximum increment for expanding cells was 0.1 ± 0.04 mm (south aspect) and 0.2 ± 0.04 mm (north aspect) for beech and 0.3 ± 0.1 mm (both aspects) for spruce. The maximum increment for wall-thickening cells was 0.2 ± 0.1 mm (south aspect) and 0.1 ± 0.07 mm (north aspect) for beech and 0.4 ± 0.3 mm (south aspect) and 0.5 ± 0.3 mm (north aspect) for spruce. The maximum increment of expanding cells therefore remained below 0.2 mm (beech) and 0.5 mm (spruce) and the maximum increment of wall-thickening cells below 0.8 mm (spruce) and 0.4 mm (beech), although the final tree-ring widths reached several millimeters.
Variation of phenology and xylogenesis with elevation
Elevational responses of all leaf phenological and xylogenesis parameters are listed in Table 1. In leaf phenology, spring onset dates were delayed with elevation for both species and beech autumn onset dates were advanced with elevation (Fig. 3, Table 1). Elevational responses of spring leaf development were higher at the north aspect transect for beech, but similar for spruce (Table 1). Leaf coloring (BBCH 93) of beech expressed an elevational gradient only at the south aspect transect. Later senescence phases (BBCH 95 and BBCH 97) had stronger responses at both transects. All elevational responses of senescence phases at the south facing aspect were higher or similar to the responses at the north facing aspect. The total foliated period of beech (BBCH 7 to BBCH 97) was shortened by –4.7 ± 2.2 days (south aspect) and −8.0 ± 1.8 days (north aspect) per 100 m elevation (Table 1).
In xylogenesis, most of the onset dates of the different cell types showed elevational responses, but only few were significant (see Table 1 for all further details). However, a clear pattern existed for both species where leaf phenology spring onset dates and elevational responses of xylogenesis start dates were more pronounced at the northern aspect. Similarly to leaf phenology, start dates of expanding cells were delayed with elevation, whereas the response got weaker for wall thickening and mature cells. The end dates of expanding cells were advanced (on average −11.4 days 100 m−1 for beech, −3.1 days 100 m−1 for spruce) and the end dates of wall thickening and mature cells less advanced with elevation. This results in a strongly reduced growth period for expanding cells with elevation and a weaker reduction in period for wall thickening and mature cells for both species, but especially for beech. At the north aspect transect, end dates for mature cells in spruce even turned positive, meaning that maturing of the cells in high elevation lasted longer than in the valleys. It is noticeable that the reduction in xylem growth period in beech was mainly due to strongly advanced end dates of xylogenesis with elevation rather than delayed start dates. For spruce, however, end dates contributed less to the shortening of xylem growth period with elevation compared to the start dates. Combining a temperature lapse rate of 0.47 °C 100 m−1 and transect mean elevational responses for the end of expanding cells, the end dates of cell formation were delayed by about three weeks per degree for beech, but only three days for spruce.
For both species, not only the total tree-ring width was reduced with elevation, but also the maximum increment of expanding and wall-thickening cells (Fig. 3). Although the maximum tree-ring increment was smaller at the south aspect transect for both species, especially for spruce (see before), their elevational response did not differ between aspects. The maximum growth rate did not show any clear response to elevation. The date of maximum growth rate and the date of maximum increment (expanding and wall-thickening cells) were advanced with elevation for beech, but were delayed with elevation for spruce. Responses of maximum growth rate dates were always stronger at the north facing aspect for spruce but not for beech (see Table 1 for all mentioned details).
Linkages between leaf phenology and xylem formation
The beginning of xylogenesis (start dates of expanding cells) occurred shortly before or simultaneously with bud burst for spruce (Fig. 3). More precisely, both onset dates (expanding cells and BBCH 7) revealed similar significant responses to elevation with about four days delay per 100 m (Table 1). Combined with the May temperature lapse rate of −0.47 °C 100 m−1, this would result in an advance of spring onset dates of about 8.5 days per degree. The maximum growth in spruce occurred shortly after the beginning of needle elongation (BBCH 11) and equally revealed a similar, significant response to elevation of about 3 days per 100 m (Table 1).
In beech, first xylem cells were formed after bud burst about the time of leaf unfolding (BBCH 11). However, the elevational response rate of the start dates of expanding cells was more similar to the elevational response of bud burst (about 4.5 days 100 m−1 or 9.5 days °C−1, significant values in Table 1 for beech at the north aspect). The maximum growth in xylem formation occurred in beech when leaves were fully developed (BBCH 17) in high elevations or some weeks after the BBCH 17 onset date in the valley and showed a negative response to elevation (Fig. 3, Table 1). In autumn, xylem formation in beech ended before leaf coloring (south aspect and high elevations at north aspect) or shortly after (low elevations at north aspect) and was clearly advanced with elevation (Table 1).
Summarizing, elevational (thus temperature) responses of onset dates were remarkably similar in leaf and xylem phenology of spruce over the total growing season, but only at the beginning of the growing season for beech.
Discussion
Onset dates of leaf and xylem phenological phases
In 2011, beech bud burst in our study area occurred from the beginning of April at the lowest site to beginning of May at the highest site. Bud burst of spruce started about three weeks later (end-April in the valley to end-May at the higher sites). Leaf senescence of beech started mid-September and lasted to the beginning of November. We could determine clear responses of leaf phenological phases to elevation, since forcing temperature and winter chilling are dominant triggers (e.g., Menzel and Fabian 1999; Heide 2003; Körner 2007). However, there are several additional factors influencing the onset of leaf phenological phases such as precipitation, photoperiod, CO2 concentrations, nutrient availability, date of snowmelt, life traits, and stress caused by heat, drought, frost, shading, UV-radiation, ozone or pathogens.
Xylogenesis in spruce started between beginning and mid-April for spruce at our lowest sites at 800 m a.s.l.. We determined mean start date of wall thickening to occur 15 days and the mean start date of mature cells to be 40 days after the start date of xylem formation in spruce. Comparable values were reported by Swidrak et al. (2014; Norway spruce, Austria) and Rossi et al. (2013; nine conifer species, Canada and Europe), which support our findings. The average xylem formation period of 118 days for spruce in our study also matches reported values (100 to 120 days in Norway spruce in different years, Rossi et al. 2007; 105 to 120 days for conifer species, Rossi et al. 2013).
First beech xylem cells in our study started to expand in May at the time of leaf unfolding, a few weeks after bud burst. Cell wall thickening of beech xylem cells in our study area followed about 10 days and maturation about 35 days after the time when first cells were formed. Averaged tree-ring development period (start to end date of total xylem) was 116 days. In a comparable study (850 m a.s.l., 2011, Eastern Carpathians), xylem cell formation in beech started mid-May, the onset of lignification at DOY 143, first mature cells at DOY 165, and the completion of the tree ring at DOY 268 in 2011. The period for complete annual ring formation in beech was 137 days (Semeniuc et al. 2014).
The response of the beginning of beech xylem formation to elevation was more similar to the response of bud burst than of leaf unfolding, although cell formation and leaf unfolding occurred approximately at the same time. This suggests a temporal link between the onset of wood formation and bud burst, and that xylem growth only began after the new leaves started photosynthesis. Michelot et al. (2012) also found beech radial growth dependent on leaf phenology and suggested that fast maturation in leaves likely promoted fast tree-ring formation. Compared to spruce, wood formation in beech in our sites started about 1 month later, but the process of wall thickening and lignification was faster.
Averaged maximum growth rates occurred mid-June for both aspects and both species, which matches the hypothesis of synchronization with the maximum day length (Rossi et al. 2006b). Similarly, Jyske et al. (2014) did not find a clear trend of the peak of cell production along a latitudinal transect and concluded that photoperiod is the main controlling factor, followed by temperature and water availability. Since in our study both species showed a more or less significant response to elevation in the date of maximum growth rate, we can conclude that temperature played an important role in the timing of maximum cell growth. Other studies also reported variation in the date of maximum growth between May and June in both beech (e.g., Michelot et al. 2012) and spruce (e.g., Gričar et al. 2014). Beech radial growth was additionally positively triggered by wet and cool June (Di Filippo et al. 2007, Eastern Alps) and the peak of cell production in spruce by the warmest period of the growing season in July (Jyske et al. 2014, Finland). In our study, the average maximum growth rate was slightly higher in beech (0.05 mm day−1) than in spruce (0.04 mm day−1), which is also reflected in other studies (e.g., Michelot et al. 2012; Gričar et al. 2014).
Differences between aspects
Several observed effects in the elevational timing of leaf and xylem phenology may be attributed to the different temperature regimes at the north and the south facing transects. At the north facing slopes, snow lasted longer in higher elevations when temperatures in the valley had already increased and the snow on high elevations at the south facing slopes already vanished. Thus, the temperature gradient with elevation was pronounced here. This translated into strong slopes for start dates and in the higher deviations of the mean onset dates at the northern aspect transect (both xylogenesis and leaf phenology). This strong temperature gradient at the north aspect transect compared to a moderate temperature gradient at the south aspect transect presumably also led to the surprising result of earlier xylogenesis onset dates at the north aspect transect. Moreover, the pronounced temperature gradient at north aspect transect obviously influenced beech development since it started bud burst about 3 weeks earlier than spruce. Hence, elevational responses of xylogenesis and spring leaf phenology in beech were stronger at the north facing slope and revealed less significance at south aspect. The elevational response of the foliated period in beech (BBCH 7–BBCH 97) was also stronger at the north facing slope. The same effect, not between aspects but in time, could be found in stronger responses of early leaf and xylem phenology phases to elevation compared to later phases, since the temperature gradient was more emphasized early in the year.
The mean growth periods for all cell types were always shorter at the south aspect transect for both species (differences of one to two weeks were within the standard deviations). Thus, the mean total tree-ring increment was slightly smaller in beech and only about half in spruce at the south aspect transect. These results highlight the length of the growing period as an important modulator of tree-ring increment rather than growth rate (Michelot et al. 2012), since maximum growth rates in our study did not reveal a clear response to elevation. In contrast to our results, Rossi et al. (2007) reported shorter growth periods in conifers at northern compared to southern aspects, resulting in smaller tree rings. The smaller tree-ring increment in spruce at our south aspect transect could be caused by its sensitivity to summer drought (Zang et al. 2011; Hartl-Meier et al. 2014a). Martinez del Castillo et al. (2016) showed reduced xylogenesis duration at the southern distribution limit of beech under the warm and dry Mediterranean climate. Similarly, the slightly smaller increment in beech at the southern aspect could be caused by a less optimal water balance since beech tree-ring growth was significantly limited by high summer temperatures accompanied by low precipitation (Zang et al. 2011). Although our alpine study area is characterized by high annual precipitation, higher insolation and a relatively low soil depth at the south aspect transect could have reduced soil water availability.
Variation of phenology and xylogenesis with elevation
In our study, leaf phenological phases showed advanced spring and delayed autumn events with lower elevation and warmer temperature, resulting in a prolongation of the foliated period. These temperature-driven responses along elevational transects and in time series are expected under global warming conditions (e.g., Menzel and Fabian 1999; Estrella and Menzel 2006; Vitasse et al. 2009; Schuster et al. 2013).
Similarly, the general pattern of xylogenesis onset dates revealed (as expected) delayed start and advanced end dates with elevation for both species, resulting in shorter growth periods with colder conditions. This pattern also was demonstrated along a latitudinal gradient (Jyske et al. 2014) and thus is mainly triggered by temperature. In our study, especially the growth period for expanding cells was reduced drastically with elevation along our transects for beech and for spruce, which was the principal explanation for reduced tree-ring increment with elevation. In beech, the reduction of the growth period was mainly influenced by strongly advanced end dates of xylogenesis with elevation. This variation of the growth period with elevation was the most obvious difference between spruce and beech. In a comparable study in the Northern Limestone Alps (Hartl-Meier et al. 2014b), the decrease in tree growth with elevation was also more pronounced for beech compared to spruce. Similarly, Michelot et al. (2012) reported the tree-ring increment in beech was largely influenced by the growth duration, especially by the end dates, but not on onset dates. It must be mentioned that in contrast to spruce, beech almost reached its elevational distribution limit in the study area (about 1500 m a.s.l.) at the highest sampling site (1400 m a.s.l.), which could also contribute to the pronounced reduction in tree-ring growth. Moreover, the onset dates for maximum increment in expanding and wall-thickening cells as well as the date for maximum xylem growth rate were advanced with elevation in beech, but delayed in spruce. These results were closely linked to the explicit pronounced reduction of the end of the growth period in beech and reveal another clear difference between beech and spruce.
The reduction of tree-ring increment with elevation has been well known for long (e.g., Kozlowski et al. 1991; Dobbertin and Giuggiola 2006). We could demonstrate for both species that not only total xylem (final tree ring) but also the maximum increment of expanding cells and wall-thickening cells decreased significantly or almost significantly with elevation. Thereby, elevational response rates for the growth periods of expanding, wall thickening and mature cells were gradually reduced for both species. These results imply that in high elevations, lower temperatures in the end of the xylogenesis process were compensated by a comparatively longer period of maturation. The longer period for cell maturation was especially detectable for spruce at the “colder” northern aspect where the elevational response of mature cells even turned positive. For high elevation sites, several studies reported formation of new cells to cease earlier, but the process of enlargement, wall formation and lignification to last for a longer time (Rossi et al. 2007; Gričar and Čufar 2008; Gryc et al. 2012).
Elevational responses of both phenology onset dates and tree-ring increments vary between years. In particular, in years with extreme weather conditions, relationships could be very different. The sample year 2011 was no such extreme year, but the results of our study covering only one vegetation period could be improved by data of longer time-series.
Potential links between xylogenesis and phenology
The sequence and timing between parameters of intra-annual tree-ring growth and phenology differed between the species studied. In spruce, xylogenesis started about the time of bud burst, which indicates that both xylogenesis and leaf phenology begin simultaneously when environmental temperatures are suitable. Spruce also showed similar elevational responses of xylogenesis and leaf phenological onset dates, which also indicates a similar response to temperature. Moreover, at the north aspect transect the onset dates of maximum growth rate and the end dates of xylogenesis showed comparable values in their response to elevation. This could mean that the timing of differentiation phases in spruce xylogenesis depends on each other, especially on the first phenological event, a hypothesis suggested by Rossi et al. (2013).
For beech, the start date of xylem formation took place about three weeks after bud burst (BBCH 7) during the time of leaf unfolding (BBCH 11). Michelot et al. (2012) reported the beginning of beech growth occurring shortly after bud burst and the maximum growth rate in June when leaves were mature, concluding that beech radial growth was highly dependent on leaf phenology. In our study, the maximum growth rate occurred in when leaves were fully unfolded (BBCH 17) at high elevations, but several weeks later in the valley. The date of maximum xylem growth rate and later xylogenesis onset dates seemed to be uncoupled from (spring) leaf phenology. For example, xylogenesis ended when leaf coloring had already begun at the two lower sites (but before the end of the senescence process) and before or with the beginning of leaf coloring at the higher sites. In other studies, leaf coloring occurred up to two month after cessation of wood production (Prislan et al. 2013; Čufar et al. 2015). Photosynthesis products produced after the end of wood formation were considered to be deposited as reserves, although it is not clear if and how these affect leaf and cambial phenology the next year (Čufar et al. 2015).
Xylogenesis onset dates reveal high year-to-year variation (e.g., Jyske et al. 2014; Martinez del Castillo et al. 2016), which can only be partly captured by different elevational temperature regimes in our one-season study. Our results help to understand responses of leaf and xylem phenology to temperature, but how climate change and a subsequent longer growing season (e.g., Menzel and Fabian 1999; Menzel et al. 2006) will translate into changes in secondary stem growth cannot be clearly answered with our results. Responses are definitively species-dependent (as we showed in beech versus spruce in our study) and site-specific. Additionally, risks associated with increasing lack of winter chilling (Heide 2003; Caffarra and Donnelly 2011), changes in water availability (increased evapotranspiration and changes in precipitation; Jyske et al. 2014), and more frequent and intense summer droughts (e.g., Christensen et al. 2007; Engler et al. 2011) further complicate solid assessments.
Conclusion
We could quantify various responses in leaf and xylem phenological parameters to elevation in the Bavarian alpine forest. Early spring onset dates of leaf and xylem phenological phases advanced with rising temperature in similar magnitudes for both beech and spruce. In spruce, later phenological phases likewise revealed similar response rates compared to early phases, suggesting that they are dependent on each other. In beech, only early phases (especially bud burst, and start of xylem cell expanding) showed similar responses to changes in temperature, implying that they are similarly sensitive to temperature and/or dependent on each other to some extent. For later phenological phases in beech, there are no hints to connections between wood growth and leaf phenology. Our results confirm the period and especially the end dates of xylem cell formation as main modulator for final tree-ring increment, rather than variations in the start dates or the maximum growth rate. With sufficient water supply, rising temperature might extend the wood growth period (and therefore the tree-ring increment) stronger in beech than in spruce. Spruce may extend the growth period for cell forming only marginally, but reduce the time necessary for completion of cell maturing. Our results based on elevational transect data offer insights into the variation of leaf and xylem phenological phases under different temperature regimes. However, both leaf phenology and xylogenesis highly depend on season, site, species and individuals. In particular, in years with extreme weather conditions, response rates of phenological onset dates and tree-ring increment to elevation could be very different. More long-term studies, including different tree species and a high number of sampled trees, are necessary to complete our understanding of potential changes within forest ecosystems under global change scenarios. Particularly, tree growth under both changing regional temperature and precipitation patterns—and additional extreme events—deserves intensive investigation.
References
Beniston M (2003) Climatic change in mountain regions: a review of possible impacts. Clim Change 59:5–31. doi:10.1023/A:1024458411589
Briffa KR, Osborn TJ, Schweingruber FH, Jones PD, Shiyatov SG, Vaganov EA (2002) Tree-ring width and density data around the Northern Hemisphere: part 1, local and regional climate signals. Holocene 12:737–757. doi:10.1191/0959683602hl587rp
Caffarra A, Donnelly A (2011) The ecological significance of phenology in four different tree species: effects of light and temperature on bud burst. Int J Biometeorol 55:711–721. doi:10.1007/s00484-010-0386-1
Christensen JH, Hewitson B, Busuioc A, et al. (2007) Regional climate projections, climate change, 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. University Press, Cambridge, pp 849–940
Čufar K, De Luis M, Prislan P, Gričar J, Črepinšek Z, Merela M, Kajfež-Bogataj L (2015) Do variations in leaf phenology affect radial growth variations in Fagus sylvatica? Int J Biometeorol 59:1127–1132. doi:10.1007/s00484-014-0896-3
Deslauriers A, Rossi S, Anfodillo T, Saracino A (2008) Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol 28:863–871. doi:10.1093/treephys/28.6.863
Di Filippo A, Biondi F, Čufar K, De Luis M, Grabner M, Maugeri M, Presutti Saba E, Schirone B, Piovesan G (2007) Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: spatial and altitudinal climatic signals identified through a tree-ring network. J Biogeogr 34:1873–1892. doi:10.1111/j.1365-2699.2007.01747.x
Dobbertin M, Giuggiola A (2006) Baumwachstum und erhöhte Temperaturen. Forum für Wissen 2006: 35–45. Eidg. Forschungsanstalt für Wald, Schnee und Landschaft WSL
Engler R, Randin CF, Thullier W, Dullinger S, Zimmermann NE, Araújo MB, Pearman PB, Le Lay G, Piedallu C, Albert CH, Choler P, Coleda G, De Lamo X, Dirnböck T, Gégout J-C, Gómez-García D, Grytnes J-A, Heegaard E, Høistad F, Nogués-Bravo D, Normand S, Puşcaş M, Sebastià M-T, Stanisci A, Theurillat J-P, Trivedi MR, Vittoz P, Guisan A (2011) 21st century climate change threatens mountain flora unequally across Europe. Glob Change Biol 17:2330–2341. doi:10.1111/j.1365-2486.2010.02393.x
Estrella N, Menzel A (2006) Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Climate Res 32:253–267. doi:10.3354/cr032253
Ewald J (1997) Die Bergmischwälder der Bayerischen Alpen. Dissertationes Botanicae 290, J. Cramer, Berlin
Frank D, Esper J (2005) Characterization and climate response patterns of a high-elevation, multi-species tree-ring network in the European Alps. Dendrochronologia 22:107–121. doi:10.1016/j.dendro.2005.02.004
Gamache I, Payette S (2005) Latitudinal response of subarctic tree lines to recent climate change in eastern Canada. J Biogeogr 32:849–862. doi:10.1111/j.1365-2699.2004.01182.x
Gričar J, Čufar K (2008) Seasonal dynamics of phloem and xylem formation in silver fir and Norway spruce as affected by drought. Russ J Plant Physl 55:538–543. doi:10.1134/S102144370804016X
Gričar J, Prislan P, Gryc V, Vavrčík H, De Luis M, Čufar K (2014) Plastic and locally adapted phenology in cambial seasonality and production of xylem and phloem cells in Picea abies from temperate environments. Tree Physiol 34:869–881. doi:10.1093/treephys/tpu026
Gričar J, Prislan P, De Luis M, Gryc V, Hacurová J, Vavrčík H, Čufar K (2015) Plasticity in variation of xylem and phloem cell characteristics of Norway spruce under different local conditions. Front Plant Sci. doi:10.3389/fpls.2015.00730
Gryc V, Hacura J, Vavrčík H, Urban J, Gebauer R (2012) Monitoring of xylem formation in Picea abies under drought stress influence. Dendrobiology 67:15–24
Hadwiger H (1940) Eine analytische Reproduktionsfunktion für biologische Gesamtheiten. Scand Actuar J 1940:101–113. doi:10.1080/03461238.1940.10404802
Hartl-Meier C, Dittmar C, Zang C, Rothe A (2014a) Mountain forest growth response to climate change in the Northern Limestone Alps. Trees 28:819–829. doi:10.1007/s00468-014-0994-1
Hartl-Meier C, Zang C, Dittmar C, Esper J, Göttlein A, Rothe A (2014b) Vulnerability of Norway spruce to climate change in mountain forests of the European Alps. Clim Res 60:119–132. doi:10.3354/cr01226
Heide OM (2003) High autumn temperature delays spring bud burst in boreal trees, counterbalancing the effect of global warming. Tree Physiol 23:931–936
IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Jyske T, Mäkinen H, Kalliokoski T, Nöjd P (2014) Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric For Meteorol 194:241–254. doi:10.1016/j.agrformet.2014.04.015
Körner C (2003) Alpine plant life—functional plant ecology of high mountain ecosystems. Springer, Heidelberg
Körner C (2007) The use of altitude in ecological research. Trends Ecol Evol 22:569–574. doi:10.1016/j.tree.2007.09.006
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic Press, Toronto
Kullman L (2007) Tree line population monitoring of Pinus sylvestris in the Swedish Scandes, 1973-2005: implications for tree line theory and climate change ecology. J Ecol 95:41–52. doi:10.1111/j.1365-2745.2006.01190.x
Martinez del Castillo E, Longares LA, Gričar J, Prislan P, Gil-Pelegrin E, Čufar K, Luis M (2016) Living on the edge: contrasted wood-formation dynamics in Fagus sylvatica and Pinus sylvestris under Mediterranean conditions. Front Plant Sci 7:370. doi:10.3389/fpls.2016.00370
McCarty JP (2001) Ecological consequences of recent climate change. Conserv Biol 15:320–331. doi:10.1046/j.1523-1739.2001.015002320.x
Meier U (1997) Growth stages of mono- and dicotyledonous plants. Blackwell, Berlin
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659. doi:10.1038/17709
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kubler K, Bissolli P, Braslavska O, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl A, Defila C, Donnelly A, Filella Y, Jatcza K, Mage F, Mestre A, Nordli O, Penuelas J, Pirinen P, Remisova V, Scheifinger H, Striz M, Susnik A, van Vliet AJH, Wielgolaski FE, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976. doi:10.1111/j.1365-2486.2006.01193.x
Michelot A, Simard S, Rathgeber C, Dufrene E, Damesin C (2012) Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol 32(8):1033–1045. doi:10.1093/treephys/tps052
Moser L, Fonti P, Büntgen U, Esper J, Luterbacher J, Franzen J, Frank D (2010) Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol 30:225–233. doi:10.1093/treephys/tpp108
Nogués-Bravo D, Araújo MB, Errea MP, Martínez-Rica JP (2007) Exposure of global mountain systems to climate warming during the 21st century. Global Environ Chang 17:420–428. doi:10.1016/j.gloenvcha.2006.11.007
Piovesan G, Di Filippo A, Alessandrini A, Biondi F, Schirone B (2005) Structure, dynamics and dendroecology of an old-growth Fagus forest in the Apennines. J Veg Sci 16:13–28. doi:10.1111/j.1654-1103.2005.tb02334.x
Prislan P, Gričar J, De Luis M, Smith KT, Čufar K (2013) Phenological variations in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agr For Meteorol 180:142–151. doi:10.1016/j.agrformet.2013.06.001
R Development Core Team (2013) R: A language and environment for statistical computing. R foundation for statistical computing. Vienna. http://www.R-project.org/. Accessed 20 Feb 2016
Rosenzweig C, Karoly D, Vicarelli M, Neofotis P, Wu QG, Casassa G, Menzel A, Root TL, Estrella N, Seguin B, Tryjanowski P, Liu CZ, Rawlins S, Imeson A (2008) Attributing physical and biological impacts to anthropogenic climate change. Nature 453:353–357. doi:10.1038/nature06937
Rossi S, Anfodillo T, Menardi R (2006a) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27(1):89–97. doi:10.1163/22941932-90000139
Rossi S, Deslauriers A, Anfodillo T, Morin H, Saracino A, Motta R, Borghetti M (2006b) Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol 170:301–310. doi:10.1111/j.1469-8137.2006.01660.x
Rossi S, Deslauriers A, Anfodillo T, Carraro V (2007) Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152:1–12. doi:10.1007/s00442-006-0625-7
Rossi S, Anfodillo T, Čufar K, Cuny HE, Deslauriers A, Fonti P, Frank D, Gričar J, Gruber A, King GM, Krause C, Morin H, Oberhuber W, Prislan P, Rathgeber CBK (2013) A meta-analysis of cambium phenology and growth: linear and non-linear patterns in conifers of the northern hemisphere. Ann Bot 112(9):1911–1920. doi:10.1093/aob/mct243
Rossi S, Girard M-J, Morin H (2014) Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Glob Chang Biol 20(7):2261–2271
Schuster C, Estrella N, Menzel A (2013) Shifting and extension of phenological periods with increasing temperature along altitudinal transects in southern Bavaria. Plant Biology 16(2):332–344. doi:10.1111/plb.12071
Semeniuc A, Popa I, Timofte A, Gueran DM (2014) Xylem phenology of Fagus sylvatica in Rarău Mountains (Eastern Carpathians, Romania). Not Bot Horti Agrobo 42(1):275–279
Studer S, Appenzeller C, Defila C (2005) Inter-annual variability and decadal trends in alpine spring phenology: a multivariate analysis approach. Clim Change 73:395–414. doi:10.1007/s10584-005-6886-z
Swidrak I, Gruber A, Oberhuber W (2014) Xylem and phloem phenology in co-occurring conifers exposed to drought. Trees 28:1161–1171. doi:10.1007/s00468-014-1026-x
Tegel W, Seim A, Hakelberg D, Hoffmann S, Panev M, Westphal T, Büntgen U (2014) A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur. J For Res 133(1):61–71. doi:10.1007/s10342-013-0737-7
Vitasse Y, Porte AJ, Kremer A, Michalet R, Delzon S (2009) Responses of canopy duration to temperature changes in four temperate tree species: relative contributions of spring and autumn leaf phenology. Oecologia 161:187–198. doi:10.1007/s00442-009-1363-4
Zang C, Rothe A, Weis W, Pretzsch H (2011) Tree suitability under climate change conditions: susceptibility of major forest tree species from tree-rings widths. Allg Forst Jagdztg 182. Jg, 5/6:98–112
Acknowledgments
Our gratitude is directed to the Bavarian State Ministry of the Environment and Consumer Protection for funding the project “Auswirkungen des Klimawandels in den Alpen - Erfassung mittels Höhengradienten” (KLIMAGRAD) within the “Klimaprogramm Bayern 2020.” The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Program (FP7/2007–2013)/ERC grant agreement n° [282250]. Supported by the Technische Universität München—Institute for Advanced Study, funded by the German Excellence Initiative.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dr. Christian Ammer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. Supplement
Gompertz fits on the development of total xylem increment in the year 2011 for Fagus sylvatica and Picea abies on all sites (elevations from 800 to 1400 m a.s.l. and north and south aspect transects). (TIFF 274 kb)
Rights and permissions
About this article
Cite this article
Kraus, C., Zang, C. & Menzel, A. Elevational response in leaf and xylem phenology reveals different prolongation of growing period of common beech and Norway spruce under warming conditions in the Bavarian Alps. Eur J Forest Res 135, 1011–1023 (2016). https://doi.org/10.1007/s10342-016-0990-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-016-0990-7