Abstract
Dwarf mistletoes are widely studied because of their significant impact on host populations; however, few studies have focused on sympatric species. The understanding of their coexistence is important because it can provide useful knowledge for future management plans. We investigated the incidence, severity, and aggregation patterns of Arceuthobium vaginatum and A. globosum coexisting in Central Mexico. We estimated the correlation between both species incidences (N = 75 plots), the incidence–severity correlation (N = 47 plots) for each species, and the effect of biotic (host and non-host species abundance) and abiotic (altitude and slope) factors on the mistletoe incidence. In addition, we compared the hierarchical aggregation among and within plots of the two mistletoe species with a χ 2 test. There is a clear dominance of A. vaginatum in the area, and both species incidences are negatively correlated with each other (r s = − 0.54, P < 0.05) and host abundance (r = − 0.26, P < 0.05). The remaining factors were non-significant. Both species have a linear relationship between incidence and severity, i.e., they show a uniform increase in severity with incidence, which could help diagnose the degree of tree infection from incidence measurements. The species are aggregated within plots, but only A. globosum shows an aggregation among plots (χ 2 = 82.25, P < 0.001); aggregation has not been shown previously for two sympatric dwarf mistletoe species. Our results can improve the scientific basis for forest management planning to control dwarf mistletoe and maintain biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dwarf mistletoes (Arceuthobium spp., Viscaceae) are the most important parasitic plants in the coniferous forests of North America because their infection causes a reduction in growth (both in height and in basal area) and fitness in host trees (Madrigal et al. 2007; Geils and Vásquez 2002; Cibrián et al. 2007; Shaw et al. 2008). Branch and stem deformations are common symptoms for some host species in addition to the reduction in water use efficiency and higher water stress (Hawksworth and Wiens 1996; Meinzer et al. 2004). However, dwarf mistletoes add vertical complexity to the community because the clumps and deformations offer refuge and nesting sites and nourishment to different animals (Watson 2001).
Dwarf mistletoe population dynamics depend on spread and intensification events. Spread refers to the dispersion to new hosts (aloinfections), whereas intensification is the increase in severity within the tree (autoinfection) (Shaw et al. 2005). A distinctive feature of dwarf mistletoes is the explosive discharge of their seeds, which restricts the dispersal to short distances, usually not more than 14 or 15 m (Escudero and Cibrián 1985; Robinson and Geils 2006; Mathiasen et al. 2008). This pattern of dispersion, combined with host availability, may confer an aggregated distribution of mistletoes (Robinson and Geils 2006). Environmental factors that influence mistletoe spread and survival include elevation, latitude, slope, and climate conditions (Weir 1918; Graham and Leaphart 1961; Queijeiro-Bolaños et al. 2013). Host architecture is an important factor in mistletoe establishment, affecting the likelihood that seeds arrive at secure sites with enough light availability (Shaw and Weiss 2000; Godfree et al. 2003; Shaw et al. 2005).
Dwarf mistletoes are considered important species in Mexican forests. Because 22 of the 47 known species of Arceuthobium are distributed in Mexico, this region is considered the largest diversity center of the genus (Hawksworth and Wiens 1996); moreover, dwarf mistletoes are observed in 30 of the 32 states, primarily in the largest mountain chains (Cibrián et al. 2007). There are two species of special consideration in Mexico. Arceuthobium vaginatum subsp. vaginatum (Willd.) is the most damaging species for forestry and is widely distributed from Northern to Central Mexico. It has the broadest known host range in the genus (13 species of Mexican pines; Hawksworth and Wiens 1996). Arceuthobium globosum subsp. grandicaule (Hawks. and Wiens) is the most abundant species in Central Mexico; it is also distributed in Central America. It has the second broadest host range (12 species of Mexican pines; Hawksworth et al. 2002). These two species are extremely similar, except some phenotypic and phenological differences (Table 1). The distribution of the two species converges on the Trans-Mexican Volcanic Belt, Central Mexico, where they are the only mistletoe species parasitizing Pinus hartwegii (Hawksworth and Wiens 1996). According to their distribution, the two species can coexist in other areas where other suitable host species are present (Hawksworth and Wiens 1996), but there are no records of a sympatric parasitism. These species occur not only in the same stands but also on the same individual trees, where they show a differential distribution over the tree stem (Queijeiro-Bolaños et al. 2011). Infection process is extremely similar for both species; they depend almost entirely on balistocoric dispersal of the seeds, where these may travel no more than 15 m (depending on slope and wind), and most of these (91 %) do not travel farther than 8.7 m (Escudero and Cibrián 1985). Although it is unknown for these two species, it has been registered for other dwarf mistletoes that some animals, such as squirrels and birds, may be long-distance vectors by passively taking some of the seeds on their fur and feathers (Hawksworth and Wiens 1996). These species form localized infections and produce aerial shoots throughout the year (Hawksworth et al. 2002).
A great deal of research has been conducted on dwarf mistletoes, particularly on the impacts of disease on timber production; however, few studies have looked at sympatry. Even when the sympatry of two dwarf mistletoes has been observed (Hawksworth and Wiens 1996), there are no scientific reports on the incidence, severity, and aggregation patterns of two coexisting dwarf mistletoe species. The aim of this study was to investigate the intensity (i.e., incidence and severity) and aggregation patterns of A. vaginatum and A. globosum in a Natural Protected Area in Central Mexico. Our specific objectives were to (1) assess the incidence of both species and the correlation between them, (2) investigate the relationship between incidence and severity rates, (3) determine the effect of abiotic factors (altitude and slope) over the mistletoe incidence, and (4) determine the hierarchical aggregation pattern of the two species. This study is a preliminary step in the research of the dwarf mistletoe associations.
Materials and methods
Study site
The study site is located in the Zoquiapan National Park (ZNP; 19°15–29′N, 98°37–45′W), in Central Mexico. ZNP has an area of 19,400 ha and is part of the Popocatépetl–Iztaccíhuatl biological corridor (Arriaga et al. 2000). This region is important because it comprises a significant gradient of ecosystems due to the wide altitude range observed in the area (2,850–4,150 m; Vargas 1997; Arriaga et al. 2000). The climate is classified as temperate subhumid with the rainy season from June to September (Arriaga et al. 2000). The mean annual temperature is 9.7 °C, and the average annual rainfall is 941.3 mm (SMN 2010). The vegetation of the area is classified as “temperate forests” and is dominated by P. hartwegii, but other abundantly represented genera include Pinus, Cupressus, Quercus, Alnus, and Abies. The forest understory is primarily represented by tillering grasses such as Muhlenbergia macroura and Festuca tolucencis (Obieta and Sarukhán 1981; Arriaga et al. 2000). This area can be considered as an infection center because it accumulates the large infection rates of A. vaginatum and A. globosum (Hawksworth and Wiens 1996; Queijeiro-Bolaños et al. 2013).
Data collection
In July 2009, we selected 75 plots, each 0.33 ha in size, based on map of the area; the selected plots should accomplish the following criteria: clear dominance of P. hartwegii, minimum area to fit a plot, accessibility, and non-fragmented by roads or logged areas. The plots were at least 100 m apart, centerline to centerline. The plots varied in altitude from 3,021 to 3,650 m and slope from relatively flat areas to high steepness (Table 2). For each plot, we counted the total number of pines and the number of trees parasitized by A. globosum and/or A. vaginatum. Mistletoe incidence was calculated as the percentage of pines infected by each species.
In addition, we obtained the dwarf mistletoe rating (DMR) for a subset of 47 of the 75 previously selected plots. DMR system is a qualitative measurement of the degree of infection per tree (Hawksworth 1977), and it is suitable as a measure of severity, which is the quantity of disease affecting entities within a sample unit (Seem 1984). The DMR system rates a tree by dividing the tree into thirds; each third is visually classified from 0 to 2, where 0 represents the absence of infection, 1 represents <50 % of the main branches infected, and 2 represents more than 50 % of main branches infected. The DMR for each tree is calculated as the sum of the score of the thirds. The mean DMR for each plot was calculated as the average of the DMRs of all trees, including infected and uninfected ones (Hawksworth et al. 2002).
Statistical analysis
Field data were analyzed to evaluate the following:
Incidence and severity relationships
A χ 2 test was completed to determine whether the frequencies (number of observed infected trees) differed between mistletoe species. To evaluate the relationship between the incidence of one species and the incidence of the second one (incidence–incidence relationship), a Spearman’s rank correlation was computed for the plots where both species coexisted. To know the relationship between the incidence (percentage of infected trees) and severity of the infection (mean DMR), we performed a linear regression for each species. This relationship is useful to diagnose the disease because it can predict the rate of increase in severity with increasing incidence. Furthermore, incidence is a measure that can be taken in an easier and precise manner, and it would permit the estimation of severity for disease assessment (Seem 1984). These analyses were carried out with Statistica 8.0 (StatSoft Inc. 2007) software.
Factors affecting dwarf mistletoe incidence
We constructed one structural equation model (SEM) for each mistletoe species that estimated the causal relationships between the mistletoes incidence and other variables. From a previous work (Queijeiro-Bolaños 2007), we anticipated what causal paths may be important. For all models, we considered plot altitude and slope to be abiotic factors. The biotic factors we examined were abundance of non-host species, host tree abundance, and mistletoe incidence.
Parameters of the models were estimated using maximum likelihood, and goodness of fit was analyzed with a χ 2 test. The fitted models were compared with the Akaike information criterion (AIC). The models were performed with the lavaan package for R (Rosseel 2011).
Aggregation
To evaluate the aggregation at two different scales, we used the statistical method developed by Boulinier et al. (1996) and adopted by Rist et al. (2011) for parasitic plants. This method allows us to distinguish the aggregation at two hierarchical levels: among plots and among trees within the plots, testing whether the mistletoes are distributed in a random fashion on both within- and among-plot scales (Boulinier et al. 1996). This method was applied for the DMR values obtained for 47 plots, for each mistletoe species.
If mistletoes are distributed among plots in a random fashion, according to a Poisson distribution, then
where N is the total number of plots, X j is the mean DMR of plot j, and X m is the mean DMR pooling all plots. The degrees of freedom are calculated as N − 1. If mistletoes were distributed within plots in a random fashion, according to a binomial distribution, then
where N p is the number of plots with at least two pines and one infected pine, n j is the number of pines in plot j, and x ij is the DMR of the pine i of the plot j. The degrees of freedom are calculated as n p − N p , where n p denotes the number of pines in N p .
To define the strength of the aggregation among and within plots, we used the measure J, which gives the global measure of aggregation (Boulinier et al. 1996). It is the sum of the aggregation among plots, J k , and within plots, J j . Each of these measures, J k and J j , gives us the contribution to global aggregation; therefore, J k /J equals the proportion of aggregation due to among-plot patterns and J J /J the proportion due to aggregation within-plot patterns (Boulinier et al. 1996).
Results
Incidence–severity relationships
Arceuthobium vaginatum was detected in 67 plots, and A. globosum occurred in only 19 of the 75 plots (Fig. 1). Only four plots were free of infestation, and the two species coexisted in 15 plots. The incidences among plots were highly variable. The maximum incidence of A. vaginatum was 88 % of the trees infected and the minimum 1 %. In contrast, the maximum incidence of A. globosum was 62 % of the trees infected and the minimum 1 % (Table 2). The prevalence, i.e., the incidence of all plots pooled, was 35.46 % (N = 11,100 trees); it was determined that 27.6 % of the trees were infected by A. vaginatum, 6.7 % by A. globosum, and 1.15 % by both species.
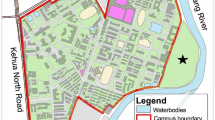
Location and incidence of the plots (N = 75) in ZNP. On each pie chart, white = percentage of non-infested trees, black = percentage of Arceuthobium vaginatum, light gray = percentage of A. globosum, and dark gray = percentage of trees infected with both species. Note that the size of the charts does not represent the size of the plot
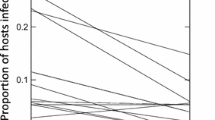
Few non-infested plots occurred, and the trees infected by A. vaginatum were significantly more frequent than those affected by A. globosum (χ 2 = 26.8, df = 1, P < 0.001). In addition, in plots where both mistletoe species were present, they had a significant negative correlation (r s = − 0.54, p < 0.05, N = 15; Fig. 2).
For the 47 plots where DMR was measured, we observed that the most common classes of infection are the lightest ones (1 and 2) for both species (Fig. 3). It is also clear that A. vaginatum is considerably more abundant than A. globosum. Mean DMR was highly variable among plots, with A. globosum ranging from 0.002 to 1.18 and A. vaginatum from 0.08 to 2.05.
The two species have a clearly linear relationship between incidence and mean DMR of the plots (Fig. 4). The regression equation for A. globosum is DMR = − 0.0014 + 0.0063 (incidence) (r = 0.99, r 2 = 0.97, P < 0.001), where the slope coefficient indicates the rate of increment of DMR with the increment of incidence. The regression equation for A. vaginatum is DMR = − 0.0403 + 0.0073 (incidence) (r = 0.93, r 2 = 0.87, P < 0.001).
Factors affecting dwarf mistletoe incidence
The SEM proposed for the two species had a good fit [Table 3; model (a) for A. globosum and model (b) for A. vaginatum]. Figure 5 shows the partial correlation coefficients for each path. The abundance of both mistletoe species was negatively correlated with each other and with the abundance of host trees. These models explained 21 and 29 percent of the variation in the incidence of A. globosum and A. vaginatum, respectively.
Fitted paths for models (a) and (b). Solid paths are statistically different from 0 (P < 0.05), whereas dashed paths are not. Dotted paths are marginally significant (P < 0.08). Path widths are proportional to the standardized regression coefficients. Only the coefficients for significant paths are shown. The R 2 of the endogenous variables are inside the variable boxes
Abundance of non-host species showed a negative, but marginally significant, relationship with A. vaginatum incidence. The abiotic factors, i.e., altitude and slope, were not significantly correlated with the incidence of the two mistletoe species. A full detail of path coefficients is presented in “Appendix.”
Dwarf mistletoe aggregation
Arceuthobium vaginatum exhibited an aggregated pattern within plots (χ 2 = 354,903, df = 5,938, P < 0.0001), yet had a random distribution among plots (χ 2 = 17.67, df = 46, P > 0.05); within-plot aggregation represented the 63 % of the total aggregation. In contrast, A. globosum had an aggregated pattern within (χ 2 = 7,324, df = 1,328, P < 0.0001) and among plots (χ 2 = 82.25, df = 46, P < 0.001); within-plot aggregation explained 55 % of the total aggregation. Only a small number of trees had a heavy infection (Fig. 3), which coincides with the aggregation pattern within trees. While only a few trees were infected with classes 1–5 of A. globosum, A. vaginatum infection was more prominent in these classes. When the two mistletoes species were infecting the same tree, the DMR of each species rarely reached a class above 3 (Table 4).
Discussion
Our study indicates that A. vaginatum is more likely to be found in the Zoquiapan National Park than A. globosum, revealing its clear dominance in the surveyed zone. The overall prevalence of both species in the zone was 35 % of the trees, which is observed within the range reported in the literature for several mistletoe species. The prevalence values are highly variable not only among systems, places, and species but also within species in an area, for both Loranthaceae and Viscaceae families. In some cases, the incidence can range from 18.0 to 33.6 % in the same area (A. oxycedri on Juniperus excelsa; Sarangzai et al. 2010). Previous surveys have indicated that Arceuthobium spp. can infest 3–77 % P. hartwegii trees in ZNP (Andrade and Cibrián 1980; Hernández-Benítez et al. 2005), whereas in Nevado de Toluca (state of Mexico), they infest up to 94 % pines P. montezumae (Ramírez-Dávila and Porcayo-Camargo 2009). Likewise incidence, the mean DMR of both species is consistent with what has been found in studies performed in other areas, where it ranges between 1 and 3 (Andrade and Cibrián 1980; Trummer et al. 1998; Sarangzai et al. 2010; Maloney and Rizzo 2002).
Both species exhibited a linear relationship between incidence and mean DMR, showing a uniform increase in DMR with incidence. According to Seem (1984), this type of pattern can be explained by a greater predominance of autoinfection due to a short-range dispersal mechanism. These species do not form a systemic infection; nevertheless, they have an explosive dispersal of the seeds that allow them to spread to no more than 15 m apart. Therefore, this species should have a slow spread and a faster intensification. This relationship is useful to predict the DMR on a stand from taking only measurements of incidence, which simplifies the diagnosis. But these models are only applicable to this zone because of the high variability among areas and species.
According to our SEM models, there is a significant relationship between the incidences of both species. The incidence–incidence relationship between two different species of dwarf mistletoes is a non-explored subject, although the dual parasitism has been described in another 12 cases (Hawksworth and Wiens 1996). This kind of association is infrequent for dwarf mistletoes, but is fairly common for other species such as Phoradendron and Loranthaceae members (see, for example, Genini et al. 2012). The sympatry of two Arceuthobium species is not unusual, but dual parasitism (two different species parasitizing a single host tree) is a rare event (Hawksworth and Wiens 1996). Hawksworth (1969) defined the term “competitive host exclusion” where if an Arceuthobium species is present in an area, other dwarf mistletoes species will rarely infest the same host. Nevertheless, the mechanism that controls this phenomenon has not been explained nor has been subjected to research, as stated by Hawksworth and Wiens (1996). In the case of A. globosum and A. vaginatum, they dual-parasitize P. hartwegii, a principal host for both species. But A. globosum was observed in a lower proportion than A. vaginatum, and the model shows that the two species have a negative relationship, possibly depicting some kind of host competitive exclusion process and a differential dispersal or recruitment patterns. All of these require further investigation. For example, when A. americanum and A. vaginatum subsp. cryptopodum are sympatric, the first one only parasitizes 13 % trees of P. ponderosa (secondary host) and the second one 64 % trees (principal host) (Hawksworth 1969).
The abundance of P. hartwegii was also a significant and negative variable, affecting the incidences of both mistletoe species, i.e., less hosts and more infection. Host availability and host demography are important factors determining the incidence of an obligate parasite (Donohue 1995). The negative correlation with host abundance has been observed for other mistletoe species, such as Phoradendron pauciflorum on Abies concolor (Maloney and Rizzo 2002). In low-density stands, the host trees have less competition for resources and have a better performance; thus, these stands are more appealing than the high-density stands (Bickford and Kolb 2005). In addition, the higher light incidence in less-dense stands favors the production of aerial shoots of the mistletoes (Shaw and Weiss 2000; Bickford and Kolb 2005; Robinson and Geils 2006).
Non-host species abundance is positively related to A. vaginatum incidence, although it was only marginally significant. This could be evidence that the non-host distribution also plays an important role in the creation of infection centers, similar to that reported in previous studies (Trummer et al. 1998; Maloney and Rizzo 2002), where the non-host forms a barrier, impeding the seed dispersal, and thus favoring the concentration of seeds among the same trees (Trummer et al. 1998).
Altitude and slope were not observed to be significant factors in determining the incidence of mistletoes and are, therefore, not informative in predicting where could we find each species or which areas are more susceptible for infestation. Ramírez-Dávila and Porcayo-Camargo (2009) reported that Arceuthobium spp. can be distributed equally within 2,850 and 3,150 masl and with any slope percentage. Even though the environmental variables are important determinants of the mistletoe species distribution on regional scales (Hawksworth 1969; Williams et al. 1972; Smith and Wass 1979), several microclimatic aspects should also be considered. These include the conditions that influence seedling establishment, such as optimal temperature, light exposure, and humidity, as shown for A. tsugense (Deeks et al. 2001) and A. americanum (Brandt et al. 2005).
Both species showed an aggregation pattern within trees, which coincides with the creation of infection centers. A. vaginatum did not show any aggregation at the plot level and presented a random distribution among areas. The patterns of aggregation that we observed are comparable with other reports on parasitic plants. These studies indicate that the majority of hosts are affected by a small amount of parasites, and only a few trees bear a large load of parasites (Norton et al. 1995; Aukema 2003; Ramírez-Dávila and Porcayo-Camargo 2009; Rist et al. 2011). The seed dispersal mechanisms have a strong influence on the spatial arrangement of parasitic plants. In case of dwarf mistletoes, the aggregation within the host is probably the result of the ballistic dispersal of the seeds, which promotes the short-distance spread or intensification within the same tree (Hawksworth and Wiens 1996). It has been reported for other species that 40 % of dispersed seeds are intercepted by trees (Hawksworth and Wiens 1996); from experimental data, we know that for A. globosum, the germination rate is of 55 %, and for A. vaginatum, it is 32 %, while the rest of these get desiccated. Furthermore, of these, only 2 % are successfully established on new trees (Queijeiro-Bolaños and Cano-Santana, unpublished data). The latter may confer an aggregated distribution within neighboring trees and the intensification on those already infected. In contrast, the aggregation among plots may have several causes, such as microhabitat preference, host defense mechanisms, habitat heterogeneity (including local disturbances), and biotic interactions (Medel et al. 2004). It could be a host exclusion mechanism, indicating a local process that reduces species overlap (Genini et al. 2012). We have evidence from 15 plots that when the two species do not coexist, they achieve a greater DMR rate. In contrast, when they share the same host, they did not accomplish a larger infection rate (Queijeiro-Bolaños, Cano-Santana, and García-Guzmán, unpublished data).
The local history of the site may have a great influence on dwarf mistletoe distribution and aggregation patterns. ZNP is an area that suffers from several types of disturbance such as natural and induced fires, legal and illegal unsustainable logging, landfill sites, and cattle grazing (Obieta and Sarukhán 1981; Arriaga et al. 2000). Disturbance can have an impact on mistletoe populations, and it is well known that fire has an influence on mistletoes occurrence and distribution patterns (Knight 1987; Kipfmueller and Baker 1998; Swanson et al. 2006). Although the present study is not addressed toward disturbance effects, we know from previous work that there is some evidence supporting the hypothesis that fire and logging may favor dwarf mistletoe incidence (Queijeiro-Bolaños 2007; Queijeiro-Bolaños et al. 2013). The plots on this work did not have evidence of logging (or this was minimum); however, we do not know the historical fire regime of the 75 plots. Nevertheless, fire in the past may have had an influence on the aggregation patterns of these two species, and this aspect needs further consideration.
Aggregation is a known pattern for parasitic plants, but it has never been shown for two sympatric dwarf mistletoe species, and this brings a new insight into dual parasitism. There are other significant ecological factors, playing an important role over the mistletoes incidence, such as the host and non-host species disposition; thus, we suggest that the study of the tree disposition (host and non-host) and seed dispersal may be relevant. Although some authors have modeled the ballistic and contagion of dwarf mistletoes (e.g., see Robinson and Geils 2006), their models did not include two sympatric and possibly competing species. Therefore, it is important to perform studies of distribution patterns, spread and intensification of dwarf mistletoe species in coexistence, and manipulative experiments that are focused on testing competitive interactions directly to give more insights into sympatric parasitism.
References
Andrade V, Cibrián D (1980) Evaluación de poblaciones de muérdago enano (Arceuthobium globosum Hawks. y Wiens y A. vaginatum Willd) en bosques de Pinus hartwegii Lindl en Zoquiapan, Edo. de México. In: Proceedings of the Simposio Nacional sobre Parasitología Forestal, Uruapan, Mich., 18–19 February 1980, pp 238–253
Arriaga L, Espinoza J, Aguilar C, Martínez E, Gómez L, Loa E (2000) Regiones terrestres prioritarias de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City
Aukema JE (2003) Vectors, viscin, and Viscaceae: mistletoes as parasites, mutualists, and resources. Front Ecol Environ 1:212–219
Bickford C, Kolb T (2005) Host physiological condition regulates parasitic plant performance: Arceuthobium vaginatum subsp. cryptopodum on Pinus ponderosa. Oecologia 146:179–189
Boulinier T, Ives AR, Danchin E (1996) Measuring aggregation of parasites at different host population levels. Parasitology 112:581–587
Brandt JP, Hiratsuka Y, Pluth DJ (2005) Germination, penetration, and infection by Arceuthobium americanum on Pinus banksiana. Can J For Res 35:1914–1930
Cibrián D, Vázquez I, Cibrián J (2007) Muérdagos enanos del género Arceuthobium. In: Cibrián D, Alvarado D, García S (eds) Enfermedades forestales en México. Universidad Autónoma de Chapingo, Mexico City, pp 357–395
Deeks S, Shamoun SF, Punja ZK (2001) In vitro germination and development of western hemlock dwarf mistletoe. Plant Cell Tiss Organ 66:97–105
Donohue K (1995) The spatial demography of mistletoe parasitism on a Yemeni acacia. Int J Plant Sci 156:816–823
Escudero M, Cibrián D (1985) Determinación del periodo de dispersión de Arceuthobium globosum grandicaule en la región central de México. In: Proceedings of the II Simposio Nacional de Parasitología Forestal. Saltillo, Coah., pp 342–351
Geils BW, Vásquez I (2002) Loranthaceae and Viscaceae in North America. In: Geils BW, Cibrián J, Moody B (eds) Mistletoes of North America conifers. USDA For. Serv. Res. Pap. RMRS-GTR-98, Fort Collins, pp 1–8
Genini J, Côrtes MC, Guimarâes PR, Galetti M (2012) Mistletoes play different roles in modular host–parasite network. Biotropica 44:171–178
Godfree RC, Tinnin RO, Forbes RB (2003) Relationships between dwarf mistletoe and the canopy structure of an old-growth lodgepole pine forest in central Oregon. Can J For Res 33:997–1009
Graham DP, Leaphart CD (1961) Larch and lodgepole pine dwarf mistletoes attack Scotch pine. J For 59:375–376
Hawksworth FG (1969) Ecological aspects of dwarf mistletoe distribution. In: Proceedings, 16th annual western international forest disease work conference. Coeur d’Alene, ID, 28 October-1 November 1, pp 74–82
Hawksworth FG (1977) The 6-class dwarf mistletoe rating. USDA For. Serv. Gen. Tech. Rep. RM-48, Fort Collins
Hawksworth FG, Wiens D (1996) Dwarf mistletoes: Biology, pathology and systematics. USDA For. Ser. Handb. 709., Washington, DC
Hawksworth FG, Wiens D, Geils BW (2002) Arceuthobium in North America. In: Geils BW, Cibrián J, Moody B (eds) Mistletoes of North America conifers. USDA For. Serv. Res. Pap. RMRS-GTR-98, Fort Collins, pp 29–56
Hernández-Benítez R, Cano-Santana Z, Castellanos-Vargas I (2005) Incidencia de infestación de Arceuthobium globosum grandicaule (Hawksw. & Wiens) en Pinus hartwegii Lindl. Rev Cien For Mex 30:79–86
Kipfmueller K, Baker W (1998) Fires and dwarf mistletoe in a Rocky Mountain lodgepole pine ecosystem. For Ecol Manag 108:77–84
Knight D (1987) Parasites, lightning, and the vegetation mosaic in wilderness landscapes. In: Turner MG (ed) Landscape heterogeneity and disturbance. Springer-Verlag, New York, pp 59–83
Madrigal S, Vásquez I, Velasco E (2007) Obtención de parámetros dasométricos para evaluar efecto causado por Arceuthobium vaginatum en Pinus hartwegii del Nevado de Colima. In: Proceedings of the VII Congreso Mexicano de Recursos Forestales, Morelia, Mich., 28–31 October 2005, pp 1–7
Maloney PE, Rizzo DM (2002) Pathogens and insects in a pristine forest ecosystem: the Sierra San Pedro Mártir, Baja, Mexico. Can J For Res 32:448–457
Mathiasen RL, Nickrent DL, Shaw DC, Watson DW (2008) Mistletoes. Pathology, systematics, ecology and management. Plant Dis 92:988–1006
Medel R, Vergara E, Silva A, Kalin-Arroyo M (2004) Effects of vector behavior and host resistance on mistletoe aggregation. Ecology 85:120–126
Meinzer FC, Woodruff DR, Shaw DC (2004) Integrated responses of hydraulic architecture, water and carbon relations of western hemlock to dwarf mistletoe infection. Plant Cell Environ 27:937–946
Norton D, Hobbs R, Atkins L (1995) Fragmentation, disturbance, and plant distribution: mistletoes in woodland remnants in the Western Australian Wheatbelt. Conserv Biol 9:426–438
Obieta MC, Sarukhán J (1981) Estructura y composición de la vegetación herbácea de un bosque uniespecífico de Pinus hartwegii. I. Estructura y composición florística. Bol Soc Bot Mex 41:75–124
Queijeiro-Bolaños M (2007) Interacciones entre dos especies de muérdago enano (Arceuthobium spp.) y Pinus hartwegii en el Parque Nacional Zoquiapan, Estado de México: El papel del disturbio. Dissertation, Facultad de Ciencias, Universidad Nacional Autónoma de México, Mexico City, Mexico
Queijeiro-Bolaños M, Cano-Santana Z, Castellanos-Vargas I (2011) Distribución diferencial de dos especies de muérdago enano sobre Pinus hartwegii en el Área Natural Protegida “Zoquiapan y Anexas”, Estado de México. Acta Bot Mex 96:49–57
Queijeiro-Bolaños M, Cano-Santana Z, Castellanos-Vargas I (2013) Does disturbance determines the prevalence of dwarf mistletoe (Arceuthobium, Santalales: Viscaceae) in Central Mexico? Rev Chil Hist Nat 86:181–190
Ramírez-Dávila JF, Porcayo-Camargo E (2009) Estudio comparativo de la distribución espacial del muérdago enano (Arceuthobium sp.) en la ladera norte del Parque Nacional Nevado de Toluca, México. Bosque 31:28–38
Rist L, Shaanker RU, Ghazoul J (2011) The spatial distribution of mistletoe in a southern Indian tropical forest at multiple scales. Biotropica 43:50–57
Robinson D, Geils BW (2006) Modelling dwarf mistletoe at three scales: life history, ballistics and contagion. Ecol Model 199:23–38
Rosseel Y (2011) LAVAAN: latent variable analysis. R package version 0.4–8. http://www.r-project.org. Accessed 1 June 2010
Sarangzai AM, Khan N, Wahab M, Kakar A (2010) New spread of dwarf mistletoe (Arceuthobium oxycedri) in Juniperus forests, Ziarat, Balochistan, Pakistan. Pak J Bot 42:3709–3714
Seem RC (1984) Disease incidence and severity relationships. Annu Rev Phytopathol 22:133–150
Servicio Meteorológico Nacional (SMN) (2010) Normales climatológicas. Mexico. http://smn.cna.gob.mx/. Accessed 1 June 2010
Shaw D, Weiss S (2000) Canopy light and the distribution of hemlock dwarf mistletoe (Arceuthobium tsugense [Rosendahl] G.N. Jones subsp. tsugense) aerial shoots in an old-growth Douglas-fir/western hemlock forest. Northwest Sci 74:306–315
Shaw D, Chen J, Freeman EA, Braun DM (2005) Spatial and population characteristics of dwarf mistletoe infected trees in an old-growth Douglas fir-western hemlock forest. Can J For Res 35:990–1001
Shaw D, Huso M, Bruner H (2008) Basal area growth of dwarf mistletoe on western hemlock on an old-growth forest. Can J For Res 38:576–586
Smith RB, Wass EF (1979) Infection trials with three dwarf mistletoe species within and beyond their known ranges in British Columbia. Can J Plant Pathol 1:47–57
Statsoft Inc. (2007) STATISTICA (data analysis software system), version 8.0. www.statsoft.com. Accessed 1 June 2010
Swanson M, Shaw D, Marosi T (2006) Distribution of Western Hemlock Dwarf Mistletoe (Arceuthobium tsugense [Rosendahl] G.N. Jones subsp. tsugense) in Mature and Old-growth Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) Forests. Northwest Sci 80:207–217
Trummer LM, Hennon PE, Hansen EM, Muir PS (1998) Modeling the incidence and severity of hemlock dwarf mistletoe in 110-year-old wind-disturbed forests in Southeast Alaska. Can J For Res 28:1501–1508
Vargas MF (1997) Parques Nacionales de México. Vol. I. Zonas Centro, Occidente y Oriente. Instituto Nacional de Ecología, Mexico City
Watson DM (2001) Mistletoe—a keystone resource in forest and woodland worldwide. Annu Rev Ecol Syst 32:219–249
Weir JM (1918) Experimental investigations on the genus Razoumofskya. Bot Gaz 66:1–31
Williams WT, Fortier F, Osborn J (1972) Distribution of three species of dwarf mistletoe on their principal pine hosts in the Colorado Front Range. Plant Dis Rep 46:223–226
Acknowledgments
The authors thank Isael Victoria-Salazar, Omar Becerra-Soria, and Emmanuel Zeno for field assistance; Iván Castellanos-Vargas for technical support; Agustín Tagle and Omar Maldonado from the Izta-Popo Zoquiapan National Park office; and Dr. Tulio Méndez-Montiel and Octavio Pérez for permission and facilities to work at the Zoquiapan Experimental Station and its surroundings. This study was supported by UNAM-DGAPA-PAPIIT IN220912 grant to ZC-S and financial aids from the Programa de Posgrado en Ciencias Biológicas, the Facultad de Ciencias, and the Instituto de Ecología from the Universidad Nacional Autónoma de México (UNAM). MQ-B was supported by a competitive grant from the Consejo Nacional de Ciencia y Tecnología (CONACYT-220652) for postgraduate studies while she studied at the Posgrado en Ciencias Biológicas from the UNAM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Matyssek
Appendix
Appendix
See Table 5.
Rights and permissions
About this article
Cite this article
Queijeiro-Bolaños, M., Cano-Santana, Z. & García-Guzmán, G. Incidence, severity, and aggregation patterns of two sympatric dwarf mistletoe species (Arceuthobium spp.) in Central Mexico. Eur J Forest Res 133, 297–306 (2014). https://doi.org/10.1007/s10342-013-0762-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-013-0762-6